The fractionated Toona sinensis leaf extract induces apoptosis of human
ovarian cancer cells and inhibits tumor growth in a murine xenograft model
Hsueh-Ling Chang
a,b,c, Hseng-Kuang Hsu
d, Jinu-Huang Su
e, Pei-Hui Wang
d,
Yueh-Fang Chung
a,b, Yi-Chen Chia
f, Li-Yu Tsai
g,
Yang-Chang Wu
c, Shyng-Shiou F. Yuan
a,b,⁎
a
Department of Medical Research, E-DA Hospital, I-Shou University, Kaohsiung 824, Taiwan, R.O.C.
b
Department of Obstetrics and Gynecology, E-DA Hospital, I-Shou University, Kaohsiung 824, Taiwan, R.O.C.
c
Graduate Institute of Natural Products, Kaohsiung Medical University, Kaohsiung 807, Taiwan, R.O.C.
dDepartment of Physiology, Kaohsiung Medical University, Kaohsiung 807, Taiwan, R.O.C.
eDepartment of Obstetrics and Gynecology, Kaohsiung Medical University Hospital, Kaohsiung 807, Taiwan, R.O.C. fDepartment of Food Science and Technology, Tajen Institute of Technology, Pingtung, Taiwan
gDepartment of Clinical Laboratory, Kaohsiung Medical University, Kaohsiung 807, Taiwan, R.O.C.
Received 17 August 2005 Available online 8 February 2006
Abstract
Objective. Aqueous extract from the leaves of Toona sinensis Roem. has been shown to have an anti-proliferative effect on human lung cancer
cells. In this study, we analyzed the anti-cancer activity/effect of different extraction fractions of the extract from T. sinensis leaves on ovarian
cancer cells.
Methods. Cell viability was determined by XTT cell proliferation assay and cell survival assay. Apoptotic effect was detected by morphological
analysis and immunoblotting. Cell cycle effect was evaluated by flow cytometry analysis and immunoblotting. In vivo therapeutic effect was
evaluated by the subcutaneous inoculation of SKOV3 cells in nude mice (Foxnlnu/Foxnlnu) model.
Results. TSL2 of T. sinensis was more cytotoxic than other fractions and exhibited selectivity for ovarian cancer cell lines. TSL2 arrested
SKOV3 ovarian cancer cells at the G2/M phase and induced cancer cells go through apoptotic pathway. Ex vivo xenograft study of nude mice
showed that intraperitoneal injection of TSL2 was able to suppress the proliferation of ovarian cancer cells without significant nephrotoxicity, liver
toxicity, or bone marrow suppression.
© 2005 Elsevier Inc. All rights reserved.
Keywords: Toona sinensis; Ovarian cancer; Apoptosis; Cell cycle
Introduction
Toona sinensis Roem. or Cedrela sinensis, commonly known
as Chinese mahogany cedar or Chinese Toona, is a perennial
deciduous tree of the family Meliaceae
[1–3]
. Almost every part
of T. sinensis, including seeds, bark, root bark, petioles, and
leaves, has a medicinal effect
[1]
. Leaves of T. sinensis have
anti-inflammatory, antidoting, and worm-killing effects and are
useful for treating enteritis, dysentery, carbuncles, boils,
dermatitis, scabies, and tinea blanca, as well as for improving
body health. In addition, aqueous extracts of leaves of T. sinensis
have been used as a folk medicine for lowering blood pressure
associated with diabetes. They have also been reported to
enhance glucose uptake and lipolysis in 3T3-L1 adipocytes
[4,5]
. Poon et al. also showed that crude T. sinensis is able
to inhibit Leydig cell steroidogenesis
[6]
.
One recent publication has also showed that methyl gallate
from T. sinensis can protect against hydrogen-peroxide-induced
oxidative stress and DNA damage in MDCK cells
[7]
. The
anti-cancer effects of T. sinensis for the most part remain unclear.
Aqueous extract from the leaves of T. sinensis can inhibit the
⁎ Corresponding author. Department of Obstetrics and Gynecology, E-DA Hospital, I-Shou University, 1, E-DA Road, Jiau-Shu Tsuen, Yan-Chau Shiang, Kaohsiung County, Taiwan 824, R.O.C. Fax: +886 7 6155352.
