J. CHEM. SOC. PERKIN TRANS: 2 1995 307
Photocycloaddition of Fumaronitrile t o Adamantan-2-ones and Modification of
Face Selectivity by Inclusion in p-Cyclodextrin
t
and its Derivatives
Wen-Sheng Chung,”na Nae-Jen Wang,a Yei-De Liu,a Yi-Jing Leua and Michael Y. Chiang $ s b
a Department of Applied Chemistry, National Chiao
-
Tung University, Hsinchu, Taiwan 30050, Republic of ChinaDepartment of Chemistry, National Sun Yat-sen University, Kaohsiung, Taiwan 804, Republic of China The face selectivity in 5-substituted-adamantan-2-ones (1 -Xs) can be dramatically reversed by means of inclusion into P-cyclodextrin (p-CD) and its heptakis(6-0- hydroxypropyl), heptakis(6-0- acetyl), heptakis(2,3,6-tri-O-methyl) and heptakis(2,3,6-tri- 0-acetyl) derivatives. The 5-substituents varied from fluoro, chloro, bromo, phenyl t o trimethylsilyl, and face selectivities of the oxetane formation have been found t o vary with the sizes of 5-substituents and cavities of p-CDs. A 98:2 face selectivity was achieved when I-SiMe, was used as a probe. The effect observed is interpreted by assuming that the carbonyl n-face syn t o the bulky 5-substituent is partially blocked by the torus of the host due t o complexation of I - X and CD. Information obtained from ‘H NMR titration and X-ray powder diffraction study o n the inclusion complex is consistent with the above explanation. X-ray single-crystal structure was used t o determine the oxetane structure of anti-2-SiMe,.
The chemistry of reactive intermediates (guest) within organized and constrained media (host) is of current interest.’ Cyclodextrins (CDs) are cyclic oligosaccharides consisting of six or more a-l,4-linked D-glucose units.2 These bucket-shaped macrocyclic molecules have a cylindrical cavity with a diameter larger than 5 8, and a depth of 7-8
A.
The inside wall of the macrocycle is comprised of many ether, methylene and methine groups, whereas at both ends of the cavity hydroxy groups are found in a circular arrangement. Cyclodextrins, which possess hydrophobic cavities that are able to include a variety of organic compounds in aqueous solution are one of themost commonly used host Ramamurthy and Turro
and co-workers have exploited the use of CDs as hosts to examine photochemical and photophysical processes that occur in molecules complexed within them and to compare their behaviour in aqueous solution and in the solid
Because of the rigid structure of CD molecules, the size and shape of the interior cavity are strictly constrained by the number of constituent glucose units and complex formation is highly stereoselective, that is, guest molecules or groups should fit into the host cavity, even if only partially. Chemical modification of CDs has been extensively studied for the
purpose of improving chemical and physical properties, such as solubility, stereoselective complexation, chiral recognition and catalytic powers, e t ~ . ~ , ~
We have previously reported that the photocycloaddition of 5-substituted adamantan-2-ones (1-X, Scheme 1) with fumaronitrile in acetonitrile and in water shows anti-2 : syn-2 product ratios varying in the range of 53:47 to 60:40 (Table l).” The anti-2-X formed through syn-face attack of fumaronitrile are the major products in all cases (Scheme 1). Several studies by le Noble and co-workers9.’* of a variety of reactions indicate that the reagent prefers to attack the face which is antiperiplanar to the more electron-rich vicinal bonds (syn and anti face preference in 1 when X equals an electron- withdrawing and electron-donating group, respectively, Scheme 2). These results have been reconciled within Cieplak’s transition-state hyperconjugation mode1.8”~1 When these
P-Cyclodextrin = cyclomaltoheptaose.
$ To whom any question related to the X-ray single-crystal structure should be sent.
N N
1 - x anti -2-X syn -2-X
X = F, CI, Br, etc.
Scheme 1
reactions were irradiated in aqueous p-CD, reversal of the face selectivity was o b ~ e r v e d . ~ The change in face selectivity by complex formation through p-CD was interpreted by assuming that the n-face syn to the bulky 5-substituent of an 1-X-p-CD complex is partially blocked by the torus of the host (Scheme 2).7
---t-
\
anti&: P-CDs ).
