INTRODUCTION
(1,3)-β-D-glucans (glucans) is a heterogenous group of glucose polymers present as structural elements in the cell walls of yeast, fungi and cereals. Biological activities of these molecules have been reported to inhibit tumor formation (Ohno et al., 1995), enhance defense against bacterial challenge (Onderdonk et al., 1992; Babineau et al., 1994), and increase growth performance (Schoenherr et al., 1994) in pigs. Glucans from a variety of yeast cell walls have been shown to stimulate both specific and non-specific immune responses (salmon, Robertsen et al., 1990; Pigs, Williams et al., 1994). In vitro studies have shown that these molecules influence macrophage morphology (Burgaleta et al., 1978), increase nitric oxide production (Ohno et al., 1996), and release some kind of cytokines, such as TNF-α, IL-6, IL-1 and IL-2 (Adachi et al., 1994; Chen et al., 2003). Glucans could also decrease inflammatory cytokine responses (Poutsiaka et al., 1993), thereby allowing nutrients to be partitioned toward growth demand (Klasing et al., 1987).
Due to the ban of most antibiotics growth promoters in the Europe and the expected spread of this trend to the rest of the world, finding alternative means of boosting disease resistance without the antibiotics is considerable practical
interest of animal production. In this context, the use of immune modulators for increasing host defense and immunocompetence is receiving more attention.
Although glucans have not been widely used in veterinary practice and animal production, Liu and Li (1999) reported that lentinan (a type of glucan) has effects on enhancing interleukin-2 (IL-2) production of broilers infected by Marek’s virus. It was suggested that lentinan may be useful in treating against Marek’s disease and other viral diseases because of its potential antitumor and immunostimulating activities. However, little is known about the effects of β-glucans on the immunity of broilers. The aim of the present study was to investigate the effects of dietary β-glucans on the growth performance, lymphocytes blastogensis and macrophage functions in broilers.
MATERIALS AND METHODS
Animal and diets treatment
A total of 160 day-old broilers (half male and half female) were obtained from a commercial hatchery. They were randomly allotted into four dietary treatments containing 0, 0.012, 0.025 and 0.05% β-glucan. Then birds were raised in electrically heated brooders with 10 chicks per pen, 2 pens for each treatment, and repeat once. The basal diet (Table 1) was base on corn and soybean meal and was formulated according to NRC (National Research Council, 1994). The β-1,3-glucan was derived from
Schizophyllum commune and was obtained from Taito Co.
(Tokyo, Japan). Feed and water were provided ad libitum throughout the 6 wk experimental period. Chickens were
Effects of
β-Glucan Supplementation on Lymphocyte Proliferation, Macrophage
Chemotaxis and Specific Immune Responses in Broilers
Yeong-Hsiang Cheng*, Der-Nan Lee, Chiu-Ming Wen1 and Ching-Feng Weng2Department of Animal Science, National I-Lan University, I-Lan, Taiwan, ROC
ABSTRACT : Immunomodulatory feed additives might offer alternatives to antimicrobial growth promoters in poultry production. This experiment was carried out to test the effect of β-glucan supplementation on the growth performance and immune response in broilers. Total of 160 day-old broilers were randomly assigned to 4 treatment groups fed corn-soybean diets containing 0, 0.012, 0.025 or 0.05% of β-glucan supplement in a 6 week feeding experiment. Growth performance, antibody titer against New Castle vaccine, lymphocyte blastogensis, and peritoneal macrophage chemotaxis activity of broilers were evaluated. Results showed that there were no significant differences in weight gain and feed efficiency among the treatments, and no differences in antibody titer was observed. Supplementation of β-glucan did not elevate the lymphocyte blastogensis among treatments, following stimulation with different mitogens. However, supplementation with 0.025 and 0.05% β-glucan enhanced the macrophage chemotaxis activity of broilers. These results suggest that β-glucan may enhance some cell-mediated immune responses of chickens by modulate macrophages ability. (Asian-Aust. J. Anim. Sci. 2004. Vol 17, No. 8 : 1145-1149)
Key Words : β-glucan, Lymphocyte proliferation, Macrophage chemotaxis, Immune responses, Broilers
* Corresponding Author: Yeong-Hsiang Cheng. Tel: +886-3-9357400 (808), Fax: +886-3-9369506, E-mail: yhcheng@niu. edu.tw
1 Department of Life Science, National Kao-Hsiung University,
Kao-Hsiung, Taiwan, ROC.