E-mail address: yuanssf@ms33.hinet.net (S.-S.F. Yuan).
0090-8258/$ - see front matter © 2005 Elsevier Inc. All rights reserved. doi:10.1016/j.ygyno.2005.12.023
proliferation of human lung adenocarcinoma cells A549 by
inhibiting the expression of cyclins D1 and E
[8]
.
In this study, we explored the anti-cancer activity of different
extraction fractions of leaf extracts from T. sinensis and found
that TSL2, a specific fraction of the leaf extracts from T.
sinensis, has a very potent anti-ovarian cancer activity, the
mechanism of which we also addressed.
Materials and methods
Preparation and fractionation of leaf extracts of T. sinensis
The leaves used in this preparation were obtained from T. sinensis Roem. grown in Tuku (Yunlin County, Taiwan) and were picked and washed briskly with water. Reverse osmosis water (RO water) was added to the leaves at a proportion of 4 l of RO water to 1 kg of leaves. The mixture was heated to a boil and kept boiling for 30 min and then cooled down slowly without further boiling for 2 h at room temperature. The leaves were then removed, and the remaining liquid was concentrated over low heat and was filtered with a sieve (70-mesh). At this point, we began the fractionation. The filtered concentrate was lyophilized with a Virtis apparatus to obtain a crude extract (TSL1). Through this procedure, 100 g of leaves yielded approximately 5–6 g of lyophilized TSL1 powder. The powder was then dissolved in 99.5% ethanol and was centrifuged at 3000 rpm at 4°C (Beckman AvantiTM J-30I) for 12 min to give a supernatant portion and a precipitate portion. The supernatant portion was further lyophilized with a Virtis apparatus to obtain the lyophilized powder, TSL2. The precipitate portion was further lyophilized using a Virtis apparatus and then dissolved in 50% ethanol. The 50% ethanol solution was centrifuged at 4°C and at 3000 rpm for 12 min to give a supernatant portion and a precipitate portion. The supernatant portion was further lyophilized with a Virtis apparatus to obtain an extract in the form of lyophilized powder, TSL3. The precipitate portion was lyophilized with a Virtis apparatus and then dissolved in 25% ethanol. The 25% ethanol solution was centrifuged at 4°C and at 3000 rpm for 12 min to give a supernatant portion and a precipitate portion. The supernatant portion was lyophilized to obtain the powder form, TSL4. Finally, the precipitate portion was dissolved in RO water and centrifuged at 4°C and at 3000 rpm for 12 min to give a supernatant portion, which was then lyophilized to a powder form, TSL5.
Reagents and cell culture
Ovarian cancer cell lines SKOV3 and PA-1, cervical cancer cell lines HeLa and HeLa S3, and endometrial cancer cell line RL95-2 were purchased from the American Type Culture Collection and grown in DMEM-F12 supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B. SKOV3 cells at various cell cycle phases were retrieved by the following procedures. First, we grew the SKOV3 cells for 2 more weeks after the cells became 100% confluence on T75 flask, and then the cells were dissociated by trypsinization and replated on 10 cm dishes. The replated SKOV3 cells were harvested at different time points to determine their cell cycle distribution patterns or treated with TSL2 the different time points after replating. Twenty-four hour after being replated, SKOV3 cells were treated with 0.4μg/ml Nocodazole for 10 h to retrieve M-phase-enriched SKOV3 cells[9]. The cell cycle distribution of the SKOV3 cells was determined by EPICS flow cytometer (Beckman Counter).
XTT cell proliferation assay
Because only viable cells can metabolize tetrazolium salt XTT into a formazan dye, it was used in the colorimetric assay to determine cell proliferation and viability. SKOV3 cells were plated out at a density of 5000 cells/well in 96-well microtiter plates the day before treatment with different extraction fractions began. After 72 h of being treated with different extraction fractions, the cytotoxicity of TSL2 was determined using XTT colorimetric cell proliferation assay (Roche Molecular Biochemicals). Briefly, the culture medium was removed, and 100μl of fresh culture medium and a pre-formulated
50μl XTT mixed reagent (XTT reagent:electronically coupled reagent = 50:1) were added. The culture plate was incubated at 37°C for 4 h. Light absorbance values (OD = OD490− OD650) were read at wavelengths of 490 nm and 650 nm using an ELISA reader for calculating the 50% inhibitory concentration (IC50), i.e., the cell concentration at which the light absorbance value of the experimental group is one half of that of the control group[10].