in solution in p-CD solution
Scheme 2
The effects of CDs on the face selectively are interesting; however, they are modest and are far from the maximum value that can be achieved. We are interested in finding out what will affect the inclusion complex formation of p-CD and thus change the face selectivity in bimolecular reactions. Stimulated by the pioneering work of Breslow and co-workers 3 b 4 c to improve
acylation rates within flexibly capped cyclodextrins, we used here several modified p-CDs (3a-f) with (i) flexible capping in the primary alcohol sites by hydroxypropyl ether, (ill ester and ether modifications on both primary and secondary alcohols and selected new 5-substituted adamantan-2-one (1-SiMe,) in search of better n-facial selectivities and higher solubilities of the
308 J. CHEM. SOC. PERKIN TRANS. 2 1995
Table 1
containing cyclodextrins as a function of p-CD used and in acetonitrile solution at room temperature
Stereochemical course of the Paterno-Buchi reaction of 5-substituted adamantan-2-ones (1-X) with fumaronitrile in aqueous solution
anti-oxetane," anti-2 : syn-2
Entry X CH,CN H,O 3a 3bb 3 C b 3db 3eb 3f
~~ 1 F 57:43" 53:47' 45:55' 43:57 45:55 41:59 50:50 57:43 2
c1
58:42' 57:43' 26:74' 28:72 31:69 24:76 38:62 60:40 3 Br 59:41' 56:44' 20:80' 22:78 24:76 22:78 29:71 61:39 4 Ph 65:35' 62:38' 23:77' 31:69 31:69 35:65 12:88 72:28 5 SiMe, 59:41 59:41 2:98 24:76 30:70 27:73 49:51 60:40a Analysis by VPC, error limit f 2%. Yields of 2-X are 70-90% at 50% conversion. For studies on CDs: 1 mmol dm-, I-X, 5 mmol dm-, CDs and 100 mmol dm-, fumaronitrile in water were used. 1-X: P-CDs = 1 : 10, where the plateau value was reported. ' Data from ref. 7 are confirmed within f 2% in this work except for the case of 1-Ph and 3a, where k 5% error was found.
100, 1 hydrophobic cavity 2" side -
-
-
1" side m 3 a m = 0 ; X = Y = Z = H b m = 3.5; X = Y = H, Z = -[CH,],OH c m = 7; X = Y = H, Z = -[CH,],OH d m = 7 ; X = Y = H , Z = A c e m = 7 ; X = Y = Z = C H , f m = 7 ; X = Y = Z = A cinclusion complexes. The results of such a study are presented below.
Results and Discussion
Results of the Paterno-Buchi reaction of 1-X (where X = F, C1,
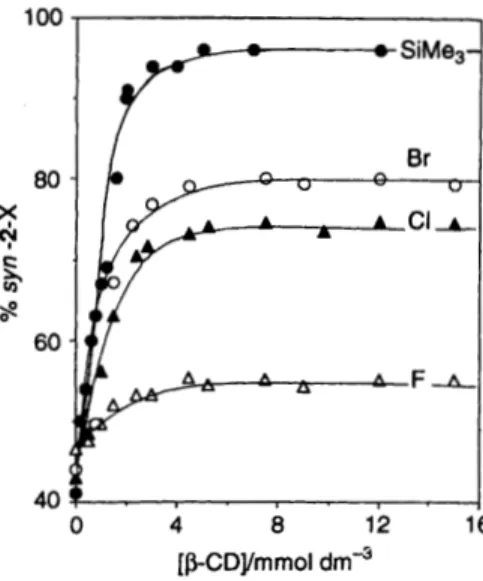
Br, Ph and SiMe,) with fumaronitrile in the presence of p-CD and its derivatives (3a-f) are presented in Table 1. For all studies, excellent yields (70-95%) for oxetanes are obtained, but the reaction was slower in the presence of p-CDs. The effect of C D complexation on the anti : syn ratio is highly dependent on the p-CDs used. For each 1-X, the product anti:syn ratio was dependent on the amount of C D employed; the more extreme values in Table 1 are from the plateau region of the titration curves, such as that shown in Fig. 1. With 1-F and p-CD (3a),
there is only a small effect on the face reversal. With 1-C1 and 1- Br and p-CD (3a), syn : anti face selectivities are reversed from those in aqueous solution, ca. 60:40 to 26:74 and 20:80, respectively. The trimethylsilyl substituent (1-SiMe,), which is the largest substituent of the series, shows the most dramatic change in syn : anti face selectivity, from cu. 60 : 40 to 2 : 98 when
p-CD is used.
For each 1-X, the reversal in face selectivity was smaller when flexibly l 2 capped p-CDs such as hydroxypropyl-modified 3b
and c were used than when 3a was used (Table 1). The decrease
in the reversal of face selectivity in modified P-CDs also showed a strong dependence on the size of the 5-substituent of 1-X. For
a small substituent such as F, there is no difference in the selectivity between different p-CDs (entry 1, Table 1). For medium-sized substituents such as C1 and Br, the reverse-face selectivity decreased slightly, from 74 and 80% in 0-CD (3a) to
72 and 78% in 3b and to 69 and 76% in 3c (entry 2 and 3, Table
1). For the largest substituent (SiMe,) of the series, however, a
remarkable effect was observed: the oxetane syn-2 (derived from anti-face attack) decreased from 98% in p-CD (3a) to 76% in 3b
1
< = S i M e 31
It'
Br n vF-l
401
0 4a
12 16 [PCDymrnol dmsFig. 1 % syn-2 oxetane from anti approach of fumaronitrile to 1-F, 1-Cl, 1-Br and 1-SiMe, as a function of p-CD concentration (data for 1-Ph are very similar to those for 1-Br and are omitted for clarity) and to 70% in 3c (entry 5 , Table 1). Similar results were found when heptakis(6-0-acetylated) p-CD, 3d, was used. These
results indicate that the face selectivities decreased when the primary alcohols of P-CDs were modified to a higher degree of 0-hydroxypropyl or 0-acetyl substitution. Conceivably, this modification, which leads to much greater solubility in water, may have either of two consequences at the molecular level. First, it may simply extend the depth of the cavity of p-CD. Secondly, if the hydroxypropyl groups turn inwards, towards the central axis of the p-CD cavity, they may form an 'intrusive floor', closing off the bottom of the cavity, as was envisaged earlier for similar functionalities.'2
In order to test the possible involvement of hydrogen bonding in complex formation between the carbonyl group of 1-X and the secondary alcohol groups of p-CDs, we also used modified P-CDs through methyl ether or acetyl ester substitutions on both the primary and the secondary hydroxy groups (i.e. C,- OH and C,-OH of the glucose units in p-CD). Modifying
p-
CDs in this way should lead to two possible results: if the cavity of the P-CDs is modified to such an extent that the volume becomes too small to accommodate the large guest molecule, one may see no reversal effect on the face selectivity compared with that in water; on the other hand, if the cavity of modifiedp-
CDs fits the guest molecule better, the face reversal selectivity should be enhanced compared with that in unaltered P-CDs. To test this idea, we employed heptakis(2,3,6-tri-O-methyl)-P-CD (3) and heptakis(2,3,6-tri-O-acetyl)-P-CD (39. The results arealso shown in Table 1.