2 Institute of Biotechnology, National Dong Hwa University,
Hualian, Taiwan, ROC.
bled form cardiac puncture at 3 and 6 wks of age. The blood was for subsequently analyzed antibody titer and lymphocyte blastogensis. Body weights, feed intake and feed conversion by pen were recorded at weekly intervals. Mortality was recorded when occurred. The experimental protocol was approved by National I-Lan University Experimental Animal Committee.
Lymphocytes blastogensis assays
Whole blood Lymphocytes proliferation assay was described as Talebi et al. (1995). Briefly, blood with anticoagulant were drained, and the separated lymphocytes (1×107 cells/0.2 ml/well) were cultured in triplicates with RPMI-1640 growth medium supplemented with 10% heat inactivated fetal bovine albumin and antibiotics (penicillin, 100 U/ml and streptomycin, 50 µg/ml), in a 96 well plate in a 39°C humidified chamber with 5% CO2 and 95% air for 72 h. To determine mitogen stimulation of lymphocytes, different mitogens were used including 20 µg/ml lipopolysaccharides (LPS, 055:B5, Sigma, St. Louis, Mo), 35 µg/ml concanavalin A (Con A), and 24 µg/ml pokeweed mitogen (PWM). The concentrations of used mitogens have been test and shown optimal proliferation in our preliminary study (Cheng and Pang, 1996). Lymphocytes were pulsed with 0.2 µCi/well 3H-thymidine (Specific activity 80 Ci/ml, Amstersham Pharmacia Biotech.,
Sweden) for another 18 h incubation, and the cells were harvested onto a glass filter. 3H-thymidine incorporation was determined by using luminescence counter (Packard, USA) and data are presented as cpm value basis, due to the previous study showed that 3H-thymidine incorporation into lymphocyte was not significantly difference from the dietary treatments. Three birds from each treatment were used for the blastogensis assay.
Harvest of macrophages
Sephadex-elicited peritoneal exudative cells (PECs), the source of macrophages, were obtained by the method described by Trembicki et al. (1984). Briefly, a 3% swollen Sephadex-G50 suspension in sterile saline (0.75%) was injected intraperitoneally into each bird at 1 ml per 100 g body wt. Approximately 42 h after injection, the birds were euthanasia, and PECs were collected by flushing the peritoneal cavity with 30 ml of sterile heparin (0.5 U/ml) saline solution. The peritoneal exudates were centrifuged at 285×g at 4°C for 15 min. The resulting PEC pellets were resuspended in 2 ml complete medium consisting of RPMI-1,640 growth medium supplemented with 10% heat inactivated fetal bovine serum and antibiotics (penicillin, 100 U/ml and streptomycin, 50 µg/ml). The viable numbers of PECs were determined by trypan blue exclusion on a hemocytometer, and the cell density was adjusted to 2×106 PECs/ml. Six birds from each treatment were used for the harvest of macrophages.
Nitric oxide production of peritoneal macrophages The macrophages from different treatments were stimulation with 1 mg/ml of LPS for 0, 3 and 6 h respectively. The accumulation of NO in culture supernatants was measured by following the Ding et al. (1988) described method. Briefly, 100 ul of supernatant was pipetted from each well into an empty 96 well plate. After the addition of 100 ul Griess reagent to each well, absorbance at 540 nm was measured by using a ELISA reader (Tecan, Maennedorf, Switzerland). NO concentration was calculated from a NaNO2 standard curve. Griess reagent was prepared by mixing one part of 0.1% naphthylethylene diamine dihydrochloride in distilled water plus one part of 1% sulfanilamide in 5% concentrated H3PO4.
