Lack of Association between
ORAI1/CRACM1 Gene
Polymorphisms and Kawasaki Disease
in the Taiwanese Children
Ho-Chang Kuo&Ying-Jui Lin&Suh-Hang Hank Juo&Yu-Wen Hsu&
Wei-Chiao Chen&Kuender D. Yang&Chi-Di Liang&Shengyu Yang&
Mei-Chyn Chao&Hong-Ren Yu&Shouyan Wang&Li-Yan Lin&Wei-Chiao Chang
Received: 18 December 2010 / Accepted: 28 March 2011 / Published online: 13 April 2011 # Springer Science+Business Media, LLC 2011
Abstract
Objective Kawasaki disease (KD) is characterized by systemic vasculitis of an unknown cause. A previous study has indicated that a polymorphism of the inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) gene is involved in the susceptibility to KD. ORAI (also known as CRACM1) is one of the components of store-operated calcium channels involved in regulating immune and inflammatory reactions. This study was conducted to investigate if polymorphisms in ORAI1/ CRACM1, a gene downstream from ITPKC, are associated with KD susceptibility and clinical outcomes.
Materials and Methods A total of 1,056 subjects (341 KD patients and 715 controls) were investigated to identify five tagging single nucleotide polymorphisms (tSNPs) in ORAI1/ CRACM1 (rs12313273, rs6486795, rs7135617, rs12320939, and rs712853) by using the TaqMan Allelic Discrimination assay.
Results No significant associations between genotype and allele frequency of the five ORAI1/CRACM1 tSNPs were observed in the KD patients and controls. In KD patients, no significant associations between ORAI1/CRACM1 polymor-phisms and coronary artery lesion (CAL) formation or
Ho-Chang Kuo and Ying-Jui Lin contributed equally to this article. H.-C. Kuo
:
K. D. Yang:
H.-R. Yu:
L.-Y. LinDivision of Allergy, Immunology and Rheumatology, Department of Pediatrics, Chang Gung Memorial Hospital–Kaohsiung Medical Center,
Kaohsiung, Taiwan
Y.-J. Lin
:
C.-D. LiangDivision of Cardiology, Department of Pediatrics,
Chang Gung Memorial Hospital–Kaohsiung Medical Center, Kaohsiung, Taiwan
H.-C. Kuo
:
K. D. Yang:
H.-R. YuGraduate Institute of Clinical Medical Science, Chang Gung University College of Medicine, Kaohsiung, Taiwan
S.-H. H. Juo
:
Y.-W. Hsu:
W.-C. Chen:
M.-C. Chao:
W.-C. Chang (*)Department of Medical Genetics, College of Medicine, Kaohsiung Medical University,
100 TzYou First Road, Kaohsiung City 807, Taiwan e-mail: wcc@kmu.edu.tw
W.-C. Chang
Center of Excellence for Environmental Medicine, Kaohsiung Medical University,
100 TzYou First Road, Kaohsiung City 807, Taiwan S.-H. H. Juo
:
W.-C. ChangCenter of Excellence for Environmental Medicine, Kaohsiung Medical University,
100 TzYou First Road, Kaohsiung City 807, Taiwan S. Yang
Comprehensive Melanoma Research Center and Department of Tumor Biology, H. Lee Moffitt Cancer Center,
Tampa, FL, USA S. Wang
Institute of Sound and Vibration Research, University of Southampton,
Southampton, UK M.-C. Chao
Division of Genetics, Endocrinology and Metabolism, Department of Pediatrics, Kaohsiung Medical University Hospital,
100 TzYou First Road, Kaohsiung City 807, Taiwan
intravenous immunoglobulin (IVIG) treatment response were observed. The results from haplotype analysis were insignificant.
Conclusions This study showed for the first time that ORAI1/CRACM1 polymorphisms are not associated with KD susceptibility, CAL formation, or IVIG treatment response in the Taiwanese population.