Morphology analysis and cell survival assay
Various cancer cell lines, grown in 6 cm petri dishes, were treated with TSL2 at different dosages (0, 10, 100, 1000μg/ml) for 24 h, and the morphological changes were observed and photographed under an inverted microscope (Nikon TS100). For cell survival assay, the cells were treated with TSL2 at a concentration of 1, 10, 100μg/ml for 24 h. After trypsinization and mixing with trypan blue at the ratio of 1:1, the surviving cells were counted under an inverted microscope[11].
FACS analysis
SKOV3 cells (2 × 105cells/35 mm dish) were treated with TSL2 at different dosages for 24 h, harvested by centrifugation, washed with ice-cold PBS, and then resuspended with ice-cold 70% ethanol for 1 h. The ethanol-treated cells were spun down and stained with 40μg/ml propidium iodide (PI) for 30 min. The DNA content was then measured by an EPICS flow cytometer (Beckman Coulter)[12].
Immunoblotting analysis
After TSL2 treatment, the cells were washed twice with PBS and lysed in EBC buffer (50 mM Tris, pH 7.6, 120 mM NaCl, 0.5% Nonidet P-40, 1 mM β-mercaptoethanol, 50 mM NaF, and 1 mM Na3VO4) followed by centrifugation.
The supernatant was collected and measured by the Bio-Rad protein assay. The detailed immunoblotting procedure was followed accordingly[11]. The proteins were detected using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech).
Nude mice assay
SKOV3 cells (2 × 106cells in 0.1 ml of PBS) were injected subcutaneously at one site of the right flank of 6-week-old female nude mice (Foxnlnu/Foxnlnu).
Table 1
Cell survival assay showing the percentage of various surviving cancer cell lines after treatment with TSL2
Cell lines Dose (μg/ml) Survival (%)
SKOVA 1 79.0 ± 8.8 10 55.4 ± 9.2 100 25.9 ± 7.3 PA-1 1 67.5 ± 4.2 10 50.4 ± 6.5 100 15.5 ± 9.7 HeLa 1 102.4 ± 5.5 10 101.6 ± 8.2 100 70.4 ± 8.7 HeLa S3 1 100.6 ± 12.6 10 100.3 ± 7.5 100 101.0 ± 8.4 RL95-2 1 100.5 ± 5.1 10 100.4 ± 5.3 100 103.1 ± 9.0
The cells were treated with 1, 10, 100μg/mL TSL2 for 24 h, and the surviving cells were determined and presented as a percentage of the untreated cells as control (the index in the control group was 100%). The experiment was repeated three times and presented as mean ± SD. One significant difference was that TSL2 was mostly cytotoxic to ovarian cell lines, PA-1 and SKOV3.
When the subcutaneous tumors became distinctively visible (approximately 3 × 3 mm in size), usually at about 2 weeks after inoculation, the mice were randomly divided into three groups (ten mice per group) and then treated with TSL2 intraperitoneally for 7 weeks. The control group, high dose group and low dose group were given PBS, 6.7μg/g body weight of TSL2 in PBS (a dose equals to one-fourth of the IC50 for SKOV3 cells), and 0.67μg/g body weight of TSL2 in PBS (a dose equals to one-tenth of the dosage for high dose group), individually. Mice were injected 5 days per week, and tumor size was measured twice a week for 7 weeks. Tumor volumes were calculated by the formula of ([1/2] × [longest dimension] × [shortest dimension]2), as described previously [12]. Complete blood count of the nude mice blood was determined by Sysmex X1-2100, and the plasma BUN, Cr, AST, and ALT levels were determined by Beckman LX20.
