EISEVIER Journal of Hazardous Materials 48 (1996) 69-82
JOURNALOF
HAZRRDOIJI
rnmERlAL5
Polycyclic aromatic hydrocarbons (PAHs) and mutagenicity
in air emissions from the two-stage incineration of
polystyrene with various metallic salt additives
Jiann-Hwa Youa,*, Pen-Chi Chiangb, Shenq-Chyi Change,S. Wang-Wuuc
“Department of Chemical Engineering, Chang Gung College of Medicine and Technology, Taoyuan. Taiwan, ROC
bGraduate Institute of Environmental Engineering, National Taiwan University, Taipei, Taiwan, ROC ‘Department of Biochemistry, National Yang-Ming University, Taipei, Taiwan, ROC
Received 16 May 1995; accepted 28 August 1995
Abstract
The yield of 14 PAHs and soots were more with BaC12 or NaCl additive than those with- out metallic chloride additives. This indicated that metallic chloride additives could promote the reaction sequence towards the growth and coagulation reaction of soots from PS pyro- lysis. When the second-stage temperature increased to the critical temperature, black tar formed and the concentration of 14 PAHs was 10 pg/g PS. Pha, Flu and Pyr were major species found among the 14 PAHs. Log K values, the ratios between the PAHs in gas phase and the PAHs in solid phases (per particulates weight; l/mg) increased as the stage-two pyrolysis tempera- ture increased. When the stage-two incineration system was controlled at 900 “C as were var- ious oxygen supplies, the maximum value of log K occurred at 0.0525 02 (nl/min) and log K
value decreased as the oxygen supply increased. When oxygen supply was increased from 0 to 0.210nl/min, the mutagenicity of particulates extracts from the PS incineration with metallic chloride additives was still more stronger.
Keywords: PAHs; Mutagenicity; Incineration; Polystyrene; Air emission
1. Introduction
With the increase in environmental consciousness among the people, traditional solid-waste handling methods, landfill and incineration, are meeting with increasing public resistance. Especially, air emissions from incineration are now releasing not only traditional air pollutants, but also air toxics, such as polycyclic aromatic com- pounds (PAHs), dioxins, and furans.
* Corresponding author.
0304-3894/96/$15.00 0 1996 Elsevier Science B.V. All rights reserved SSDI 0304-3894(95)00146-8
70 J.-H. You et al. /Journal of Hazardous Materials 48 (1996) 69-82
PAHs, which are thought to be the precursors of soots and have also been known to be mutagenic or carcinogenic, after being metabolized, are commonly produced from the incomplete combustion processes [l-6]. The formation of PAHs and soot particulates in the incineration of organic compounds not only depends on temper- ature, residence time and oxygen supply, but also depends on additives, e.g. Cl2 and metallic salts. Since complex mechanisms are involved in the incineration (or com- bustion) processes, their influence on PAHs and soot particulates formation is not yet clearly understood and various hypotheses, such as ionic chemical mechanisms, hydroxyl radical equilibrium and electrostatic effects are proposed based on various experimental systems and results [7-131.
Dependence of PAHs-induced mutagenicity on the bay region of the molecule and on the activating cytochrome P-450 enzyme has already been studied [ 141. The results showed that the mutagenic activity of PAHs without a bay region was largely inde- pendent of the source of activating enzyme. Some researchers indicated that nucleo- philic epoxides were formed from a metabolic oxidation of PAHs and then the epoxide ring would react with a nucleophilic group in celluar DNA. With its DNA thus altered, the cell was unable to reproduce normally [15]. According to the exper- imental results of a prototype/laboratory-scale rotary kiln under suboptimal opera- tion conditions, Demarini suggested that the mutagenic emission factors may depend as much as or more on the operating conditions of the incinerator than on the feed stock [16].
The objectives of this research work are to investigate the mutagenicity and PAHs in air emissions from the incineration of polystyrene with various metallic salt addi- tives under various stage-two pyrolysis temperatures and oxygen supplies.
