High birefringence and wide nematic range bis-tolane liquid crystals
Shin-Tson Wu, C.-S. Hsu, and K.-F. ShyuCitation: Applied Physics Letters 74, 344 (1999); doi: 10.1063/1.123066
View online: http://dx.doi.org/10.1063/1.123066
View Table of Contents: http://scitation.aip.org/content/aip/journal/apl/74/3?ver=pdfcov
Published by the AIP Publishing
Articles you may be interested in
Modeling the solid-liquid phase transition in saturated triglycerides
J. Chem. Phys. 132, 054502 (2010); 10.1063/1.3276108
Temperature-dependent optical constants and birefringence of nematic liquid crystal 5CB in the terahertz frequency range
J. Appl. Phys. 103, 093523 (2008); 10.1063/1.2913347
Tumbling instability in a shearing nematic liquid crystal: Analysis of broadband dielectric results and theoretical treatment
J. Chem. Phys. 118, 4253 (2003); 10.1063/1.1542597
Wide nematic range alkenyl diphenyldiacetylene liquid crystals
Appl. Phys. Lett. 77, 957 (2000); 10.1063/1.1288600
Photothermal effects in liquid crystals on dye-doped polymer films
J. Appl. Phys. 83, 4957 (1998); 10.1063/1.367297
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Thu, 01 May 2014 08:47:24
High birefringence and wide nematic range bis-tolane liquid crystals
Shin-Tson Wua)
HRL Laboratories, 3011 Malibu Canyon Road, Malibu, California 90265 C.-S. Hsu and K.-F. Shyu
Department of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan 30050, Republic of China
~Received 23 June 1998; accepted for publication 9 November 1998!
Several dialkyl and fluoro bis-tolane liquid crystals were synthesized and their physical properties evaluated. These highly conjugated liquid crystals exhibit a wide nematic range, small heat fusion enthalpy, high birefringence, and relatively low viscosity. Their excellent compatibility with commercial cyano mixtures makes these liquid crystals attractive for many electro-optic applications. © 1999 American Institute of Physics.@S0003-6951~99!02103-8#
Liquid crystals~LCs! with low melting and high clearing temperature are particularly attractive for automobile and outdoor display applications. Inside a car or at outdoor envi-ronment, temperature could vary wildly depending on the geographic locations. Generally speaking, the nematic range of a single LC substance is rather limited. Forming eutectic mixture is a common approach for lowering the melting point. From the Schroder–Van Laar equation,1,2 the indi-vidual LC components possessing a low melting (Tm p) and
high clearing point, and small heat fusion enthalpy (DH) are better candidates for widening the nematic range of the eu-tectic mixture.
In addition to wide nematic range, high birefringence (Dn) is also desirable for cholesteric LC displays3and opti-cal phased arrays for laser beam steering.4For a cholesteric display using Bragg reflection,3 highDn widens the reflec-tion bandwidth and improves the display brightness. For a LC-based optical phased arrays,4 highDn helps to shorten response time through thinner cell gap requirement. The bi-refringence of an LC is mainly determined by thep-electron conjugation, differential oscillator strength, molecular shape, and order parameter.5 Thus, a more linearly conjugated LC would exhibit a larger optical anisotropy. A general problem of these highly conjugated molecules is that their melting temperature is usually very high.
Dialkyl diphenyl-diacetylene LCs6 are found to possess a high Dn, low melting temperature, and small heat fusion enthalpy. They are excellent candidates for making eutectic mixtures, except their dielectric anisotropy is a little too small (De;0.8). To reduce operation voltage, some polar compounds need to be added. When these LCs are mixed with more than 10% cyano-biphenyls, they tend to form 1:1 complexes and limits the solubility of the polar compounds.7 Polytolane8–10is another interesting series of high bire-fringence LC. A major problem of polytolane is its high melting point. In this letter, we report a new series of bis-tolane LCs with wide nematic range, highDn, low viscosity, and excellent compatibility with commercial cyano mixtures. More than 20 dialkyl and fluoro bis-tolanes with molecular structures shown below were synthesized.