As can be seen from the results using these two p-CDs (3e and
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:56:33.
J. CHEM. SOC. PERKIN TRANS. 2 1995 T c
-
309 ( a ) P-CD ( d ) 1-Br:pCD = 1:l physical mixture c ( c ) 1-Br:pCD = 1:l inclusion complex v -100 1 1 80 Er
4J
v3s
60 40 0 4 8 12 [Sa-fymmol dm-3-
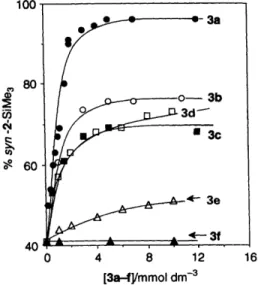
16Fig. 2 % syn-2 oxetane from anti approach of fumaronitrile to l-SiMe, in modified p-CDs (3a-3f) as a function of concentration at room temperature 10
3
-10 -20 4-H 2-H 5-H 0 1 2 3 4 p-CD:l -Ph Fig. 3 function of p-CD : l-Ph ratio'H NMR chemical shift differences of p-CD protons as a
f), our predictions for both situations (vide supra) are confirmed. The first situation occurred for all guest molecules (l-Xs) when
heptakis(2,3,6-tri-O-acetyl)-P-CD (30 was used (cf. columns of
data under H,O and 3f in Table 1). The results indicate that when the cavity of 3f is too small for any of the substituents, inclusion into it will be very unlikely and therefore the face selectivity is similar to that in an homogeneous solution.§ The effect of such a modified p-CD 3f on the reactions of l-X resembles that of an a-CD in our previous studies.' The second situation did not occur for any of the modified P-CDs (3b39 and l-Xs except for the combination of l-Ph and 3e (entry 4, Table 1).
The results are remarkable for the guest molecule with the largest 5-substituent7 l-SiMe, (entry 5, Table 1). The syn :anti
face selectivities were dramatically reversed from those in aqueous solution, ca. 60:40 to 2:98 if p-CD (3a) is added. Concentration dependent titration on l-SiMe, also shows the usefulness of it in probing the cavity size and depth of P-CDs. The effect of CD complexation on the syn : anti selectivity is highly dependent on the p-CD used. Results for photocyclo- addition of l-SiMe, with fumaronitrile in various P-CDS' aqueous solutions are shown in Fig. 2. The product ratio varies 6 These two cyclodextrins were ordered from Cyclolab (Budapest), Hungary. CPK models also confirm that l-Xs do not fit snugly into the cavity of heptakis-(2,3,6-O-acetyl)-P-CD (30.
Table 2 complexes a
300 MHz 'H NMR chemical shifts of p-CD protons in
Compound l-H 2-H 3-H 4-H 5-H 6-H
p-CD 1523.6 1097.3 1191.6 1077.5 1157.1 1166.4 p-CD and l-Ph 1523.6 1097.6 1182.4 1080.2 1144.2 1155.9 p-CD and l-Br 1526.8 1101.7 1169.6 1084.6 -b 1169.6 * Chemical shifts are expressed in Hz with reference to external standard Me,Si in CDCI,, sample was in D,O. 5-H was buried under 6-H.