2. Materials and methods
Polystyrene (PS) (0.2g) was incinerated at various temperatures and oxygen supplies in a two-stage incinerator. The two-stage incinerator consisted of two furnaces and two quartz tubes (D = 3 cm, L = 30 cm and L = 50 cm). The feed gas, which consisted of oxygen and nitrogen, was first introduced through two tubes (D = 5.0 cm, L = 30 cm) containing 1 M BaC12 or 3 M NaCl solution, and then car- ried BaC12 (1.3x 106mmol/s) or NaCl (1.1 x lo5 mmol/s) into the second-stage incin- erator. The feed rate was controlled at 2.0nl/min and at various oxygen ratios by using the flotation flowmeter and needle valves. The rate of temperature increase in the stage-one incinerator was controlled at 35-40”C/min, and the maximum temperature was controlled at 500°C. The temperature in the stage-two incinerator was controlled at 700 ‘C to 1200 “C. The theoretical residence time was 2-4 s.
The glass-fiber filter was used for sampling the particulates and PAHs which were defined in the solid phase. PUF (polyurethane foam) was used for sampling the PAHs which were defined in the gas phase. The sampling time was 5 min and the sampling flow time was 5 nl/min. The samples were pretreated in a series process: extraction, concentration and purification. Then the GC/MS (HP5890-11, HP G1034C MS Chemistation; NIST/EPA/NIH Mass Spectral Database-Envelop)
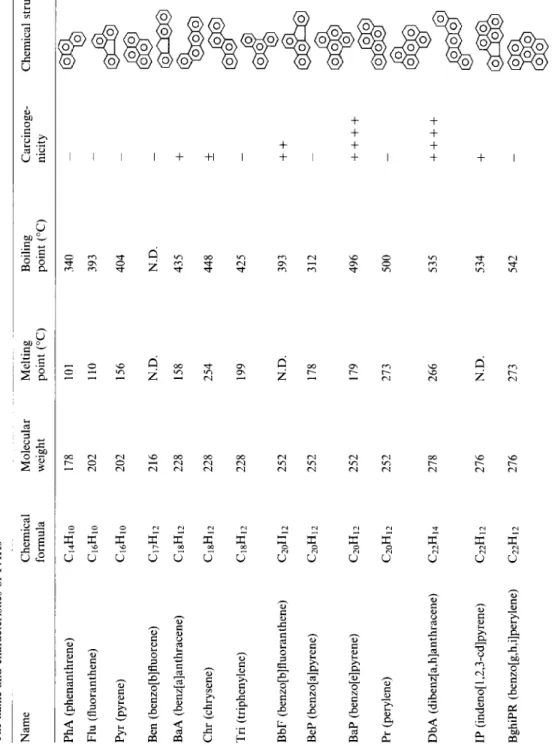
Table 1 The name and characteristics of PAHs Name Chemical formula Molecular weight PhA (phenanthrene) Flu (fluoranthene) Pyr (pyrene) Ben (benzo[b]fluorene) BaA (benz[a]anthracene) Chr (chrysene) Tri (triphenylene) C14H10 C16HlO Cl6HIO 178 101 340 202 110 393 _ 202 156 404 C17H12 C18Hl2 C18Hl2 216 N.D. 228 158 228 254 ClSHl2 228 BbF (benzo[b]fluoranthene) BeP (benzo[a]pyrene) C20H12 C2oH12 252 252 BaP (benzo[e]pyrene) Pr (perylene) C20H12 C20H12 252 252 DbA (dibenz[a,h]anthracene) C22H14 278 266 IP (indeno[l,2,3_cd]pyrene) C22H12 276 BghiPR (benzo[g,h,i]perylene) C22H12 276 Melting point (“C) Boiling point (“C) Carcinoge- nicity Chemical structure 199 N.D. 178 179 273 N.D. 273 N.D. _ 435 + 448 * 425 _ 393 ++ 312 496 ++++ 500 _ 535 ++++ 534 + 542 _ Note: -, Not carcinogenic; + , uncertain or weakly carcinogenic; + + , carcinogenic; + + + + very strongly carcinogenic, 00 % 0 0