Results
Cytotoxicity of different extraction fractions from T. sinensis
leaves on various cancer cell lines
Using ovarian cancer cell line SKOV3, XTT cell
prolifer-ation assay was performed to screen the cytotoxicity of
different extraction fractions from the leave extracts of T.
sinensis on cancer cells. Fraction 2 of the leaf extracts of T.
sinensis (TSL2) was found to have the highest dose-dependent
cytotoxicity with IC50 of 26
μg/ml (data not shown). The
IC50 is more than 100
μg/ml for other extraction fractions
from T. sinensis leaves.
One previous report showed that T. sinensis leaf extracts
were cytotoxic to human lung adenocarcinoma cells A549.
In this study, we further explored the cytotoxicity of TSL2,
which had the highest anti-ovarian cancer cell activity
among different extraction fractions from the leave extracts
of T. sinensis, on different cancer cells derived from ovarian
cancer (PA-1 and SKOV3), cervical cancer (HeLa and HeLa
S3), and endometrial cancer (RL95-2). The growth inhibition
for various cancer cell lines, determined by cell survival
assay, is shown in
Table 1
. Twenty-four hours after treatment
with 1, 10, and 100
μg/ml of TSL2, we found that TSL2
had the most potent activities against ovarian cancer cells
PA-1 and SKOV3, with IC50 of 11.0 and 26.4
μg/ml,
respectively. In contrast, under the same dosage and time
period, TSL2 had a very low cytotoxicity on cervical cancer
cells HeLa and HeLa S3 as well as endometrial cancer cells
RL95-2. Therefore, we chose SKOV3, which was derived
from the most common type ovarian cancer, epithelial
ovarian cancer, to further study the antitumor mechanism
of TSL2 in vitro and in vivo.
Induction of pro-apoptotic and anti-apoptotic genes upon
treatment with TSL2 on SKOV3 cells
To dissect the detailed mechanism for TSL2-induced
apoptotic cell death, the pro-apoptotic and anti-apoptotic genes
were explored for their possible involvement in the cytotoxicity
of T. sinensis. TSL2 was able to induce the expression of Bax at 1
h after treatment and the proteolytic cleavage of procaspase 3
and PARP at the dosage of 500
μg/ml (
Fig. 1
).
Fig. 1. Immunoblotting analysis showing the expression of the pro-apoptotic proteins in SKOV3 cells upon treatment with TSL2 at the dosage of 500μg/ml for different time periods. (C) Untreated control; (1, 4, 8, 12, 24) treated with TSL2 for 1, 4, 8, 12, and 24 h individually.
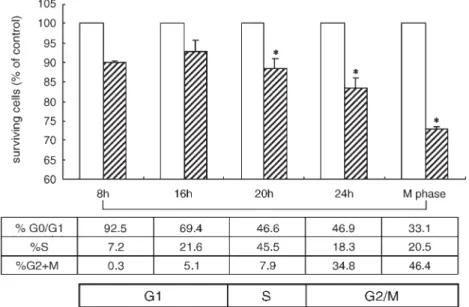
Fig. 2. TSL2 was more cytotoxic to M-phase-enriched SKOV3 cells than cells at other phases. Various cell-cycle-phase-enriched SKOV3 cells (see Materials and methods) were treated with TSL2 at the dosage of 100μg/ml, and the surviving cells were determined by cell survival assay at 4 h after treatment. The experiment was repeated three times and presented as mean ± SD. *Significantly different from the control group (Pb 0.05).
The cytotoxicity of TSL2 on SKOV3 cells at different cell cycle
phases
Cell-cycle-phase-enriched SKOV3 cells were achieved by
density arrest (please see Materials and methods for detail) and
were treated with TSL2 for 4 h to assess the cytotoxicity of
TSL2 on SKOV3 cells at different phases. Interestingly, TSL2
had an enhanced cytotoxicity on SKOV3 cells enriched at M
phase (
Fig. 2
).
The effects of TSL2 on cell cycle distribution and
cell-cycle-related proteins
To study the effect of TSL2 on cell cycle distribution,
unsynchronized SKOV3 cells were treated with TSL2 for 24
h and analyzed by fluorescence-activated cell sorter (FACS).
Interestingly, SKOV3 cells enriched at G2/M phase were
increased after TSL2 treatment in a dose-dependent manner
(
Table 2
).