Measurements of antibody titers
Antibody titers of Newcastle disease (ND) were determined by ELISA reader using the Idexx test kit (IDEXX, Maine, USA). For blood biochemical assay, blood from 3 or 6 week-old chicks per treatment (8 birds/ treatment) were collected by cardiac puncture, and the blood were centrifuged at 2,654×g for 10 min.
Table 1. The composition and calculated analysis of basal diet
(%)
Ingredients (0-3 wks) Growers (4-6 wks) Finishers
Corn 46.00 56.90 Soybean meal 43.41 34.13 Soybean oil 6.94 5.30 Dicalcium phosphate 1.58 1.40 Limestone 1.35 1.50 Salt 0.40 0.40 DL-methionine 0.15 0.07 Choline chloride 0.02 0.05 Vitamin premix 0.10 0.10 Mineral premix 0.10 0.10 Coccidiostat 0.05 0.05 Total 100.00 100.00
Calculated nutrient composition
Crude protein 23 20 Metabolizable energy, kcal/kg 3,200 3,200 sulfur-containing amino acid
(Met+cys)
0.84 0.71
Lysine 1.30 1.10
Calcium 1.00 1.00
Available phosphorus 0.45 0.40
A Supplied per kg diet: vitamin A, 8,000 IU; vitamin D
3, 1,200 ICU; vitamin E, 10 IU; vitamin K, 2 mg; thiamin, 2 mg; riboflavin, 5 mg; pyridoxine, 0.2 mg; vitamin B12, 0.03 mg; Ca-pantothenate, 10 mg; niacin, 50 mg; biotin, 0.1 mg; folic acid, 0.5 mg; choline, 300 mg; Mn, 50 mg; Zn, 60 mg; Cu, 10 mg; Se, 0.15 mg; Fe, 80 mg.
Statistical analysis
The data were analyzed statistically according to the General Linear Model procedure of SAS (SAS, 1989). The percentage data were subjected to arc sin transformation prior to analysis of variance and differences among were separated by using Duncan’s multiple range test.
RESULTS AND DISCUSSION
Growth performance
The effects of β-glucans supplementation on the growth performance of broilers are summarized in Table 2. No significant differences were observed among treatments in feed intake, weight gain and feed efficiency. These results imply that β-glucans had no effect on broiler performance even up to dosage of 0.05%. Similarly, Wu et al. (1997) observed no significant effects of β-glucans in daily gain feed intake in weanling pigs. But Dritz et al. (1995) found that β-glucans incorporated at 0.025% significantly improved the daily gain of weanling pigs than that of control group. Based on the nutrient distribution for growing chicken and immune response, nutrient flow will predominantly supply for antibody and cytokine synthesis (i.e. IL-1and IL-2) when immune responses are primed. The final result will be growth depression (Klasing et al., 1987; Chen et al., 2003).
Lymphocytes blastogensis
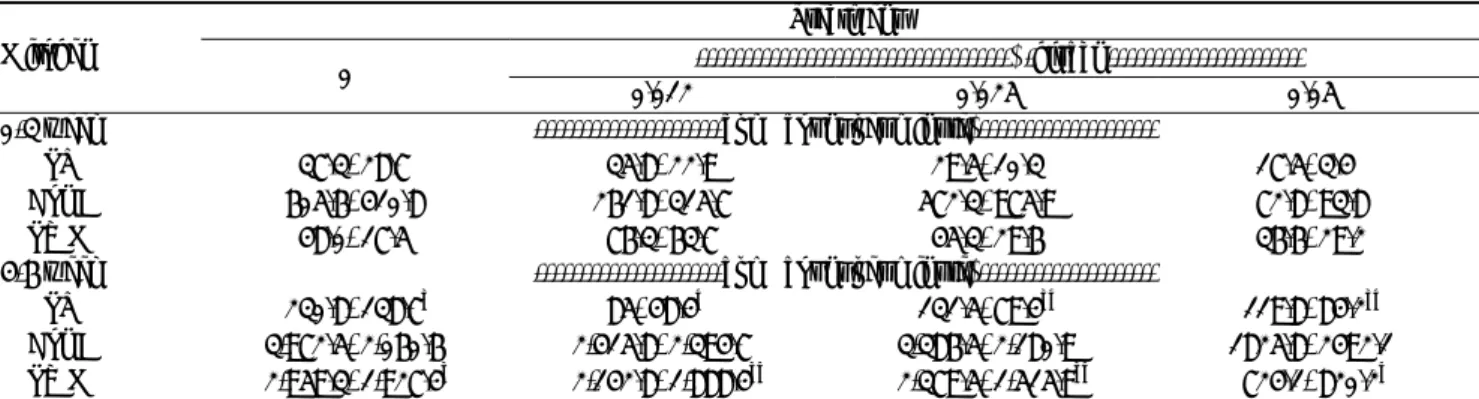