&I
0 0 000
&
0 000833
00083
00 043
00 0 0c&l
00 0 00
000 0 00cx!B
000 0 000R9
0 0H
000 0 00aa
0 00 00 00 0029
12 J.-H. You et al. /Journal of‘ Hazardous Materials 48 (1996) 69-82
Table 2
The regression equations of GC/MS calibration curves of 14 PAHs
PAH Regression equation R2
1. PhA 2. Flu 3. Pyr 4. Ben 5. BaA 6. Tri + Chr I. BbF 8. BeP 9. BaP 10. Pr 11. IP 12. BghiPr 13. DbA y = 2.551 x 10’~ + 1.362 x lo5 0.984 y = 4.753 x 10*x + 3.511 x 104 0.984 y = 2.793 x 10’~ - 2.314x IO4 0.996 y = 1.095 x 108x - 4.005 x 105 0.998 y = 1.700x 10*x - 1.681 x 105 0.990 y = 5.208x 10’~ - 1.532x lo5 0.999 y= 1.818x10sx- 1.078~10~ 0.998 y = 1.647x 10’~ - 6.384 x lo5 0.999 y = 3.363 x 10*x - 3.876 x lo5 0.997 y = 1.759 x 108x - 5.394 x 104 0.999 y = 2.141 x 10*x - 2.451 x lo5 0.999 y = 2.310 x 10*x - 1.001 x lo5 0.992 y = 1.214x 10*x - 1.592x lo5 0.997
Note: (1) y, signal area; x: PAHs(ug).
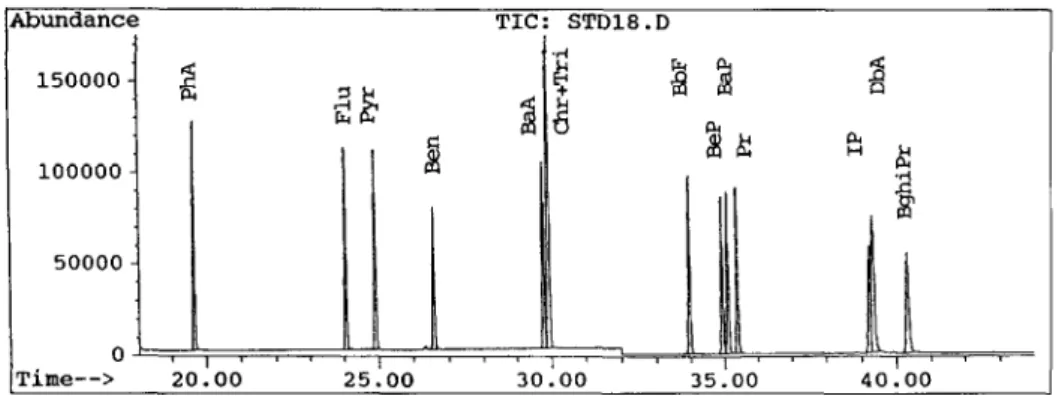
(2) R2, correlation coefficient. 150000 - g 227 100000 - Li 50000 - 0’ , , , I , 1 , ;, , Time--> 20.00 25.00 30.00 35.00 40.00
Fig. 1. GC/MS spectrum of 14 PAHs standards.
was used to analyze the 14 species of PAHs as shown in Tables 1, 2 and Fig. 1. During the extraction process, samples were placed and extracted in a soxhlet extrac- tor with 250 ml of dichlormethane at 40 “C for about 16 h. After the extraction process, the dichloromethane residue was concentrated to l-2ml by using a rotary evaporator. The extract residue was purified with 100 ml of hexane- dichloromethane (7 : 3, v/v) in the tube purifier (D = 1 cm, L = 20 cm) which was packed with 2 g of activated aluminum and 3 g of silica gel. Finally, the purified samples were concen- trated to 2 ml by using a rotary evaporator, and dried to 1 ml by using nitrogen.