We also analyzed, by immunoblotting, the gene expression
of several cell cycle regulatory proteins, including Chk1, Chk2,
Cdc25c, and Cdc2 in SKOV3 cells upon TSL2 treatment. At 1
h and the later time points after treatment, TSL2 was found to
have significantly enhanced the expression of phosphorylated
Chk1 and Chk2 (p-Chk1 and p-Chk2) in SKOV3 cells, which
then phosphorylated and inactivated Cdc25C phosphatase, a
key activator of Cdc2/cyclin B that controls M-phase entry in
eukaryotic cells (
Fig. 3
). The inactivated Cdc25C was unable
to dephosphorylate Cdc2 and caused the increase of
phos-phorylated Cdc2, an inactive form of Cdc2, in SKOV3 cells
(
Fig. 3
).
Tumoricidal effect of TSL2 on ovarian cancer cells using nude
mice model
To verify whether TSL2 had an anti-cancer effect in vivo, we
used a nude mice xenograft model (see Materials and methods
for the detailed information). After the subcutaneous tumors had
developed to at least 3 mm, which took about 2 weeks, the nude
mice were given intraperitoneal injections of either TSL2 (low
dose group and high dose group) or PBS (control group). After
7 weeks of TSL2 treatment, the tumor growth was significantly
suppressed by TSL2 in a dosage-dependent manner, while the
tumors continued to grow in the control group (
Fig. 4
).
Moreover, after intraperitoneal injection of TSL2 for 7 weeks,
no significant impairment of hematopoiesis or renal function
was observed in the mice (
Table 3
). Although liver enzymes
AST and ALT were elevated in the high dose group, no
statistical significance was reached (
Table 3
).
Discussion
In this study, we showed that fraction 2 of T. sinensis (TSL2)
had significant cytotoxic effect on ovarian cancer cells both in
vitro and in xenograft studies. Furthermore, we illustrated the
molecular mechanisms for the anti-cancer effects of TSL2 on
SKOV3 ovarian cancer cells.
Table 2
TSL2 arrested SKOV3 cells at G2/M phase
Dosage Percentage (mean ± SD) of ovarian cancer cells at each cell cycle phase after treatment with TSL2 for 24 h
G1 S G2M
0μg/ml 59.0 ± 0.2 27.3 ± 0.9 13.8 ± 0.7 10μg/ml 53.7 ± 1.3 ⁎ 32.1 ± 1.4 14.2 ± 1.1 100μg/ml 54.7 ± 1.6 ⁎ 20.9 ± 0.9 ⁎ 25.0 ± 2.5 ⁎ SKOV3 cells were treated with TSL2 at different dosages for 24 h and cell cycle distribution was determined by flow cymmetry. The experiment was repeated four times and presented as mean ± SD.
⁎ Significantly different from the control group (P b 0.05).
Fig. 3. Expression of cell cycle checkpoint proteins upon TSL-2 treatment. SKOV3 cells were treated with TSL2 at a dosage of 500μg/ml for different time periods and then harvested for immunoblotting analysis. (C) Untreated control; (1, 4, 8, 12, 24) treated with TSL2 for 1, 4, 8, 12, and 24 h individually.
Fig. 4. TSL2 suppressed the ex vivo ovarian cancer cell growth in a dose-dependent manner. Two million SKOV3 ovarian cancer cells were injected subcutaneously into the nude mice, and, after the tumor mass became noticeable, TSL2 was given by intraperitoneal injection 5 days per week for a total duration of 7 weeks at the dosage of 0.67μg/g body weight (low dose) and 6.7 μg/g body weight (high dose). The tumor volumes were measured for each group and presented as mean ± SD.
Poly(ADP-ribose) polymerase (PARP) is a multifunctional
protein and is involved in several crucial cellular processes:
DNA replication, transcription, DNA repair, genome stability,
and apoptosis
[13–16]
. On the other hand, caspases cause PARP
cleavage and inactivation during apoptosis
[17–19]
. Our study
shows that TSL2 decreased the expression of PARP and arrested
the repairing function of PARP (
Fig. 1
).
The expression of pro-apoptotic proteins Bax was enhanced
by at 1 h and the later time points after TSL2 treatment (
Fig.