Lymphocyte proliferation is an important indicator of lymphocyte function. In this study, different mitogens (LPS, Con A, and PWM) were applied to stimulate the proliferation of blood lymphocytes, therefore, mainly referring to the B, T, and B/T-lymphocyte proliferation, respectively. The influence of β-glucans on lymphocytes blastogensis is summarized in Table 3. Date showed that LPS significantly (p<0.05) decreased the cpm value (85±48.4) compared to the other two mitogens at 0.012% β-glucans level at 6weeks of age. Significant decrease in cpm value (724.2±820.2) also noticed after PWM challenge at 0.05% β-glucans level at 6 weeks of age. Overall our data suggest that β-glucans feeding for a 6 week period had no effect on lymphocytes proliferation following challenge with different mitogens, although, some dosages depressed the proliferation.
Recent reports show that lentinan (a type of β-glucans) could promote lymphocyte proliferation in broilers (Chen et al., 2003).However, lymphocyte proliferation indices were not found to be different between control and treatment groups (0.03% β-glucans) in weanling pigs in a 4 week feeding experiment (Hiss and Sauerwein, 2003). These inconsistent results may be due to the differences in the dosage and the type of β-glucans used. The mechanism by which β-glucans promotes lymphocyte proliferation is unclear and remains to be elucidated.
Table 2. Effects of β-glucan supplementation on the growth performance of broilers
0%
0.012% 0.025% 0.05% 0-3 week
Feed intake 1 1.78±1.20 1.95±1.35 1.85±1.33 1.81±1.25 Body weight gain 1 0.61±0.04 0.67±0.03 0.66±0.03 0.62±0.004
Feed efficiency 2 0.46±0.33 0.44±0.29 0.48±0.36 0.45±0.31
4-6 week
Feed intake 1 1.81±1.31 1.86±1.32 1.91±1.30 1.72±1.19
Body weight gain 1 1.60±0.36 1.67±0.53 1.78±0.46 1.75±0.40
Feed efficiency2 1.09±0.59 1.06±0.47 1.11±0.52 1.23±0.62
1 (kg/bird), 2 weight gain/kg feed. Data presented as mean±SD (n=10). None of the differences among treatments were statistically significant (p>0.05).
Table 3. Effects of β-glucan on supplementation lymphocytes blastogensis in broilers
Treatment
---β-glucan--- Mitogen
0%
0.012% 0.025% 0.05% 0-3 week ---cpm (count per minute)---
LPS 37.3±28.7 35.8±22.9 29.5±10.3 17.5±3.4
Con A 605.6±410.8 261.8±315.7 572.3±975.9 72.8±93.8 PWM 48.0±17.5 76.3±63.7 45.3±29.6 36.6±29.2 4-6 week ---cpm (count per minute)---
LPS 230.8±138.7a 85±48.4b 131.5±79.4ab 119.8±84.2ab
Con A 3,972.5±2,060.6 2,415.8±2,394.7 3,386.5±2,180.9 1825.8±2492.1 PWM 2,959.3±1,927.4a 2,142.8±1,888.4ab 2,379.5±1,515.9ab 724.1±820.2b
Data presented as mean±SD (n=6). Lipopolysaccharide (LPS), 20 ug/ml; concanavalin A (Con A), 35 ug/ml; pokeweed mitogen (PWM), 24 ug/ml. a, b Means in the same row without common superscripts differ significantly (p<0.05 ).