For simplicity, these alkyl-alkyl and fluoro-alkyl compounds are abbreviated as PTP~3-Me!TP-nm and PTP~3-Me!TP-Fm, respectively; here P stands for the phenyl ring, T for the carbon–carbon triple bond, and P~3-Me! for the middle phe-nyl ring with a methyl group sticking out at the 3 position. The general synthesis schemes can be found in Refs. 8–10, and the detailed synthetic procedures for the described bis-tolanes will be published elsewhere.11
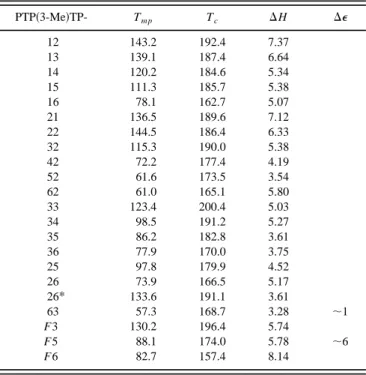
The phase transition temperature and heat fusion en-thalpy of these bis-tolane LCs are listed in Table I. A com-pound ~PTPTP-26! without the methyl group in the middle phenyl ring is also included for comparison. From Table I, we find several intriguing phase transition phenomena:
a!Electronic mail: swu@hrl.com
TABLE I. Phase transition temperatures~in °C!, heat fusion enthalpy ~DH, in kcal/mol!, and extrapolated ~in E63 host! dielectric anisotropy ~De! of the dialkyl and fluoro PTP~3-Me!TP liquid crystals. Here, 26* stands for PTPTP-26 LC; no methyl group in the middle phyenyl group.
PTP~3-Me!TP- Tm p Tc DH De 12 143.2 192.4 7.37 13 139.1 187.4 6.64 14 120.2 184.6 5.34 15 111.3 185.7 5.38 16 78.1 162.7 5.07 21 136.5 189.6 7.12 22 144.5 186.4 6.33 32 115.3 190.0 5.38 42 72.2 177.4 4.19 52 61.6 173.5 3.54 62 61.0 165.1 5.80 33 123.4 200.4 5.03 34 98.5 191.2 5.27 35 86.2 182.8 3.61 36 77.9 170.0 3.75 25 97.8 179.9 4.52 26 73.9 166.5 5.17 26* 133.6 191.1 3.61 63 57.3 168.7 3.28 ;1 F3 130.2 196.4 5.74 F5 88.1 174.0 5.78 ;6 F6 82.7 157.4 8.14
APPLIED PHYSICS LETTERS VOLUME 74, NUMBER 3 18 JANUARY 1999
344
0003-6951/99/74(3)/344/3/$15.00 © 1999 American Institute of Physics
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Thu, 01 May 2014 08:47:24
~1! These bis-tolane possess a very high clearing
tempera-ture ranging from 160 to 200 °C. Some homologues
@e.g., PTP~3-Me!TP-52, 62, and 63# even exhibit a
rela-tively low melting point~;60 °C! and small heat fusion enthalpy~,4 kcal/mol!. Thus, these compounds are very useful for formulating eutectic mixtures with a wide nematic range.
~2! The asymmetric compound generally shows a much
lower melting point than the symmetric one. From Table I, the Tm pof PTP~3-Me!TP-33 is 123.4 °C. Keeping the
total side-chain carbon number unchanged except break-ing their symmetry leads to a much lower Tm p. For
in-stance, the Tm p of PTP~3-Me!TP-42 is reduced to
72.2 °C. This effect was also clearly observed in the diphenyl diacetylene system as well.6 However, if one side has a too short alkyl chain, such as in PTP ~3-Me!TP-15, then the advantage is not obvious. Then Tm p
of PTP~3-Me!TP-15 remains as high as 111.3 °C.
~3! Since the methyl group is sticking out at the 3 position of
the middle phenyl ring, it is more favorable to have a longer alkyl chain on the left-hand side than on the right. This is demonstrated in the @PTP~3-Me!TP-52 and -25#, and @PTP~3-Me!TP-62 and -26# pairs. The Tm p of
PTP~3-Me!TP-52 and -62 homologues is 36 and 13 °C lower than that of PTP~3-Me!TP-25, and -26 homo-logues, respectively.