Keywords Kawasaki disease .ORAI1/CRACM1 . coronary artery lesions . intravenous immunoglobulin
Abbreviations
KD Kawasaki disease
IVIG Intravenous immunoglobulin CAL Coronary artery lesions
CRACM1 Calcium release-activated calcium (CRAC) modulator 1
ITPKC Inositol 1,4,5-trisphosphate 3-kinase C
Introduction
Kawasaki disease (KD) is characterized by acute, febrile, systemic vasculitis and was first described by Kawasaki et al. in 1974 [1]. In developed countries, KD is the leading cause of acquired heart diseases in children [2,3]. KD occurs worldwide particularly in Japan, Korea, and Taiwan, and mainly affects children less than 5 years of age [4–6]. The most serious complication of KD is the occurrence of coronary artery lesions (CAL) [7, 8]. The prevalence of KD in children younger than 5 years is the highest in Japan, followed by Korea and Taiwan, and lowest in Europe. Previous studies have either failed to identify the causative pathogen for KD or reported discrepant results [9–11]. Therefore, it is possible that genetic background plays an important role in KD pathogenesis. Several lines of evidence support T-cell-mediated cytokine release as a key regulator in the onset of KD [7,12–14]. Our previous studies have shown that eosinophil and IL-5 levels are associated with intravenous immunoglobulin (IVIG) responsiveness. Data on CAL formation also indicated that T-cell-mediated immunity is involved in KD pathogenesis [7,15,16].
Inositol 1,4,5-trisphosphate 3-kinase C (ITPKC), a negative regulator of the nuclear factor of activated T cells (NFAT) signaling pathway, functions in immune modula-tion by phosphorylating inositol 1,4,5-trisphosphate (IP3)
[12, 13]. A functional ITPKC SNP (rs28493229) is associated with susceptibility to KD and CAL formation in this disease [13]; however, the controversial results from independent groups revealed genetic association between ITPKC (rs28493229) and the susceptibility to KD in the Taiwanese population [17, 18]. Recently, another study
indicated that A allele of CASP3 (rs72689236) is a risk allele in the development of aneurysm in patients with KD in the Taiwanese population [19].
The calcium influx through IP3-mediated store-operated
calcium channel is significant to a variety of physiological functions in non-excitable cells such as T cells and mast cells [20,21]. ORAI1/CRACM1 is an essential component of the store-operated calcium channels [22]. A point mutation in ORAI1/CRACM1 impairs store-operated calcium entry, which leads to severe combined immunodeficiency (SCID) in human infants [23]. However, no ORAI1/CRACM1 genetic associations with KD have been reported yet. The aim of our study was to determine if any ORAI1/CRACM1 SNPs are associated with susceptibility to KD, CAL formation, or IVIG treatment response in Taiwanese children.
Patients and Methods
Patients Studied
All study cases were children from Chang Gung Memorial Hospital, Kaohsiung Medical Center, who fulfilled the diagnostic criteria for KD between 2001 and 2009. All patients were treated with IVIG (2 g/kg) and aspirin as in our previous studies [7,8,24]. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Blood samples were collected after informed consent was obtained from parents or guardians. We excluded patients who did not meet KD diagnostic criteria. CAL formation was defined as the internal diameter of the coronary artery measuring at least 3 mm (4 mm if the subject was over the age of 5 years) or the internal diameter of a segment at least 1.5 times that of an adjacent segment, as observed in the echocardiogram [8, 25]. IVIG respon-siveness was defined as defervescence within 48 h after IVIG treatment completion and no recurrence in fever (temperature >38°C) for at least 7 days after IVIG with marked improvement or normalization of inflammatory signs [7,8].