Chemotaxis and NO production of macrophages
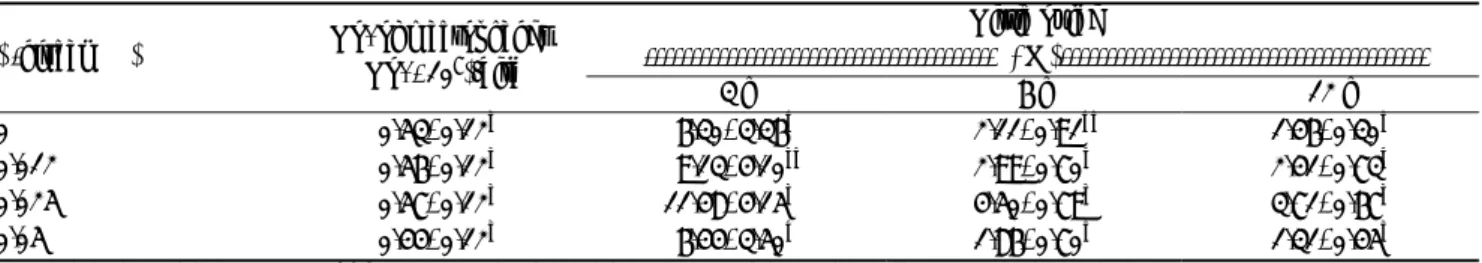
Macrophage is a lineage of mononuclear phagocytic system and plays an important role in immune-defense against pathogenic agents (Skamene and Gros, 1983). Many functions are performed by macrophages, including phagocytosis of exogenous particles (Qureshi et al., 1986). Furthermore, macrophage also can secrete prostaglandins and cytokines to modulate the activity of lymphocytes and other macrophages (Kimball, 1990). Our results showed that the numbers of harvested peritoneal macrophages were not influenced by β-glucans supplementation (Table 4). The bacterial killing ability of macrophages was not evaluated in present study.
The NO concentration of macrophages reached plateau at the level of 0.025% glucan during 3-12 h in vitro culture, and then declined at 0.05% level (Table 4). Nitric oxide is a chemical messenger and has been recognized as an important effectors molecule for macrophages in their cytotoxic and cytostatic activity in fighting against invading pathogens and tumor cell targets (Liew, 1995). In addition, NO regulates host immunity as a modulator of T-lymphocyte response. The production of large amounts of NO by activated macrophages was found to suppress lectin-induced lymphocyte proliferation in vitro (Albina et al., 1991). There are some controversial reports about the immunomodulation of NO. Increased NO production is thought to be the result of increased gene expression of inducible NO synthase (iNOS) in macrophages by glucan stimulation, which is responsible for the synthesis of NO by activating NF-k B transcriptional factor as proposed by Ramamoorthy et al. (1996).
Antibody titers
Specific vaccination responses, as quantified by the Newcastle disease (ND) antibody titers occurred in all birds,
but were not related to glucan feeding (Table 5). The results also indicated that antibody titers between 3 and 6 weeks of age were similar observed in Hiss and Sauerwein (2003) who challenged weanling pigs with PRRS (porcine reproductive and respiratory syndrome) vaccine after 0.03% glucan feeding for 4 weeks, and no benefit outcome was noted with glucan supplementation. But rats fed with glucan and challenge the parasite trophozoites via the intraperitoneal route increased serum IgG antibody titers by 300-fold (Navarro-Garcia et al., 2000). This dramatic induction of serum IgG antibody by IP co-administration of glucan and antigens may be due to macrophage activation. Since, our antigen challenge was by way of subcutaneous injection and glucan was provided by oral route, glucan may not have expressed its immune adjuvant characteristics. Concluded our findings, showed that β-glucan up to 0.05% did not have significant differences in weight gain and feed efficiency among the treatments, and no differences in antibody titer was observed. Supplementation of β-glucan did not elevate the lymphocyte blastogensis among treatments, following stimulation with different mitogens. However, supplementation with 0.025 and 0.05% β-glucan enhanced the macrophage chemotaxis activity of broilers. These results suggest that β-glucan may enhance some cell-mediated immune responses of chickens by modulate macrophages ability. In summary, by manipulating the immune response through the use of different routes, glucan might be used to achieve different effects on the protective immunity to different pathogens.