~4! The middle methyl group plays an important role in
low-ering the melting point. For example, the Tm p of PTPTP-26 is 133.6 °C while the Tm p of PTP ~3-Me!TP-26 drops to 73.9 °C. This is because the methyl group slightly increases the width of the molecule and then decreases the molecular packing density. As a re-sult, the required temperatures to melt and to clear the LC clusters are both lower. If we replace the methyl with an ethyl group, the melting point will further decrease. However, its viscosity will slightly increase.
The dialkyl bis-tolanes are nonpolar so that their dielec-tric anisotropy is small (De;1). A small De applies to a high operation voltage, as the Freedericksz transition thresh-old voltage12 of a homogeneous cell is determined by the dielectric anisotropy and splay elastic constant (K11) of the
LC as Vth5p@K11/e0De#1/2. To lower Vth, one can either
use fluoro compounds shown in Table I or mix the nonpolar bis-tolane compounds with commercial polar mixtures. The extrapolated De of fluoro bis-tolane is about 6. However, their melting temperature is relatively high so that their solu-bility in a dialkyl bis-tolane mixture is limited to;10%. For such a low concentration, its contribution toDeenhancement is limited. Thus, we have selected to mix dialkyl bis-tolanes with a Merck cyano-biphenyl mixture, E63. The most im-pressive feature of E63 is that is does not freeze at 240 °C. Its clearing temperature is;87 °C, Dn;0.21 at l5589 nm and T522 °C, and De;14.6 at f 52 kHz sine wave fre-quency.
In experiment, we have mixed 10%, 20%, and 30% of PTP~3-Me!TP-63 to the host E63 mixture and evaluated their clearing point, threshold voltage, visco-elastic coefficient, and birefringence. The phase transition temperatures of the mixtures were measured using a Mettler differential scanning calorimeter. Due to the high clearing point of PTP
~3-Me!TP-63, the mixture’s clearing temperature increases in propor-tion to the dopant concentrapropor-tion. For example, adding a 30% of PTP~3-Me!TP-63 to E63 increases the mixture’s clearing point to 103.3 °C while preserving the desirable low melting temperature.
To characterize the threshold voltage, we prepared a ho-mogeneous cell and measured its voltage-dependent phase retardation at voltages slightly above the threshold. A HeNe laser with l5633 nm was used for this study. The indium-tin-oxide coated glass substrates were evaporated with SiOx
layers to produce uniform homogeneous LC alignment with pretilt angle of ;2°. The cell gap was controlled to be ;6
mm. Experimental data were collected at every 10 mVrms.
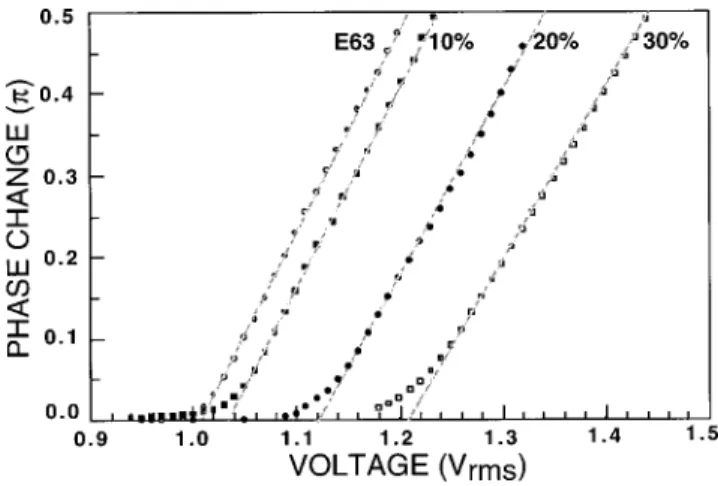
The sine wave frequency of 2 kHz was set for all the mea-surements reported here. Results are depicted in Fig. 1 for E63 mixture alone, and E63 containing 10%, 20%, and 30% of PTP~3-Me!TP-63 dopant. In the vicinity of threshold, the phase retardation is linearly proportional to the applied volt-age as expected from theory.13 Through a linear extrapola-tion, the threshold voltage is determined as shown in Fig. 1. Since PTP~3-Me!TP-63 has a much smaller Dethan E63, the mixture’s threshold voltage increases as the dopant concen-tration increases. As shown in Fig. 2, the Vthof E63 is 1.01
Vrms. As the PTP~3-Me!TP-63 concentration increases to
FIG. 1. The voltage-dependent phase change of four homogeneous LC cells: E63 alone, and E63 containing 10%, 20%, and 30% of PTP~3-Me!TP-63.