DNA Extraction and Genotyping
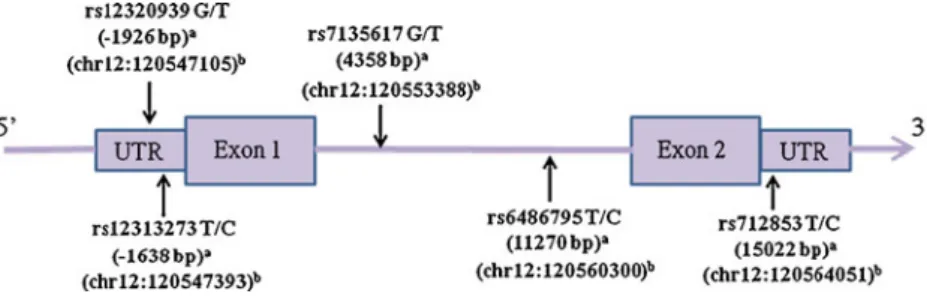
DNA was extracted from blood cells by using Gentra extraction kit, followed by 70% alcohol precipitation as described in our previous report [26]. Five tSNPs of ORAI1/CRACM1 (rs12313273, rs6486795, rs7135617, rs12320939, and rs712853) with minor allele frequency (MAF) >10% and r2>0.8 were selected from chromosomal region 120,545,838 to 120,561,329 in the Han Chinese in Beijing (CHB) population from the HapMap database (http://www.hapmap. org, HapMap Data Rel 27 PhaseII+III, Freb09, on NCBI B36 assembly, dbSNP b126). A graphical overview of physical
and chromosomal location information of the five SNPs is shown in Fig.1. Two ORAI1/CRACM1 polymorphisms are located in promoter area (rs12313273 and rs1232093), two in the intron (rs6486795 and rs7135617), and one in the 3′ untranslated region (UTR) (rs712853).
Genotyping was conducted by using the TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster city, CA, USA). Polymerase chain reaction (PCR) was per-formed in a 96-well microplate and with the ABI 9700 Thermal Cycler. The thermal cycle conditions were as follows: denaturation at 95°C for 10 min, 40 cycles of denaturation at 92°C for 15 s, and annealing and extension at 60°C for 1 min. After PCR, fluorescence was measured and analyzed using the System SDS software version 1.2.3.
Statistical Analysis
SAS 9.1 for Windows was used for analysis. Statistical differences between case and control in genotype and allele frequency were assessed using the χ2 test or the Fisher’s exact test. Statistical differences in genotype and allele frequency of KD patients with/without CAL formation and patients with IVIG resistance/responsiveness were assessed
using the χ2test. The Bonferroni test was used to correct for multiple tests.
Results
No Association between ORAI1/CRACM1 tSNPs and Susceptibility to KD
A total of 341 KD patients and 715 controls were included in this study. The distribution of ORAI1/CRACM1 geno-types was in accordance with the Hardy–Weinberg equilib-rium for both cases and controls (Table I). However, none of the tSNPs were significantly associated with the genotype or allele frequency of controls and KD patients under three genetic models (Dominant, Recessive, or Allelic models).
No Association in KD Patients between ORAI1/CRACM1 tSNPs and CAL Formation or IVIG Treatment Response
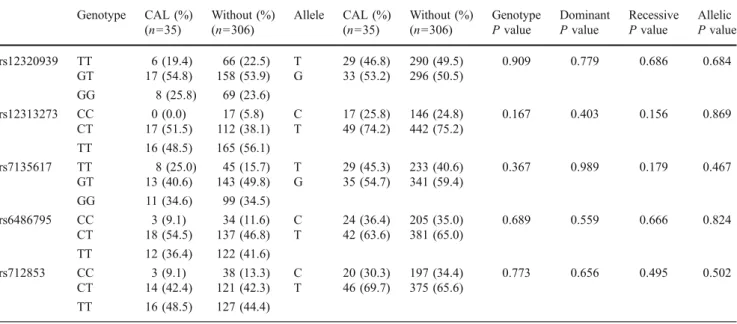
A total of 341 KD patients were included; 35 patients (10.3%) showed CAL formation and 43 patients (12.6%) Fig. 1 Graphical overview of
the genotyped human ORAI1/ CRACM1 gene polymorphisms in relation to its exon/intron structure. a Distance from the transcriptional start site. b Location of SNPs on chromosome 12