with the concentration of 3a-f in the way expected, given that the solution will approach saturation if the concentration of
p-
C D is made sufficiently high (e.g. in the manner shown in Figs. 1 and 2).Evidence for the Format ion of
p-
Cy clodex tr in-adaman tan-
2- one Complexes (P-CD-l-Xs).-The complexes of p-CD with 1- Ph and l-Br were chosen as model systems for the structural analyses in aqueous solution. The 300 MHz 'H NMR spectra of aqueous solutions of p-CD and solutions containing various ratios of the host to the guest were recorded. The chemical shifts of p-CD protons in the uncomplexed and in the complexed forms were utilized for drawing conclusions regarding the nature of the complex. Fig. 3 shows a plot of the chemical shift differences for p-CD protons as a function of the ratio of p-CD to l-Ph. In order to obtain information regarding the relative structures of the complexes of 5-substituted-adamantan-2-ones (1-X), we recorded their 'H NMR spectra. The results are tabulated in Table 2. From Table 2 it is evident that the cyclodextrin protons 1-, 2- and 4-H are virtually unaffected while the inner protons 3-, 5- and 6-H are shifted upfield to various extents. These upfield shifts of protons provide evidence for the inclusion of the guest molecule into the hydrophobic cavity of p-CD in aqueous solution. In the l-Ph case, 5-H is shifted upfield to a greater extent (- 12.9 Hz) compared with 3-H (-9.2 Hz) and 6-H (- 10.5 Hz). The above chemical shift behaviour for the cyclodextrin protons definitely establishes that the phenyl ring of l-Ph is positioned within the cyclodextrin cavity. Similar results in 'H NMR spectroscopy in the studies of p-CD complexes with dibenzyl ketone were o b ~ e r v e d . ~ ~ , ~ A recent report from MM2 calculations and NOE ('H NMR) experiments by Jaime et al.', also supports the 1 : 1 inclusion complex of 1 -bromoadamantane and p-CD. Their results also predict that the bromine atom points into the p-CD cavity.
Complexes formed between 1-Xs and 3a-f most probably have 1 : 1 stoichiometries, as found in several instances by X-ray ~rystallography.'~"~~ Since the complexes of l-Xs with
p-
cyclodextrin did not yield suitable single crystals for X-ray crystallographic studies, no such investigation was pursued. Formation of solid inclusion complexes between cyclodextrinPublished on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:56:33.
310 J. CHEM. SOC. PERKIN TRANS. 2 1995 and 1-Br was evident from X-ray powder photographs. The X-
ray powder pattern of the precipitated solid differed from those of the guest and the host or their 1 : 1 physical mixture (Fig. 4). Stability and Possible Structure of the Complexes.-I f the relative ratio of the product is employed as a binding parameter, binding constants for these complexes of p-CD can be obtained.7 Binding constants of 1-Xs in p-CD have been .determined previously’ to be (8.7 k 3.5) x lo2 (for 1-F),
(8.7 k 2.6) x lo2 (for 1-Cl), (8.4 f 3.5) x lo2 (for 1-Br) and
(1.33 k 0.39) x lo3 dm3 mol-’ (for 1-Ph). The binding constant for the newly added guest, 1-SiMe,, was determined to be (1.27 4 0.18) x lo3 dm3 mol-’. Note that the magnitudes of the reversal selectivities described here do not parallel the stabilities of the CD-1-X complexes, but can be explained on the basis of the stereochemistry of the CD-1-X complexes (Scheme 3 ) . 3 b * “ 3 7 Substituents of our probe molecules (1-Xs) may be
with bulky X
Complex A Complex B
Scheme 3
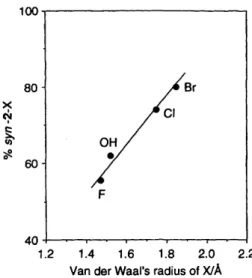
divided into three categories: spherical ball (e.g. halogen atoms), planar structure (e.g. phenyl group) and three dimensional tetrahedron (%Me3 or tert-butyl), and they probably will have different stereo requirements in the cavity of p-CDs. In p-CD solutions when the percentage syn-2-X was plotted us. the van der Waals radii of the 5-substituents of 1-X for the same group of elements (e.g. F, C1 and Br),” a clear linear correlation was observed: the larger the substituent the higher the anti face selectivity (Fig. 5).
Our interpretation for results of photocycloaddition reac- tions of 1-Xs in P-CDs (3a-f) is that p-CD or its derivative
complexes the 1-Xs (with the bulkier X) so that the normally preferred carbonyl face is protected by the torus of the host (complex B, Scheme 3). With relatively ‘large’ substituents of 1- X (e.g. 1-SiMe,), complex B should, in fact, be favoured in the
equilibrium between complex A and B;3c complex A should increase in importance when the 5-substituents are ‘small’ (e.g. 1-F). Besides varying sizes of guest molecules (1-Xs), we also varied the cavity depths (narrow end) and its wide end (C-2 and -3 hydroxy group) through chemical modification of the host molecule.
Modifying a P-CD’s primary alcohol groups by hydroxy- propyl or methyl ether should form an intrusive floor in the narrow-end of the p-CD cavity. The crystal structure of
heptakis(2,3,6-tri-O-methyl)-P-CD (3e) with various guests has
been reported l 6 in which five of the seven O(6)-methyl groups
are inclined toward the inside of the cavity and make the O(6) end of the cavity narrower and capped (e.g. 3b3e). If complex B in Scheme 3 is really the inclusion complex that determines the face attack, then a flexibly capped p-CD should not affect the inclusion of ‘small’ substituents (such as 1-F and 1-C1) but should obstruct the full inclusion of ‘large’ substituents (such as 1-Br and 1-SiMe,). The results in Table 1 are fully consistent
’T[ In general, one might use the differences in optical density or the differences in fluorescence intensity to determine the binding constants. The UV-absorption experiment was performed, but the small difference in optical density at the highest CD concentration studied (ca. 15 mmol dnr3 in H , O ) made estimating the binding constant very difficult. The fluorescence experiment was hampered by the impurities in the commercially available j3-CD.