1
). In our study, although procaspase 3 expression was
decreased at 1 h and the later time points after TSL2
treatment, the P12 and P17 subunits of caspase 3 did not
appear after TSL2 treatment in SKOV3 cells (
Fig. 1
). A
similar observation has been reported by Seo and Surh, who in
one study treated human promyelocytic leukemia HL-60 cells
with Eupatilin
[20]
. However, the reason for this observation
is currently unclear.
Checkpoint controls ensure chromosomal integrity through
the cell cycle. Chk1 and Chk2 are effector kinases in the
G2-phase checkpoint activated by damaged or unreplicated DNA
[21,22]
. Chk1 and Chk2 inhibit Cdc2 by phosphorylation and
inactivation of Cdc25, the phosphatase that normally activates
Cdc2
[23,24]
. Cdc25 is a protein phosphatase responsible for
dephosphorylating and activating Cdc2, a crucial step in
regulating the entry of all eukaryotic cells into the M-phase of
the cell cycle
[25
–27]
. In this study, we showed that TSL2
enhanced the expression of the kinases of both Chk1 and Chk2,
which phosphorylated/inactivated Cdc25C and inhibited the
activation of Cdc2 (
Fig. 3
). These results were consistent with
our findings that the treatment of TSL2 enriched the cell
population of G2 phase in SKOV3 cells (
Table 2
). Our study
clearly demonstrated that growth of transplanted human ovarian
cancer cells in nude mice was inhibited after intraperitoneal
injection of TSL2 (
Fig. 4
). We also found injection of TSL2 into
the nude mine to have no significant toxicity to the bone marrow,
kidneys, or liver (
Table 3
).
In summary, we demonstrated that TSL2, a fractionated
extract from the leaves of T. sinensis, arrested ovarian cancer
cell growth at the G2 phase and also caused significant
cytotoxicity to cancer cells at the G2/M phase. In vivo xenograft
study showed that TSL2 suppressed the growth of ovarian
cancer cells without significant nephrotoxicity, liver toxicity, or
bone marrow suppression. Our results indicate that TSL2 could
someday be developed into a promising anti-ovarian cancer
drug and that it is worthy of further study.
Acknowledgment
This work was supported by a grant from Department of
Health, Taiwan, ROC to SSFY (DOH92-TD-1004 and
DOH93-TD-1015).
References
[1] Edmonds JM, Staniforth M. Toona sinensis (Meliaceae). Curtis's Bot Mag 1998;15:186–93.
[2] Luo XD, Wu SH, Ma YB, Wu DG. Limonoids and phytol derivatives from Cedrela sinensis. Fitoterapia 2000;71:492–6.
[3] Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine 2003;10:544–51.
[4] Hsu HK, Yang YC, Hwang JH, Hong SJ. Effects of Toona sinensis leaf extract on lipolysis in differentiated 3T3-L1 adipocytes. Kaohsiung J Med Sci 2003;19:385–90.
[5] Yang YC, Hsu HK, Hwang JH, Hong SJ. Enhancement of glucose uptake in 3T3-L1 adipocytes by Toona sinensis leaf extract. Kaohsiung J Med Sci 2003;19:327–33.
[6] Poon SL, Leu SF, Hsu HK, Liu MY, Huang BM. Regulatory mecha-nism of Toona sinensis on mouse leydig cell steroidogenesis. Life Sci 2005;76:1473–87.