Table I Genotype and allele frequencies of theORAI1/CRACM1 gene in patients with Kawasaki disease and controls Genotype Case (%) (n=341) Control (%) (n=715) Allele Case (%) (n=341) Control (%) (n=715) Genotype P value Dominant P value Recessive P value Allelic P value rs12320939 TT 72 (22.2) 165 (24.3) T 319 (49.2) 664 (48.9) 0.359 0.352 0.469 0.889 GT 175 (54.0) 334 (49.2) G 329 (50.8) 694 (51.1) GG 77 (23.8) 180 (26.5) rs12313273 CC 17 (5.2) 53 (7.7) C 163 (24.9) 377 (27.4) 0.323 0.465 0.141 0.238 CT 129 (39.5) 271 (39.4) T 491 (75.1) 999 (72.6) TT 181 (55.3) 364 (52.9) rs7135617 TT 53 (16.6) 113 (16.4) T 262 (41.1) 564 (41.0) 0.997 0.991 0.940 0.974 GT 156 (48.9) 338 (49.1) G 376 (58.9) 812 (59.0) GG 110 (34.5) 237 (34.5) rs6486795 CC 37 (11.3) 98 (14.3) C 229 (35.1) 511 (37.4) 0.429 0.653 0.194 0.331 CT 155 (47.6) 315 (46.1) T 423 (64.9) 857 (62.6) TT 134 (41.1) 271 (39.6) rs712853 CC 41 (12.9) 96 (13.9) C 217 (34.0) 490 (35.5) 0.816 0.566 0.648 0.513 CT 135 (42.3) 298 (43.2) T 421 (66.0) 890 (64.5) TT 143 (44.8) 296 (42.9)
showed resistance to the initial IVIG treatment. However, no tSNPs were significantly associated with genotype or allele frequency in KD patients with or without CAL formation (Table II). Additionally, the ORAI1/CRACM1 polymorphisms tested in this study failed to show any significant associations with genotype or allele frequency in KD patients who showed IVIG treatment response (TableIII). We also calculated pairwise linkage disequilib-rium (LD) of the SNPs and analyzed the relationship between five common haplotypes of OARI1/CRACM1 and
clinical KD status. However, none was significantly associated with the phenotype.
Discussion
Several genetic associations with susceptibility to KD and CAL formation have been reported, but the results are inconsistent [13,14, 17, 18]. Previous genetic association studies indicate that the intronic SNP (rs28493229) of Table II Genotype and allele frequencies ofORAI1/CRACM1 gene in Kawasaki disease patients with or without lesion formation in the coronary artery Genotype CAL (%) (n=35) Without (%) (n=306) Allele CAL (%) (n=35) Without (%) (n=306) Genotype P value Dominant P value Recessive P value Allelic P value rs12320939 TT 6 (19.4) 66 (22.5) T 29 (46.8) 290 (49.5) 0.909 0.779 0.686 0.684 GT 17 (54.8) 158 (53.9) G 33 (53.2) 296 (50.5) GG 8 (25.8) 69 (23.6) rs12313273 CC 0 (0.0) 17 (5.8) C 17 (25.8) 146 (24.8) 0.167 0.403 0.156 0.869 CT 17 (51.5) 112 (38.1) T 49 (74.2) 442 (75.2) TT 16 (48.5) 165 (56.1) rs7135617 TT 8 (25.0) 45 (15.7) T 29 (45.3) 233 (40.6) 0.367 0.989 0.179 0.467 GT 13 (40.6) 143 (49.8) G 35 (54.7) 341 (59.4) GG 11 (34.6) 99 (34.5) rs6486795 CC 3 (9.1) 34 (11.6) C 24 (36.4) 205 (35.0) 0.689 0.559 0.666 0.824 CT 18 (54.5) 137 (46.8) T 42 (63.6) 381 (65.0) TT 12 (36.4) 122 (41.6) rs712853 CC 3 (9.1) 38 (13.3) C 20 (30.3) 197 (34.4) 0.773 0.656 0.495 0.502 CT 14 (42.4) 121 (42.3) T 46 (69.7) 375 (65.6) TT 16 (48.5) 127 (44.4)