l5633 nm, f 52 kHz sine waves, and T522 °C.
FIG. 2. Concentration effect of PTP~3-Me!TP-63 on the threshold voltage
~left! and visco-elastic coefficient ~right! of the E63 cells.
345
Appl. Phys. Lett., Vol. 74, No. 3, 18 January 1999 Wu, Hsu, and Shyu
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Thu, 01 May 2014 08:47:24
30%, the mixture’s Vth increases to 1.21 Vrms.
Using the same cell, we are able to measure the mix-ture’s visco-elastic coefficient (g1/K11) through the
time-dependent phase decay method.14 Results are plotted in the right-hand side of Fig. 2. From Fig. 2, the viselastic co-efficient of the mixtures is;15% lower than that of the E63 host indicating that the viscosity of PTP~3-Me!TP-63 is lower than that of E63. This result is encouraging because for such highly conjugated LC molecules, their viscosity would be normally quite high.15,16The observed low viscos-ity makes these compounds very attractive for many electro-optic applications.
The refractive indices of E63 and three mixtures contain-ing 10%, 20%, and 30% of PTP~3-Me!TP-63 were measured using the Jelly–Leitz refractometer. The birefringence of such guest-host system can be approximated as: (ne,o)gh
5x(ne,o)g1(12x)(ne,o)h; where x is the concentration of
the guest dopant, and neand noare the refractive indices for
the extraordinary and ordinary rays, respectively. By measur-ing E63’s refractive indices, the refractive indices of PTP ~3-Me!TP-63 can be extrapolated. And the results are: ne
51.889, no51.507, or Dn50.382 at l5589 nm and T
522 °C. This high birefringence originates from the linear
conjugation of the bis-tolane structure. We also measured the birefringence dispersion of a 20% PTP~3-Me!TP-63 in E63 over the entire visible region. Results are shown in Fig. 3. From Fig. 3, theDn enhancement is more pronounced in the blue region than that in the near infrared ~IR!. This is be-cause blue is closer to the PTP~3-Me!TP-63’s electronic ab-sorption wavelength.
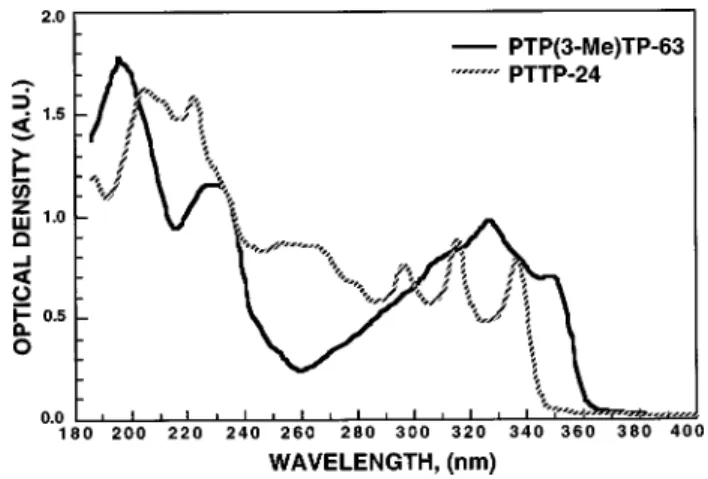
The ultraviolet ~UV! absorption spectra of PTP~3-Me!TP-63 was measured against a commonly employed diphenyl-diacetylene PTTP-24. In experiments, we mixed 1% of PTP~3-Me!TP-63 and PTTP-24 in a UV transparent LC mixture ZLI-2359. The mixture E63 absorbs strongly at wavelengths below;350 nm. So, ZLI-2259 is a better host. The computer-controlled Lambda 9 spectrophotometer was used for the absorption measurements. Two homogeneously aligned LC cells made of quartz substrates were prepared: the reference cell consists of a 6mm ZLI-2359 LC layer and the sample cell contains 1% dopant dissolved in ZLI-2359.