The mutagenic activity of the particulates and PUF extracts were tested by the Ames Salmonella/microsomal assay system [17, 181. From the mutagenicity research of ambient particles, it was noted that the mutagenic response of strain TA98, which was used to detect frame-shift substitution mutation, was significantly higher than that of strain TAlOO, which was used to detect base-pair
J.-H. You et al. /Journal of Hazardous Materials 48 (1996) 69-82 13
substitution mutation, upon incubation with the dichloromethane extract residue of airborne particulates. Thus, Salmonella typhimurim strain TA98 was employed to carry out the mutagenicity test in this study.
0.8 ml of DMSO was added to the dried sample in the vial. The aliquot of sam- ple extracts in DMSO was placed in a test tube containing 2 ml of molten top agar supplemented with a 0.1 ml test solution, 0.1 ml of an overnight broth culture of the tester strain, and with or without 0.5 ml of S9 mixture. The S9 homogenate was pre- pared from the liver of Sprague-Dawley male rats pretreated for 5 days with Aroclor 1254 (500 mg/kg body weight) according to Maron and Ames et al. [17]. After 2 days incubation of the culture plates at 37 “C, histidine revertants of TA98 were scored.
3. Results and discussion
In our investigation, when the thermal temperature of the first-stage incinerator was at 350-450 “C, polystyrene was decomposed by the mechanisms of random- chain scission and end-chain scission. The long chain of polymer structure was bro- ken down to a shorter-chain structure, i.e., styrene monomer, dimer, trimer and toluene.
When the intermediate products from the first-stage incinerator were introduced to the second-stage incinerator, the intermediate products should be transformed by two reactions, cracking and addition reactions. The cracking reaction occurred dom- inantly at 500-700 “C in the second-stage incinerator. When the incineration tem- perature was greater than 1200 “C, the addition reaction occurred dominantly and the major products were soots which are more thermally stable.
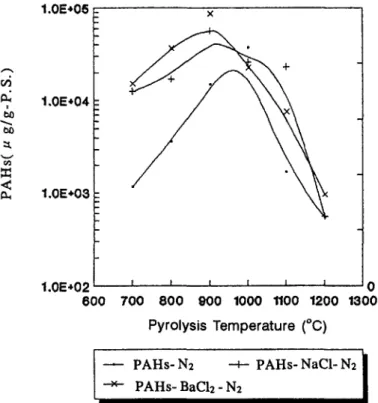
The analysis results in the 14 PAHs, as shown in Fig. 2, indicated that the criti- cal temperature for the maximum yield of 14 PAHs was shifted from 1000 “C to 900 “C, when metallic chloride (BaC12 or NaCl) was added to the incineration system. The yields of 14 PAHs and soots were more with BaC12 or NaCl additive than those without metallic chloride additives. This indicated that metallic chloride additives could promote the reaction sequence towards the growth and coagulation reaction of soots from PS pyrolysis. When the second-stage temperature increased to the critical temperature, black tar formed and the concentration of 14 PAHs was
104-IO5 ug/g PS. Pha, Flu and Pyr were major species found among the 14 PAHs, as shown in Fig. 3. When the stage-two temperature was increased, the concentra- tion of the 14 PAHs formed was decreased. The reason was that the thermal tem- perature provided enough energy for the low-ring PAHs to grow to high-ring PAHs and soot particulates. At incineration temperature above 1000 “C, the soot particu- lates of black powder formed and the weight of soot particulates formed increased as the stage-two pyrolysis temperature increased.