Table III Genotype and allele frequencies of theORAI1/CRACM1 gene in Kawasaki disease patients who did or did not respond to intravenous immunoglobulin treatment Genotype Resistant (%) (n=43) Responsive (%) (n=298) Allele Resistant (%) (n=43) Responsive (%) (n=298) Genotype P value Dominant P value Recessive P value Allelic P value rs12320939 TT 8 (20.0) 64 (22.5) T 38 (47.5) 281 (49.5) 0.933 0.845 0.718 0.741 GT 22 (55.0) 153 (53.9) G 42 (52.5) 287 (50.5) GG 10 (25.0) 67 (23.6) rs12313273 CC 3 (7.1) 14 (4.9) C 19 (22.6) 144 (25.3) 0.448 0.360 0.543 0.601 CT 13 (31.0) 116 (40.7) T 65 (77.4) 426 (74.7) TT 26 (61.9) 155 (54.4) rs7135617 TT 7 (17.1) 46 (16.6) T 34 (41.5) 228 (41.0) 0.996 0.961 0.933 0.938 GT 20 (48.8) 136 (48.9) G 48 (58.5) 328 (59.0) GG 14 (34.1) 96 (34.5) rs6486795 CC 7 (16.3) 30 (10.6) C 29 (33.7) 200 (35.3) 0.178 0.269 0.274 0.770 CT 15 (34.9) 140 (49.5) T 57 (66.3) 366 (64.7) TT 21 (48.8) 113 (39.9) rs712853 CC 7 (16.7) 34 (12.3) C 33 (39.3) 184 (35.5) 0.567 0.347 0.428 0.274 CT 19 (45.2) 116 (41.9) T 51 (60.7) 890 (64.5) TT 16 (38.1) 127 (45.8)
ITPKC reduces gene expression by altering splicing efficiency, and the C allele contributes to immune hyper-reactivity in KD patients [13]. Although the functional effects of ITPKC in regulating store-operated calcium channels (SOC) are elusive, changes in ITPKC expression levels may influence the phosphorylation of IP3, which in
turn controls SOC activation [13,20,21]. SOC is encoded by the ORAI gene family, including ORAI1, ORAI2, and ORAI3 (also known as CRACM1, CRACM2, and CRACM3). Mutations of ORAI1 (E106D and E190Q) result in changed calcium channel ion selectivity, which indicates that ORAI1/CRACM1 is a pore subunit of store-operated CRAC channels [27]. Two other non-synonymous SNPs (rs3741596 and rs75603737) have been observed within the ORAI1/CRACM1 locus in the NCBI SNP database; however, the MAFs of the two SNPs are less than 1% in the Taiwanese population. We found no significant associations between genotype and phenotypes of KD susceptibility and clinical outcomes (data not shown).
The stromal interaction molecule 1 (STIM1) is an intracel-lular Ca2+ sensor that initiates SOC activation [28]. In addition, STIM2 is an intracellular Ca2+ regulator that maintains the Ca2+ balance between the cytosol and the endoplasmic reticulum [29]. We also tested KD patients for SNPs in STIM1 (rs35637264), STIM2 (rs4505809), and calcium sensor receptor (CASR) (rs17251221). No significant findings were observed (data not shown). We acknowledge that the SNPs selected for this study were not adequate to investigate the entire SOC pathway and that further studies on additional genetic polymorphisms of this pathway are needed. We systematically investigated five ORAI1/CRACM1 tSNPs (rs12313273, rs6486795, rs7135617, rs12320939, and rs712853) in the Taiwanese children. None of these SNPs reached statistical significance. Our moderate sample size may not have provided sufficient power to detect minor genetic effects. Therefore, we cannot exclude rare causal genetic polymorphisms at ORAI1/CRACM1. Application of direct ORAI1/CRACM1 sequencing in larger samples may be useful to identify new SNPs in the ORAI1/CRACM1 gene and to clarify the effects of ORAI1/CRACM1 poly-morphisms on KD susceptibility.