ACKNOWLEDGEMENTS
We are grateful to Mr. C. R. Shen and W. H. Su for their help in the care and management of the birds and in the preparation of this manuscript.
Table 4. Effects of β-glucan supplementation on the no. of harvested chickens peritoneal macrophages and nitric oxide production after
exposure to lipopolysaccharides
Nitric oxide
--- (µM)--- β-glucan (%) No. of macrophages (No. ×107)/bird
3 h 6 h 12 h
0 0.53±0.12a 6.30±3.36b 2.11±0.91bc 1.46±0.30c
0.012 0.56±0.12a 9.13±4.10ab 2.99±0.70b 2.41±0.73b
0.025 0.57±0.12a 11.48±4.15a 4.50±0.79a 3.71±0.67a
0.05 0.44±0.12a 6.44±3.50b 1.86±0.70c 1.31±0.45c
Data presented as mean±SD (n=6). a, b, c Means in the same column without common superscripts differ significantly (p<0.05).
Table 5. Effects of β-glucan supplementation on antibody titers production after Newcastle disease challenge
--- β-glucan ---
0% 0.012% 0.025% 0.05%
--- Log 2 titer
±SD---3 weeks 2.95±0.45 3.11±0.42 3.00±0.48 3.19±0.47 6 weeks 2.84±0.39 3.15±0.46 2.96±0.52 3.26±0.60
REFERENCES
Albina, J. E., J. A. Abate and W. L. Henry. 1991. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation: Role of IFN-r in the induction of the nitric oxide-synthesizing pathway. J. Immunol.147:144-148.
Adachi, Y., M. Okazaki, N. Ohno and T. Yadomae. 1994. Enhancement of cytokine production by macrophages stimulated with (1,3)-β-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 17:1554-1560. Babineau, T. J., P. Marcello, W. Swails, A. Kenler, B. Bistrian and
R. A. Forse. 1994. Randomised phase I/II trial of a macrophage-specific immunomodulator (PGG-Glucan) in high-risk surgical patients. Ann. Surg. 220:601-609.
Burgaleta, C., M. C. Territo, S. G. Quan and D. W. Golde. 1978. Glucan-activated macrophages: Functional characteristics and surface morphology. J. Reticuloendothel. Soc. 23:195-204. Chen, H. L., D. F. Li, B. Y. Chang, L. M. Gong, X. S. Piao, G. F.
Yi and J. X. Zhang. 2003. Effects of lentinan on broiler splenocyte proliferation, interleukin-2 production, and signal transduction. Poult. Sci. 82:760-766.
Cheng, Y. S. and Victor F. Pang. 1996. The cytotoxic effect of aflatoxin B1 on duck lymphocytes and the protective effect of β-carotene. J. Chinese Anim. Sci. 25:195-207.
Ding, A. H., C. F. Nathan and D. J. Stuer. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412.
Dritz, S. S., J. Shi, T. L. Kielian, R. D. Goodband, J. L. Nelssen, M. D. Tokach, M. M. Chengappa, J. E. Smith and F. Blecha. 1995. Influence of dietary beta-glucan on growth performance, nonspecific immunity and resistance to Streptococcus suis infection in weanling pigs. J. Anim Sci. 73:3341-50.
Hiss, S. and H. Sauerwein. 2003. Influence of dietary ss-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentration in pigs. J. Anim. Physiol. Anim. Nutr. 87:2-11.
Kimball, J. W. 1990. Introduction to immunology. pp. 232-345. Macmillan Publishing Co., New York; NY. USA.