The above results also indicated that PAHs would effectively react with phenyl, biphenyl, naphthyl, binaphthyl, and CzH2 and grew to larger PAHs when the pyro- lysis temperature was increased to a higher temperature of 1000 “C. Reactions of soot formation such as nucleation, condensation coagulation, aggregation mechanisms
J.-H. You et al./Journal of Hazardous Materials 48 (1996) 69-82
l.OE+02 ’ I 1 I I I I
600 700 800 900 1000 ilO 1200
130: I
Pyrolysis Temperature (“C)
- PAHs-Nz + PAHs- NaCl- N2
* PAHs- BaC12 - Nz I
Fig. 2. Formation of 14 PAHs from polystyrene incineration with or without metallic chloride additives under various pyrolysis temperatures.
occurred effectively. Since the change in free energies caused by the series reactions of PAHs and soot formation was so large, reactions became practically irreversible. This, in turn, had the effect “of pulling” the reaction sequence forward towards for- mation of larger PAHs and soot particles. It has been postulated that mechanism for coagulation is through the reactions of aryl radical + PAH, and molecule and soot active site + soot [lo].
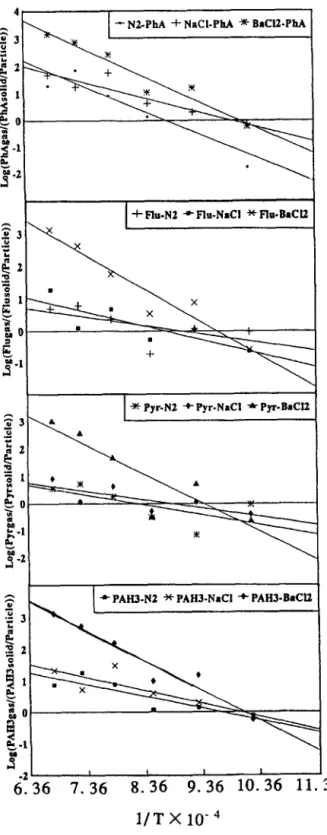
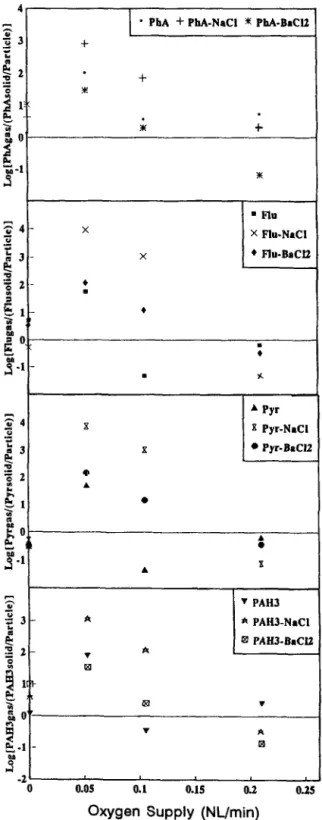
Of the 14 species of PAHs, only Pha, Flu and Pyr were always found to be in the gas phase in the PUF sample. The reason may be that the boiling points and vapor pressures were low. The various distributions of 14 PAHs between the gas and solid phases resulting from the polystyrene incineration with or without metallic chloride additives were caused by various temperatures and oxygen supplies. The Pha, Flu and Pyr distributions between the solid and gas phases at various pyrolysis temper- atures are shown in Fig. 4 and the relationship can be expressed as
1ogK = -A/T + B,
where K = PAH(,,,j(PAH(,,tid)/particulates), T is the temperature of the second- stage incinerator (K) and A, B are constants.
LogK values, the ratios between the PAHs in gas phase and the PAHs in solid phases (per particulates weight; l/mg) increased as the stage-two pyrolysis
PAHs Concentration ( ug/g-PS ) PAHs Concentration ( ug/g-PS ) PAHs Concentration ( ug/g-PS ) b b b b b b b b b b b b b b 7 7 m f 7 7 7 m m 7 7 T 7 7 m 8 2 N B P 2 k k z 8 9 B z: B z
1
76 J.-H. You et al. JJournal of Hazardous Materials 48 (1996) 69 82
* Pyr-Nf -+- Pyr-NaCI * Pyr-BaCI2
* PAHJ-N2 * PAHJ-NaCI + PAHJ-BaCl2
s
8236 7.36 , 8.36 8 9.36 I
I
10.36 11.36
l/T
X
lo-’
Fig. 4. The PAHs distributions between the solid and gas phases in air emissions from polystyrene incin- eration at various pyrolysis temperatures.