Acknowledgements I am grateful to Dr. Yoshihiro Onouchi (RIKEN) for reading this manuscript. This study was partly supported by funding from Excellence for Cancer Research Center grant, Department of Health, Executive Yuan, Taiwan, ROC (NO. DOH100-TD-C-111-002); grant from the National Science Council, Taiwan, ROC (NSC 98-2320-B-037-028-MY2 and NSC97-2314-B-182A-054-MY2); grant from the Center of Excellence for Environ-mental Medicine, Kaohsiung Medical University (KMU-EM-99-6-3); and grant from Chang Gung Memorial Hospital, Taiwan, ROC (CMRPG891441 and CMRPG891241).
Competing interests The authors declare no competing interests.
References
1. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271–6. 2. Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki
disease: infection, immunity and genetics. Pediatr Infect Dis J. 2005;24(11):998–1004.
3. Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364 (9433):533–44.
4. Park YW, Han JW, Park IS, Kim CH, Cha SH, Ma JS, et al. Kawasaki disease in Korea, 2003–2005. Pediatr Infect Dis J. 2007;26(9):821–3.
5. Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003–2006. Pediatrics. 2009;123(3):e401–5.
6. Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K, Yanagawa H. Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int. 2008;50(3):287–90.
7. Liang CD, Kuo HC, Yang KD, Wang CL, Ko SF. Coronary artery fistula associated with Kawasaki disease. Am Heart J. 2009;157 (3):584–8.
8. Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. 2010;99 (10):1578–83.
9. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191(4):499–502.
10. Chang LY, Chiang BL, Kao CL, Wu MH, Chen PJ, Berkhout B, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193(2):283–6.
11. Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192(2):351–2. author reply 3. 12. Sauer K, Cooke MP. Regulation of immune cell development
through soluble inositol-1,3,4,5-tetrakisphosphate. Nat Rev Immunol. 2010;10(4):257–71.
13. Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42.
14. Shimizu C, Jain S, Lin KO, Molkara D, Frazer JR, Sun S, et al. Transforming growth factor-{beta} signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet. 2011;4 (1):16–25.
15. Kuo HC, Yang KD, Liang CD, Bong CN, Yu HR, Wang L, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. 2007;18(4):354–9.
16. Kuo HC, Wang CL, Liang CD, Yu HR, Huang CF, Wang L, et al. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr Allergy Immunol. 2009;20(3):266–72.
17. Chi H, Huang FY, Chen MR, Chiu NC, Lee HC, Lin SP, et al. ITPKC gene SNP rs28493229 and Kawasaki disease in Taiwanese children. Hum Mol Genet. 2010;19(6):1147–51.
18. Lin MT, Wang JK, Yeh JI, Sun LC, Chen PL, Wu JF, et al. Clinical implication of the C allele of the ITPKC gene SNP rs28493229 in Kawasaki disease: association with disease susceptibility and BCG scar reactivation. Pediatr Infect Dis J. 2011;30(2):148–52.
19. Kuo HC, Yu HR, Juo SH, Yang KD, Wang YS, Liang CD, et al. CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet. 2011;56 (2):161–5.
20. Onouchi Y. Identification of susceptibility genes for Kawasaki disease. Nihon Rinsho Meneki Gakkai Kaishi. 2010;33(2):73–80. 21. Hata A, Onouchi Y. Susceptibility genes for Kawasaki disease: toward implementation of personalized medicine. J Hum Genet. 2009;54(2):67–73.
22. Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+stores and
on electrostatic interactions. Mol Biol Cell. 2009;20(1):389–99. 23. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et
al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–85. 24. Kuo HC, Wang CL, Liang CD, Yu HR, Chen HH, Wang L, et al.
Persistent monocytosis after intravenous immunoglobulin therapy
correlated with the development of coronary artery lesions in patients with Kawasaki disease. J Microbiol Immunol Infect. 2007;40(5):395–400.
25. Shulman ST, De Inocencio J, Hirsch R. Kawasaki disease. Pediatr Clin North Am. 1995;42(5):1205–22.
26. Yang KD, Chang JC, Chuang H, Liang HM, Kuo HC, Lee YS, et al. Gene–gene and gene–environment interactions on IgE produc-tion in prenatal stage. Allergy. 2010;65(6):731–9.
27. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–3.
28. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169 (3):435–45.
29. Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+levels. Cell. 2007;131(7):1327–39.