Table 3
(A) Body weight (BW) profile, (B) complete blood count and biochemical profile for the nude mice after treatment with TSL2 for 7 weeks Control (N = 10) Low dose (N = 10) High dose (N = 10)
Before treatment After treatment Before treatment After treatment Before treatment After treatment (A)
BW (g) mean ± SD 27.8 ± 1.9 26.2 ± 2.3 27.8 ± 1.9 26.2 ± 2.3 27.8 ± 1.9 26.2 ± 2.3
Control (N = 10) Low dose (N = 10) High dose (N = 10) (B) Mean ± SD WBC (×103/μl) 3.1 ± 0.5 3.6 ± 0.3 3.2 ± 0.8 RBC (×106/μl) 9.0 ± 0.4 9.4 ± 0.4 9.5 ± 0.2 HGB (g/dl) 12.2 ± 0.5 12.7 ± 0.5 12.9 ± 1.1 HCT (%) 40.3 ± 2.2 40.6 ± 1.3 41.7 ± 2.1 MCV (fl) 44.2 ± 1.6 43.4 ± 1.9 44.9 ± 1.6 MCH (pg) 13.4 ± 0.2 13.3 ± 0.5 13.6 ± 0.2 MCHC (%) 30.6 ± 0.8 30.7 ± 0.7 60.5 ± 0.7 PLT (×103/μl) 617.47 ± 108.8 687.4 ± 30.4 646.6 ± 25.8 BUN (mg/dl) 28.0 ± 3.7 25.8 ± 2.6 26.9 ± 4.8 Creatinine (mg/dl) 0.43 ± 0.11 0.47 ± 0.04 0.47 ± 0.16 AST (IU/l) 94.0 ± 22.2 96.2 ± 24.7 107 ± 8.7 ALT (IU/l) 30.3 ± 17.6 34.1 ± 7.3 33.8 ± 14.9
The data were presented as mean ± SD. No significant difference was noticed between each group, thought AST and ALT showed an elevated tendency after high dose TSL treatment.
[7] Hsieh TJ, Liu TZ, Chia YC, Chern CL, Lu FJ, Chuang MC, et al. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem Toxicol 2004;42:843–50.
[8] Chang HC, Hung WC, Huang MS, Hsu HK. Extract from the leaves of Toona sinensis Roemor exerts potent anti-proliferative effect on human lung cancer cells. Am J Chin Med 2002;30:307–14.
[9] Yuan SS, Su JH, Hou MF, Yang FW, Zhao S, Lee EY. Arsenic-induced Mre11 phosphorylation is cell cycle-dependent and defective in NBS cells. DNA Repair (Amst) 2002;1:137–42.
[10] Yuan SS, Chang HL, Chen HW, Yeh YT, Kao YH, Lin KH, et al. Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Bax- and caspase-3-related pathway. Life Sci 2003;72:2853–61.
[11] Yuan SS, Lee SY, Chen G, Song M, Tomlinson GE, Lee EY. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res 1999;59:3547–51.
[12] Yawata A, Adachi M, Okuda H, Naishiro Y, Takamura T, Hareyama M, et al. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene 1998;16:2681–6.
[13] Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem 2000;69:217–45.
[14] Huang P, Oliff A. Signaling pathways in apoptosis as potential targets for cancer therapy. Trends Cell Biol 2001;11:343–8.
[15] Nicoletti VG, Stella AM. Role of PARP under stress conditions: cell death or protection? Neurochem Res 2003;28:187–94.
[16] Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol 2002;3:275–83.
[17] Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell 2002;108:153–64.
[18] Soldani C, Lazze MC, Bottone MG, Tognon G, Biggiogera M, Pellicciari CE, et al. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res 2001;269:193–201.
[19] Duriez PJ, Shah GM. Cleavage of poly (ADP-ribose) polymerase: a sen-sitive parameter to study cell death. Biochem Cell Biol 1997;75:337–49. [20] Seo HJ, Surh YJ. Eupatilin, a pharmacologically active flavone derived
from Artemisia plants, induces apoptosis in human promyelocytic leukemia cells. Mutat Res 2001;496:191–8.
[21] Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta 2001;1519:1–12.
[22] Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol 2000;12:666–75.
[23] Gotoh T, Ohsumi K, Matsui T, Takisawa H, Kishimoto T. Inactivation of the checkpoint kinase Cds1 is dependent on cyclin B-Cdc2 kinase activation at the meiotic G(2)/M-phase transition in Xenopus oocytes. J Cell Sci 2001;114:3397–406.
[24] Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem 2004;279:25813–22.
[25] Bulavin DV, Amundson SA, Fornace AJ. p38 and Chk1 kinases: different conductors for the G(2)/M checkpoint symphony. Curr Opin Genet Dev 2002;12:92–7.
[26] Shibuya EK. G2 cell cycle arrest—a direct link between PKA and Cdc25C. Cell Cycle 2003;2:39–41.
[27] Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res 2000;4:107–14.