l*Ll
80 FI
404 . I r I . I . I . I 1.2 1.4 1.6 1.8 2.0 2.2Van der Waal’s radius of WA
Fig. 5 % anti-face attack in the reaction of 1-Xs with fumaronitrile in p-CD solution as a function of the size of the 5-substituents of 1-Xs (only same group of elements fit this line)
with this explanation. What needs to be explained is why 1-Ph in p-CD (3e) did not show the expected loss of anti-attack preference and why in fact it was highly enhanced (entry 4, Table 1). From the ‘H NMR study (see Supplementary Material
[I),
we speculate that the planar phenyl substituent in 1-Ph may still be able to penetrate deep into the cavity and is held tightly by the flexible methoxy groups, thus showing a better anti-face selectivity (88: 12).In their study of hydrolysis rates within p-CD complexes, Breslow et al. invoked similar orientations in order to explain the observed acceleration rates of (3-tert-butyl- 1 -adamantyl) pr~piolate.~‘ Improvement of the acylation rates within CD complexes was achieved by ‘capping’ the CD and by adjusting the shape of the substrate. The current results for the modified P-CDs further support the inclusion complex model proposed in Schemes 2 and 3 and owing to this complexation, the origin- ally favoured syn-face of the carbonyl group is blocked by the torus of CDs and leads to a completely reversed product select- ivity. Note that two other recent reports strongly support the idea of ‘using inclusion complexes of CDs as a steric shield’ in controlling bimolecular reactions. In the reduction of nor- bornen-7-ones7 Mehta et a1.6a found that the face selectivity can be altered or reversed by inclusion into p-CD similar to those studied here. Trost and Van Vraken 6c also used this idea in the osmium-catalysed oxidation of a benzyl oxazoline ether, in which the benzyl group was placed in the cavity of the cyclo- dextrins, thereby introducing the latter as a shield for the con- vex face. The selectivity of their reactions was modest and could probably be enhanced by using the methodology applied here.
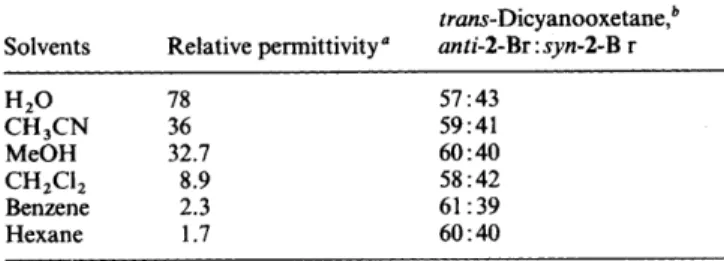
One may question whether the modification of face selectivity discussed above is really due to the formation of inclusion complexes (such as that shown in Scheme 3) or whether it may just be a solvent-polarity effect. Photocycloaddition reactions of 1-Br with fumaronitrile in solvents of different relative permittivity were carried out and the results are presented in Table 3. Solvent polarities were varied from very polar, e.g.
water ( E = 78), acetonitrile ( E = 36), methanol ( E = 33) and dichlorornethane (E = 8.9) to non-polar solvents, e.g. benzene
(E = 2.3) and hexane ( E = 1.7). Within the range of experi-
11 SuppZementary material Titration curves (similar to that of Fig. 2) of 1-F, 1-Cl, 1-Br and 1-Ph by j3-CDs (3a-e) have been deposited. For details of the deposition scheme see ‘Instructions for Authors (1995),’
J. Chem. Soc., Perkin Trans. 2, 1995, issue 1 [supp. pub. no. 57059 (6 PP-)l*
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:56:33.
J. CHEM.
soc.
PERKIN TRANS. 2 1995 311 Table 3 Face selectivities in the Paterno-Buchi reaction of 1-Br withfumaronitrile in solvents of different polarities at room temperature trans-Dicyanooxetane,b Solvents Relative permittivity" anti-2-Br :syn-2-B r
H2O 78 CH,CN 36 MeOH 32.7 CH2C12 8.9 Benzene 2.3 Hexane 1.7 57 : 43 59:41 60 : 40 58 : 42 61 :39 60 : 40
" Data of solvent relative permittivities were from C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, 2nd ed. VCH, New York, 1990. Analysis by GC, error limits k 2%. Yields of 2-Br are
2 90% at low ( I 20%) conversion. The ratios stay constant during the long irradiation periods.
Fig. 6 ORTEP drawing of structure anti-2-SiMe3
mental errors no change in face selectivity owing to solvent- polarity variations was observed.