J.-H. You et al. /Journal of Hazardous Materials 48 (1996) 69-82 ?I
Table 3
The distribution constants of PhA, Flu, and Pyr between the gas and solid phases
Materials Species A B R2 PS PhA 8882.5 Flu 2604.4 Pyr 3612.3 PAHs 3760.6 7.9 2.4 3.0 3.7 0.836 0.371 0.471 0.770 PS + BaC12 PhA 9658.8 9.8 0.939 Flu 10211.2 9.9 0.992 Pyr IO 372.7 9.8 0.771 PAHs 9330.2 9.5 0.942 PS + NaCl PhA 5523.3 5.5 0.854 Flu 4294.8 3.8 0.669 Pyr 3077.5 2.7 0.590 PAHs 4135.8 4.2 0.737
Note: (1) log K = -A/T + B; K = PAH(.s,,j(PAH(,,t,~)/particulates).
(2) PAHs = PhA + Flu + Pyr. (3) R2, correlation coefficient.
temperature increased. When the stage-two incineration system was controlled at 900 “C as were various oxygen supplies, the maximum value of log K occurred at 0.0525 02 (nl/min) and 1ogK value decreased as the oxygen supply increased, as shown in Fig. 5 and the constants A and B are given in Table 3.
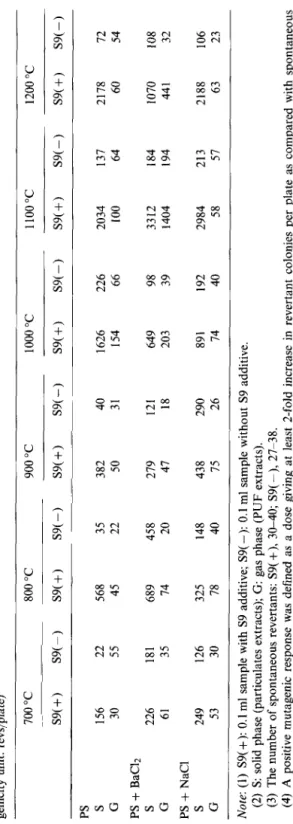
From the results of the TA98 Ames test in Table 4, when PS was pyrolyzed from 700 “C to 1200 “C, the mutagenicity was increased as the second-stage temperature increased, as shown in Fig. 6. The maximum mutagenicity of particulates extracts occurred at 1100 “C and were about 3000 revs/plate with BaClz and NaCl additives. When the second-stage temperature increased to 1200 “C, the mutagenicity of the extracts decreased.
The mutagenicity of particulates and PUF extracts at the second-stage tempera- ture of 900 “C and various oxygen supplies are given in Table 5. When oxygen supply was increased from 0 to 0.210 nl/min, the mutagenicity of particulates extracts from the PS incineration with metallic chloride additives was still more stronger. These results were surprising and they were very different from the previous results from the incineration of polystyrene without metallic chloride additives [19]. Also, the correlations between mutagenicity and PAH7 (BaP, BbF, BaA, IP, DbA, Tri and Chr) from polystyrene incineration with BaC12 or NaCl additive at various opera- tion conditions were not so good. The reason is that other toxic compounds (i.e., nitro-PAHs, dioxin and furan) might be formed and should be identified in future research work.