Figure 4 depicts the measured absorption spectra of PTP~3-Me!TP-63 and PTTP-24. From Fig. 4,
PTP~3-Me!TP-63 has a slightly longer absorption edge than PTTP-24 due to its longer molecular conjugation. The longer absorption alone is insufficient to explain the observed higher birefringence of bis-tolane. The molecular packing density and absorption anisotropy all play important roles.5 In addition, the slightly longer absorption does not necessar-ily imply that bis-tolanes should have a poorer photostability than diacetylenes.
In conclusion, we have synthesized many asymmetric bis-tolane LC compounds which possess high birefringence, low viscosity, low melting and high clearing temperatures, and small heat of fusion enthalpy. These compounds are compatible with commercial cyano mixtures. Mixing PTP ~3-Me!TP-63 LC to E63 mixture enhances the mixture’s clear-ing temperature and birefrclear-ingence while reducclear-ing its visco-elastic coefficient. The trade-off is in its slightly increased threshold voltage.
The HRL group is indebted to W. H. Smith, Jr. for the technical assistance.
1L. Schroder, Z. Phys. Chem. 11, 449~1893!. 2
J. J. Van Laar, Z. Phys. Chem. 63, 216~1908!.
3D. K. Yang, J. W. Doane, Z. Yaniv, and J. Glasser, Appl. Phys. Lett. 64, 1905~1994!.
4T. A. Dorschner, L. Friedman, M. Holz, D. P. Resler, R. C. Sharp, and I. W. Smith, Proc. IEEE International Symposium on Phased-array Systems and Technology, 15–18 Oct. 1996, Boston, MA, p. 119.
5S. T. Wu, Phys. Rev. A 30, 1270~1986!.
6S. T. Wu, J. D. Margerum, B. H. Meng, L. R. Dalton, C. S. Hsu, and S. H. Lung, Appl. Phys. Lett. 61, 630~1992!.
7
M. Neubert, Liquid Crystal Institute, Kent State University~private com-munication!.
8C. Pugh, S. K. Anderson, and V. Percec, Liq. Cryst. 10, 229~1991!. 9C. Viney, D. J. Brown, C. M. Dannels, and R. J. Tweig, Liq. Cryst. 13, 95
~1993!.
10
R. J. Twieg, V. Chu, C. Nguyen, C. M. Dannels, and C. Viney, Liq. Cryst. 20, 287~1996!.
11C. S. Hsu, K. F. Shyu, and S. T. Wu~unpublished!.
12V. Freedericksz and V. Zolina, Trans. Faraday Soc. 29, 919~1933!. 13
H. J. Deuling, ‘‘Elasticity of nematic liquid crystals,’’ Solid State Phys.
Suppl. 14: Liquid Crystals, edited by L. Liebert~Academic, New York,
1978!.
14I. C. Khoo and S. T. Wu, Optics and Nonlinear Optics of Liquid Crystals
~World Scientific, Singapore, 1993!.
15
M. A. Osipov and E. M. Terentjev, Z. Naturforsch. Teil A 44, 785~1989!. 16S. T. Wu and C. S. Wu, Phys. Rev. A 42, 2219~1990!.
FIG. 3. Birefringence dispersion of E63 and E63120% PTP~3-Me!TP-63.
T522 °C. FIG. 4. The UV absorption spectra of PTPhost5ZLI-2359; Cell gap56mm and dopant concentration~3-Me!TP-63 and PTTP-24. LC51%.
346 Appl. Phys. Lett., Vol. 74, No. 3, 18 January 1999 Wu, Hsu, and Shyu
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Thu, 01 May 2014 08:47:24