Structure Determination of the 0xetanes.-All products mentioned in this paper have been completely characterized by
'H and 13C NMR analysis and most of them have been
reported previously.' The assignment of configuration of the anti and syn isomers on the basis of 13C NMR spectroscopy has
been described elsewhere,' but was found to be useless in the assignment of anti- and syn-ZSiMe, because the calculated or observed chemical shift differences are very small for both products. The configurations of these two oxetanes were determined by the trends shown in p-CD studies and finally by single-crystal X-ray crystallography (Fig. 6).
molecules and host molecules, (iii) methylation or hydroxy- propylation in the primary alcohols of p-CD forms a flexible floor at the narrow-end, which hampers the inclusion of a large substituent, but not the small substituent, (iu) the change in face selectivity observed is not due to solvent-polarity effects. The present results, by modification of p-CD and by tuning the sizes of the substrates and hosts, have demonstrated that the attack of fumaronitrile can be directed towards the exposed anti face of the carbonyl group through complexation with p-CDs (Scheme 3), and the inclusion of 5-substituents of 1-Xs were from the wider secondary side of the C D cavities. Moreover, the depth of penetration of the guests into the cavity must be such that they barely encounter the hydroxypropyl (or acetyl) groups on the distal, primary side of p-CDs (3b, c and d). Thus, anti-face selectivity decreased for large substituents owing to shallower binding and less protection of the syn-n face of the carbonyl group (Scheme 4). Since these modified f3-CDs have much better
1
with bulky XComplex 6 in 3a Complex 6 in 3 b 4 Scheme 4
solubility** in water and other organic solvents than p-CD itself does,I7 the application of CDs for traffic control clearly has synthetic potential. We are continuing to explore the interaction of CDs with bound reactive intermediates and are
using CDs to control the regio- and stereo-chemistry of other bimolecular reactions.
Experiment a1
NMR spectra were recorded on either a Varian Unity-300 or 400 spectrometer with Me,Si in CDCl, as an internal or external standard. Powder diffractograms were recorded on a MacScience MXP3 X-ray powder diffractometer equipped with CU-Ka radiation. Gas chromatography was carried out on a Hewlett-Packard 5890 instrument equipped with an FID detector using either a 25 m HP-1 cross-linked methyl silicone column or a Carbowax column at 200 "C.
Materials.-Fumaronitrile (Merck), adamantan-2-one (Aldrich) and P-CDs (Aldrich or Merck) were used as received. Doubly distilled water was used for all the experiments and organic solvents were distilled twice prior to use. Tetrakis(6-O- hydroxypropy1)- p-CD (3b) and heptakis-(6-O-hydroxypropyl)- p-CD (3c) are not pure compounds, being available at different degrees of substitution. We used the materials provided by Amaizo which have averaged molecular weight of 1388 for 3b and 1541 for 3, respectively, corresponding to the replacement of about 3.5 and 7 primary hydroxy groups of p-CD by hydroxypropyl groups. Heptakis-(2,3,6-O-methyl)-p-CD (3e)
and heptakis-(2,3,6-O-acetyl)-P-CD (30 were purchased from Conclusions
The results presented here show that (i) there is a close relationship between the van der Waals radii of 1-Xs and the syn-oxetane selectivity in the inclusion complexes, (iz] a much higher face selectivity is achieved from size changes of guest
**
For example, at room temperature the modified p-CDs 3b and c have at least I mol dm-3 solubilities in water compared with ca. 18 mmol dm-3 for p-CD itself. The 2,3,6-O-methyl derivative of p-CD (3e) is not only soluble in organic solvents, but is also ca. 10 times more soluble in water than is the parent p-CD.312 J. CHEM.
soc.
PERKIN TRANS. 2 1995Cyclolab (Hungary) and were used as received. The synthesis of 5-trimethylsilyladamantan-2-one (l-SiMe,),' 5-phenyl- adamantan-2-one (l-Ph)," 5 - ~ h l o r o - , ' ~ 5-brom0-~' and 5-
fluoroadamantan-2-ones 9 * 2 have all been described elsewhere. All compounds mentioned in this paper have been completely
characterized by NMR analysis and most of them have been reported previously.
anti-5- Trimethylsilylspiro~adamantane-2,2'-oxetane)-trans- 3',4'-dicarbonitrile (anti-Z-SiMe,).-White solid (mp 154 "C); 2.39 (1 H, br s), 2.32 (1 H br s), 1.90-2.15 (2 H, m), 1.58-1.78 (9 H, m) and -0.086 (9 H, s); 6,(75.4 MHz, CDCl,) -5.51s (SiMe,), 19.018, 25.312, 31.256, 31.372, 32.975, 33.499, 35.218,
35.568,37.986,38.656,61.936,91.685, 115.082and 116.742.mlz
(EI, 70 eV) 300 (M', 673, 245 (6), 222 ( 5 ) and 73 (100) ( M , 300.1659. M + , 300.1645).
dH(300 MHz, CDC1-J 5.12 (1 H, d, J 5.86), 3.67 (1 H, d, J 5.86),
X-Ray Structure Analysis of anti-ZSiMe,.--(See Fig. 6 for ORTEP drawing.) Mp 154 "C, was crystallized from 10% ethyl acetate in hexane. Its structure was determined by means of single-crystal X-ray analysis on a Rigaku AFC6S diffractometer with a graphite monochromated Cu-Ka (2 = 1.541 78
A)
radiation at 296 k 1 K, with an 4 2 0 type scan at a speed of
16 deg min-' (in 10). The crystals are primitive monoclinic,
with space group P2Jc (No. 14) and unit cell dimensions a = 14.488(3), b = 9.129(3), c = 13.253(3),
B
= 101.84(2)",V = 1715.6(7)
A3,
2 = 4, pcalcd = 1.16 g cm-j, crystal size 0.16 x 0.33 x 0.42 mm, p(CU-Ka) = 12.05 cm-', F(OO0) =648, 2871 reflections, 2742 unique reflections, 1665 with I > 3.00a(I) and with 190 variable parameters. The model, which included Br, 0, N and C atoms treated anisotropically
and H atoms isotropically, was refined by the least-squares
method with weight co = 1/[a2 (F,)] to final R values of 0.079 and R , = 0.082.f.f.