However, the mutagenicity of particulates and PUF extracts caused significantly higher potence of revertants to S. typhimurium TA98 in the presence of S9 mixture than that without S9 additive. This indicates that the emitted gas from the PS incin- eration contains not only direct-acting mutagens but also indirect mutagens, which
Table 4 Comparison of the mutagenicity of particulates and PUF extracts from polystyrene incineration under various stage-two pyrolysis temperatures (muta- genicity unit: revs/plate) PS S G PS + BaCl2 S G PS + NaCl S G 700 “C 800 “C 900 “C 1000 “C 1100 “C 1200 “C S9(+) S9(-) S9(+) S9(-) S9(+) S9(-) S9(f) S9(-) S9(+) S9(-) S9(+) S9(-) 156 22 568 35 382 40 1626 226 2034 137 2178 72 30 55 45 22 50 31 154 66 100 64 60 54 226 181 689 458 279 121 649 98 3312 184 1070 108 61 35 74 20 47 18 203 39 1404 194 441 32 249 126 325 148 438 290 891 192 2984 213 2188 106 53 30 78 40 75 26 74 40 58 57 63 23 Note: (1) S9( +): 0.1 ml sample with S9 additive; S9( -): 0.1 ml sample without S9 additive. (2) S: solid phase (particulates extracts); G: gas phase (PUF extracts). (3) The number of spontaneous revertants: S9( +), 30-40; S9( -), 27738. (4) A positive mutagenic response was defined as a dose giving at least 2-fold increase in revertant colonies per plate as compared with spontaneous reversion.
J.-H. You et al. /Journal of’ Hazardous Materials 48 (1996) 69-82 19
Table 5
Comparison of the mutagenicity of particulates and PUF extracts from polystyrene incineration under
various oxygen supplies (mutagenicity unit: revs/plate)
0 nl/min 0.0525 nl/min S9(+) S9(-) S9(+) S9(-) PS S 382 40 520 155 G 50 31 125 60 PS + BaCl2 S 219 121 636 207 G 41 18 12 31 PS + NaCl S 438 251 344 322 G 15 26 49 22 0.105 nl/min 0.210 nl/min S9(+) S9(-) S9(f) S9(-) 123 59 93 58 95 57 94 24 254 101 1910 184 76 26 129 36 691 311 527 IO 82 48 39 28
Note: Same as Table 3
are usually activated by the mixed-function oxygenases in the microsomes of ani- mal liver to become ultimate mutagens whether the PS incineration system added metallic chloride or not. The mechanisms of epoxide formation and nucleophilic epoxide opening could be involved in metabolic oxidation. In the body, the epox- ide ring should react with an amino group in celluar DNA to give an altered DNA that is covalently bound to the PAH. With its DNA thus altered, the cell was unable to reproduce normally. From the recent experimental results, sister chromatid exchanges in human lymphocytes were induced by the particulates extracts from polystyrene incineration, as shown in Fig. 7.
4. Conclusions
The critical temperature in the second-stage incinerator for the maximum yield of 14 PAHs was shifted from 1000 “C to 900 “C when metallic chloride (BaC12 or NaCl) was added to the incineration system. Log K values, the ratios between the PAHs in gas phase and the PAHs in solid phases (per particulates weight; l/mg) increased as the stage-two pyrolysis temperature increased. When the second-stage furnace was controlled at 900 “C and various oxygen supplies, the maximum value of log K occurred at 00525 02 (nl/min).
Concerning the mutagenicity in the air emissions, the maximum mutagenicity of particulates and PUF extracts occurred at 1100 “C, when the pyrolysis temperature was increased from 700 “C to 1200 “C. The mutagenicity of PUF extracts was weak- er than the mutagenicity of particulates extracts. When oxygen supply was increased to 0.210 nl/min and stage-two temperature was controlled at 900 “C, the mutagenicity of particulates and PUF extracts were still higher for NaCl additive and were much higher for BaC12 additive. Moreover, the mutagenicity is much higher in the pres- ence of rat liver microsomal fraction (S9 mixture) than that without S9 additive.