Klasing, K. C., D. E. Laurin, R. K. Peng and D. M. Fry. 1987. Immunologically mediated growth depression in chicks: influence of feed intake, corticosterone and interleukin-1. J. Nutr. 117:1629-37.
Liew, F. Y. 1995. Regulation of lymphocytes function by nitric oxide. Curr. Opin. Immunol. 7:396-400.
Liu, Y. J. and Q. Z. Li. 1999. Effect of Lentinan and astragalan on IL-2 inductive activity and lymphocyte proliferation in chicks infected with vMDV. Chinese. J. Vet. Med. 25:3-5.
Navarro-Garcia, F., M. Pedroso and R. Lopez-Revilla. 2000. Immunodulation of rat serum and mucosal antibody response to Entamoeba histolytica trophozoites by β-1,3-glucan and cholera toxin. Clin. Immunol. 97:182-188.
National Research Council. 1994. Nutrient Requirements of Poultry. 9th ED. National Academy Press, Washington, DC. Ohno, N., N. Asada, Y. Adachi and T. Yadomae. 1995.
Enhancement of LPS triggered TNF-α (tumor necrosis factor-α) production by (1,3)-β-D-glucans in mice. Biol. Pharm. Bull. 18:126-133.
Ohno, N., Y. Egawa, T. Hashimoto, Y. Adachi and T. Yadomae. 1996. Effect of β-glucans on the nitric oxide synthesis by murine peritoneal macrophages in vitro. Biol. Pharm. Bull. 19:608-612.
Onderdonk, A. B., R. L.Cisneros, P. Hinkson and G. Ostroff. 1992. Anti-infective effect of poly-β1-6-glucotrioyl-β1-3-glucopyranose glucan in vivo. Infect. Immun. 60:1642-1647. Poutsiaka, D. D., M. Mengozzi, B. Sinha and C. A. Dinarello.
1993. Cross-linking of the β-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. Blood 82:3695-3700.
Qureshi, M. A., R. R. Dietert and L. D. Bacon. 1986. Genetic variation in the recruitment and activation of chicken peritoneal macrophage .Proc. Soc. Exp. Biol. Med. 181:560-566.
Rabertsen, B., G. Rorstad, R. Engstad and J. Raa. 1990. Enhancement of non-specific disease resistance in Atlantic salmon. Atlantic salmon L., by a glucan form Sachromyces cerevisiae cell walls. J. Fish Dis. 13:878-884.
Ramamoorthy, L., M. C. Kemp and I. R. Tizard. 1996. Acemannan, a β-(1,4)-acetylated mannan, induces nitric oxide production in macrophage cell line RAW 2647. Mol. Pharmacol. 50:878-884. SAS. 1989. SAS/STAT Guide for personal computers. Release
6.03th Edition. SAS Institute Inc. Cary, NC, USA.
Schoenherr, W. D., D. S. Pollmann and J. A. Coalson. 1994. Titration of MacroGardTM-S on growth performance of nursery
pigs. J. Anim. Sci. 72 (Suppl. 2):57 (Abstr.).
Skamene, E. and P. Gros. 1983. Role of macrophages in resistance against infectious disease. Clinic. Immunol. Allergy 3:539-560. Talebi, A. P., R. Torgerson and G. Mulcahy. 1995. Optimal conditions for measurement of blastogenic responses of chickens to concanavalin A in whole blood assays. Vet Immunol Immunopathol. 46:293-301.
Trembicki, K. A., M. A. Qureshi and R. R. Dietert. 1984. Avian peritoneal exudate cells: a comparison of stimulation protocols. Dev. Comp. Immunol. 8:395-402.
Williams, D. L., T. S. Stahly and D. R. Zimmerman. 1994. Impact of immune system activation on growth and amino acid needs of pigs from 6 to 114 kg body weight. J. Anim. Sci. 72:57 (Abstract).
Wu, M. C., L. C. Wung, C. C. Liao, C. C.Kuo and F. S. Chang. 1997. Effect of dietary egg white product and MacroGard on growth performance and immune response in wealing pigs. J. Anim. Ind. Res. 3:17-33.