syn-2-SiMe3.-White solid (mp 170 "C). dH(3O0 MHz, CDCI3)5.11(1H,J5.86Hz),3.67(1H,d,J5.86Hz),2.38(1H, br s), 2.32 (1 H, br s), 1.86-1.87 (1 H, m), 1.82-1.85 (4 H, m),
1.50-1.69 (6H, m) and -0.10 (9 H, s); d,(75.4 MHz, CDCI,) -5.544(SiMe3), 19.222,25.108, 31.256, 31.401, 32.800,33.266, 35.189, 35.568, 38.132, 38.569, 61.995, 91.656, 115.169 and 116.771. m/z (EI, 70 Ev) 300 (M', 673, 245 (7) and 73 (100). ( M , 300.1659. M + 300.1665).
Photocyc1oaddition.-1-Xs (1 or 5 mmol dm-3) were added to
water or to aqueous solutions of varying amounts of CDs and ultrasonically agitated for 50 min to promote the dissolution of ketones. An excess of fumaronitrile (100 mmol dm-3) was then added to 20 cm3) of the solution and dissolved by magnetic stirring for 1 h. Each sample (20 cm3) was allowed to equilibrate for 2 h at 25 "C, after which it was irradiated at ambient temperature in stoppered Pyrex tubes for 12 h by the output from a high pressure Xe-Hg lamp (1 kW) with a K,CrO, filter
solution. The solutions were clear except for those of l-Ph and 1- SiMe,, in which cloudy solutions were irradiated. The solutions were then extracted with ether (3 x 20 cm3); after drying
(Na,SO,), the ether was removed under reduced pressure to give a white solid. Under these conditions, the conversions were l0-80% and the yield of oxetanes was 70-95%. The product ratios were determined by GC. At low conversion only trans- dicyanooxetanes were formed; cis-dicyanooxetanes were obtained only after the cis-trans isomerization of fumaronitrile has set in.8 As in acetonitrile, the aqueous reactions exhibit a
tt
Crystallographic details have been deposited at the Cambridge Crystallographic Data Centre. For details of the Deposition scheme, see 'Instructions for Authors (1995),' J. Chem. Soc., Perkin Trans. 2, 1995, issue 1 .clear preference for the syn approach to form the anti-oxetane (Table 1).
Powder Diffraction of Solid Cyclodextrin Complexes.-To an aqueous solution of p-CD (0.05 mol dm-3; 10 cm3) were added equimolar amounts of the guest ketones l-Xs and the solutions were heated to 40 "C and allowed to cool to room temperature while stirring overnight. The white precipitate thus obtained was filtered, washed with cold water several times and dried in
vacuo at room temp. for 1 h. The X-ray powder diffractograms
of p-CD and complexes of p-CD and ketones l-X (where X = Br and Ph) were recorded. The powder photographs of the complexes were different from that of 0-CD or a 1 : 1 physical mixture of p-CD and ketones. On this basis it was concluded that inclusion complexes between p-CD and the guest ketones l-Xs have been formed.
N M R Studies. Sample Preparation.-Solutions containing different proportions of guest-to-host were prepared by stirring 4-5 mg of the guest molecule 1-X with 0.4,0.8,1 .O, 1.5,2,3 and 4 equiv. p-CD solution (15 mmol dm-, stock solution in D 2 0 ) in
1 cm3 D 2 0 for ca. 1 h before measurement.
The NMR spectra of all the p-CD complexes, p-CD and l-Xs in D 2 0 and CDCl, with a coaxial external standard (SiMe, in CDCl,) were recorded with a Varian Unity-300 spectrometer
equipped with a UNIX computing system. Water elimination
program (WEFT)22 provided by the instrument was used to suppress the water peak. Temperature was set to be 24.0 k 0.5 "C for all measurements. The guest-induced shifts in the p-CD protons were computed by comparing the above spectra with that of pure p-CD.
Acknowledgements
We thank the National Science Council of the ROC for financial support.
References
1 (a) Photochemistry in Organized and Constrained Media, ed. V. Ramamurthy, VCH, Weinheim, 1991; (6) V. Ramamurthy,
Tetrahedron, 1986,42,5753 and refs. cited therein.
2 (a) M. L. Bender and M. Komiyama, Cyclodextrin Chemistry, Springer Verlag, Weinheim, 1978; (b) W. Saenger, Angew. Chem.,
Znt. Ed. Engl., 1980, 19, 344; (c) I. Tabushi, Acc. Chem. Res., 1982, 15, 66; (d) R. Breslow, Science (Washington, D. C.), 1982, 218, 532;
(e) J. Szej tli, Cyclodextrins and their Inclusion Complexes, Akadkmiai Kiado, Budapest, 1982; (f) V. T. D'Souza and M. L. Bender,
Ace. Chem. Res., 1987, 20, 146; (g) J. Szejtli, Cyclodextrin Tech-
nology, Kluwer, Dordrecht, 1988; (h) Inclusion Compounds, eds. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, New York, 1991, vol. 1-5.