80 J.-H. You et aLlJournal of Hazardous Materials 48 (1996) 69-82
4
5
. PhA + PM-NaCI X PM-BaCU
33 + t $2 . .? + 1 $1:: f b -- . . $0 m + 8 Flu 5 4- X 3 X Flu-NaCI t w 3- X ??Flu-B&t e e z 2- ?? a . E l- * I ! % i OY : s-1 - . Y. z4 A Pyr 3 x X Pyr-N&I e $” X ??Pyr-B&I. ’ PAM * PAHJ-NaCI a PAH3.B&I . A ? ? 0 0.05 0.1 0.15 0.2 0.25
Oxygen Supply (NL/min)
Fig. 5. The PAHs distributions between the solid and gas phases in air emissions from polystyrene incin- eration at 900 “C and various oxygen supplies.
J-H. You et al. /Journal of Hazardous Materials 48 (I 996) 69-82 81
800 900 loo0 1100 lzoo
Pyrolysis Temperature (“C)
Fig. 6. The mutagenicity in air emissions from polystyrene incineration under various operation conditions.
Fig. 7. Sister chromatid exchanges in human lymphocytes were induced by the particulates extracts from polystyrene incineration.
82 J.-H. You et al. /Journal of Hazardous Materials 48 (1996) 69-82
Finally, from the results of this study, the metallic chloride in the municipal solid waste (MSW) should be considered as an important factor in design and operation of MSW incinerator. As for the toxic control and the risk assessment of human health, the SCE (sister chromatid exchange) test in the air emissions from various incineration conditions should also be taken into consideration and this should be worth researching in the future.
References
[l] J. Josephson, Environ. Sci. Technol., 18 (1984) 93.
[2] P.C. Chiang and J.H. You et al., Man and his Ecosystem, Proc. 8th World Clean Air Congress, Vol. 4, 1989, p. 289.
[3] 0.1. Smith, Prog. Energy Combust. Sci., 7 (1981) 252.
[4] T.R. Hendersm, J.D. Sun, A.P. Li, R.L. Hansm and W.E. Bechtold, Environ. Sci. Technol., 18 (1984) 428.
[5] R. Panlman and 0. Pelkonen, Carcinogenesis, 8 (1987) 773. [6] T. Morikawa, J. Combust. Toxicol., 15 (1978) 347. [7] T. Morikawa, Fire Sci. Technol., 4 (1984) 27.
[8] J.A. Mulholland, A.F. Sarofim, P. Sosothikul and A.L. Lafleur, Combust. Flame, 92 (1993) 161. [9] B.S. Haynes and H.G. Wanger, Prog. Energy Combust. Sci., 7 (1981) 229.
[lo] M. Frenklach, Combust. Sci. Technol., 74 (1990) 283.
[l I] M. Frenklach and J. Wamatz, Combust. Sci Technol., 51 (1987) 265.
[12] M. Frenklach, D.W. Clary, T. Yuan and W.C. Gardiner, Combust. Sci. Technol., 50 (1986) 79. [13] J.M. Smedley, A. Williams and K.D. Bartle, Combust. Flame, 91 (1992) 71.
[14] D.R. Thakker et al., in: P.W. Jones and P. Leber (Eds.), Polynuclear Aromatic Hydrocarbons, Proc. 3rd Int. Symp. on Chemistry and Biology-Carcinogenesis and Mutagenesis, Ann Arbor Science, Ann Arbor, 1979, p. 455.
[15] J. Mcmurry, Fundamentals of Organic Chemistry, Brooks/Cole, Monterey, CA, 1986, p. 250. [16] D.M. Demarini, R.W. Williams, E. Perry, P.M. Lemieux and W.P. Liank, Combust. Sci. Technol.,
85 (1992) 437.
[17] D.M. Marron and B.N. Ames, Mutat. Res., 113 (1983) 173.
[18] B.N. Ames, J. McCann and E. Yamasaki, Mutat. Res., 31 (1975) 347.