3 (a) W. Saenger, in Inclusion Compounds, eds. J. L. Attwood, J. E. Davies and D. D. MacNicol, Academic Press, New York, 1984, vol. 2, 231; (b) J. Emert and R. Breslow, J. Am. Chem. Soc., 1975,
97, 670; (c) R. Breslow, M. F. Czarniecki, J. Emert and
H. Hamaguchi, J. Am. Chem. Soc., 1980,102,762.
4 V. Ramamurthy and D. F. Eaton, Ace. Chem. Res., 1988,21,300. 5 (a) M. S. Syamala and V. Ramamurthy, Tetrahedron, 1988,44,7223;
(b) M. S. Syamala, B. N. Rao and V. Ramamurthy, Tetrahedron, 1988, 44, 7234; (c) B. N. Rao, M. S. Syamala, N. J. Turro and V. Ramamurthy, J. Org. Chem., 1987, 52, 5517; (d) G. D. Reddy, G. Usha, K. V. Ramanathan and V. Ramamurthy, J. Org. Chem.,
1986,51,3085.
6 For some recent examples see ref. 7 and refs. cited therein and (a) G. Mehta, F. A. Khan and K. A. Lakshimi, Tetrahedron Lett., 1992, 33,7977; (b) N. R. Bantu, J. G. Kotach and A. J. Lees, Tetrahedron
Lett., 1993,34,2039; (c) B. M. Trost and D. L. Van Vraken, J. Am.
Chem. Soc., 1993, 115, 445; (d) J. H. Liu and R. G. Weiss, J. Photochem., 1985,30,303.
7 W. S. Chung, N. J. Turro, J. Silver and W. J. le Noble, J, Am. Chem. Soc., 1990, 112, 1202.
8 (a) W. S . Chung, N. J. Turro, S. Srivastava, H. Li and W. J. le Noble,
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:56:33.
J. CHEM. SOC. PERKIN TRANS. 2 1995 313
J. Am. Chem. SOC., 1988, 110, 7882; (b) W. S. Chung, N. J. Turro, S. Srivastava and W. J. le Noble, J. Org. Chem., 1991,56, 5020. 9 C. K. Cheung, L. T. Tseng, M. H. Lin, S. Srivastava and W. J. le
Noble, J. Am. Chem. SOC., 1986,108, 1598; 1987,109,7239. 10 V. R . Bodepudi and W. J. le Noble, J. Org. Chem., 1994,59, 3265;
1991, 56, 2001 and earlier refs. cited therein.
1 1 ( a ) A. S. Cieplak, B. Tait and C. R. Johnson, J. Am. Chem. SOC., 1989, 111, 8447; (b) A. S. Cieplak, J. Am. Chem. SOC. 1981,103,4540. 12 For modification of the cavity of p-CD by flexible capping see refs.
3(b), 3(c) and T. A. Gadosy and 0. S. Tee, J. Chem. SOC., Perkin Trans. 2, 1994, 715 and refs. cited therein.
13 C. Jaime, J. Redondo, F. Sanchez-Ferrando, A. Virgili, A. J. Org. Chem., 1990,55,4772.
14 ( a ) J. A. Hamilton, L. K. Steinrauf and R. L. van Etten, Acta
Crystallogr., Part B, 1968, 24, 1560; (b) M. Komiyama and
M. L,. Bender, J. Am. Chem. Soc., 1978,100,2259; ( c ) M. Komiyama and S. Inoue, Bull, Chem. SOC. Jpn., 1980, 53, 2330; 3266; (d) M. Czugler, E. Eckle and J. J. Stezowski, J. Chem. SOC., Chem.
Commun., 1981, 1291; (e) J. C. Harrison and M. R. Eftink, Bipolymers, 1982,21, 1153.
15 The van der Waals radii data are from A. BonJi. J . PIij..v. ('ircw.. 1964,68,441.
16 K. Harata, in Inclusion Compoi~nds. eds. J. L. AttHood. J . I:. 11. Davies and D. D. MacNicol, Academic Press. New Y ork. I99 1 . vol. 5,311.
17 J. Szejtlil, A. Liptak, 1. Jodal, P. Fiigedi, P. Nanasi and A. Ncsmiclyi.
Starch, 1980, 32, 165.
18 M. Xie and W. J. le Noble, J. Org. Ciwu.. 1980. 54. 3836. 19 H. W. Geluk, Synthesis, 1972, 374.
20 H. Klein and R. Wiartalla, Synth. Comntioi.. 1979. 9, 825. 21 I. Tabushi and Y. Aoyama, J. Org. Client.. 1973. 28. 3447. 22 For WEFT program see One-Dimensionrrl (rnrl Two-Dii)ic.n.\.iollrrl
N M R Spectra by Modern Pulse Tcchniyiws, ed. K. Nakanishi. Kodansha, Tokyo, 1990, pp. 199-1 20.
Puper. 4/0458 5 H
Receiwd 26th Jirlj- 1994 Accepted 2 1 st Septcwiher. I994