Date 2015/May/12
Type of manuscript: Original article
Manuscript title: Zopiclone use associated with increased risk of acute pancreatitis: a case-control study in Taiwan
Running head: zopiclone and acute pancreatitis Authors' full names:
Shih-Wei Lai MD1,2, Hsueh-Chou Lai DM and MS 3,4, Cheng-Li Lin MS 1,5, Kuan-Fu Liao MD and MS 6,7
1College of Medicine, China Medical University and 2Department of Family Medicine, China Medical University Hospital, Taichung, Taiwan
3College of Chinese Medicine, China Medical University and 4Division of Hepato-gastroenterology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
5Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
6Graduate Institute of Integrated Medicine, China Medical University and 7Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung, Taiwan
(The first two authors equally contributed to this study)
Corresponding author: Kuan-Fu Liao, Department of Internal Medicine, Taichung Tzu Chi General Hospital, No.66, Sec. 1, Fongsing Road, Tanzi District, Taichung City, 427, Taiwan
Phone: 886-4-2205-2121; Fax: 886-4-2203-3986 E-mail: kuanfuliaog@gmail.com
ABSTRACT
Background. The purpose of this study was to assess the relationship between zopiclone use and the risk of acute pancreatitis in Taiwan. Methods. This was a population-based case-control study. The data source was from the database of the Taiwan National Health Insurance Program since 2000 to 2011.We identified 5169 subjects aged 20-84 years with a first-time attack of acute pancreatitis as the cases and 20676 sex-matched and age-matched subjects without acute pancreatitis as the controls. Active use of zopiclone was defined as subjects who received at least 1 prescription for zopiclone within 30 days before the date of diagnosing acute pancreatitis. The lack of zopiclone prescription was defined as “never use”. We calculated the odds ratio (OR) and 95% confidence interval (CI) to assess the risk of acute pancreatitis associated with zopiclone use by the multivariable logistic regression model. Results. After adjustment for potential confounding variables, the adjusted OR of acute pancreatitis was 2.36 for subjects with active use of zopiclone (95% CI 1.70, 3.28), as compared with those with never use of zopiclone. In further analysis, as a reference of subjects with never use of zopiclone and without alcohol-related disease and biliary stone, the adjusted OR
increased to 14.44 in those with active use of zopiclone and with alcohol-related disease or biliary stone (95% CI 7.47, 27.89). Conclusions. Subjects actively using zopiclone are associated with increased risk of acute pancreatitis. Clinicians should take acute pancreatitis risk into account when prescribing zopiclone, particularly comorbid with alcohol-related disease or biliary stone.
Keywords: zopiclone; acute pancreatitis; alcohol; biliary stone
Review criteria: how did you gather, select and analyze the information you considered in your review?
Health Insurance Program to assess this issue.
The odds ratio and 95% confidence interval was calculated to assess the risk of acute pancreatitis associated with zopiclone use by the multivariable logistic regression model.
INTRODUCTION
Zopiclone is a non-benzodiazepine hypnotic agent and has displayed great effectiveness and excellent tolerability to treat insomnia since 1980s. Its adverse reactions mainly include bitter taste, dry mouth, and difficulty arising in the morning. Although no case was reported about zopiclone-associated acute pancreatitis, the United States Food and Drug Administration (FDA) has disclosed that among 6407 people reporting to have side effects when taking zopiclone since 2008 to 2012, 25 people (0.39%) had acute pancreatitis.
Acute pancreatitis is an acute inflammatory status of the pancreas associated with high mortality. Its mortality rate would be about 6.4%-7.1% within 60 to 90 days of
admission. There is a growing body of evidence that some factors may be associated with acute pancreatitis, including alcohol, biliary stone and type 2 diabetes mellitus. In addition, drug-induced acute pancreatitis accounts for 2% of cases.
To date, although no pharmacoepidemiological study is available to support the association between zopiclone use and the risk of acute pancreatitis in scientific
journals, any reported event of acute pancreatitis potentially related to zopiclone has its clinical implication. Zopiclone has never been submitted for approval in the United States, but was exclusively licensed to be sold in the United States. . Eszopiclone, a stereoselective isomer of zopiclone, has been approved in the United States in December 2004. Therefore, zopiclone and eszopiclone can be found in the United States market. In addition, four non-benzodiazepine hypnotic agents including zolpidem, zopiclone, eszopiclone and zaleplon have been approved in Taiwan.
Zopiclone, after zolpidem, is the second frequently used non-benzodiazepine hypnotic agent in Taiwan. Given acute pancreatitis having potential mortality and massive use of zopiclone in treatment of insomnia in Taiwan, we proposed a novel hypothesis linking
zopiclone use and the risk of acute pancreatitis. Therefore, we conducted a population-based case-control study to assess this relationship.
METHODS
Design and data source
This was a population-based case-control study. The data source was from the
nationwide representative database of the Taiwan National Health Insurance Program. Shortly speaking, this program began in March 1995 and has covered approximately 99% of the whole Taiwan 23 million citizens. This program contained multiple datasets that includes registry for beneficiaries and information on outpatient care, inpatient care, emergency care, dental service, and prescription drugs. Personal information was available on sociodemographic status such as sex and date of birth. The details of the program have been well written in previous studies. This study was approved by the Institutional Review Board of China Medical University and Hospital in Taiwan (CMU-REC-101-012).
Identification of cases and controls
We identified subjects aged 20-84 years with a first-time attack of acute pancreatitis between 2000 and 2011 as the cases (International Classification of Diseases (ICD) 9th Revision, ICD-9 codes 577.0). For each case with acute pancreatitis, 4 subjects without acute pancreatitis were randomly selected as the controls. Both cases and controls were matched by sex, age (every 5 years) and index year of diagnosing acute pancreatitis. For case subject, the index date was defined as the date of diagnosing acute pancreatitis. For control subject, the index date was a randomly assigned date within the index year of case subject. Subjects with chronic pancreatitis or pancreatic cancer before the date of diagnosing acute pancreatitis were excluded from the study (Figure 1).
Zopiclone is a short-acting hypnotic agent with an elimination half-life of
approximately 5 to 6 hours and no accumulation exists on repeated use. In order to reduce biased results, subjects receiving no prescription for zopiclone within 1 year but receiving at least 1 prescription for zopiclone > 1 year before the date of diagnosing acute pancreatitis were excluded from the study. Therefore, only subjects whose final prescription for zopiclone was filled within 1 year before the date of diagnosing acute pancreatitis were included. In the U.S. FDA report, the duration from taking zopiclone to develop acute pancreatitis mainly occurred within 1 month. Therefore, we used the period of 30 days as a cut-point. Active use of zopiclone was defined as subjects who received at least 1 prescription for zopiclone within 30 days before the date of
diagnosing acute pancreatitis or the corresponding date for control subjects. Non-active use of zopiclone was defined as subjects who did not receive 1 prescription within 30 days but received at least 1 prescription for zopiclone ≥ 31 days before the date of diagnosing acute pancreatitis or the corresponding date for control subjects. The lack of zopiclone prescription was defined as “never use”.
Potential confounding variables
Variables potentially associated with the risk of acute pancreatitis before index date were identified as follows: alcohol-related disease, biliary stone, cardiovascular disease including coronary artery disease, heart failure, cerebrovascular disease and peripheral atherosclerosis, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, as well as hypertriglyceridemia. All variables were diagnosed with ICD-9 codes and the diagnosis accuracy of these comorbidities was validated in previous studies. History of prescriptions for benzodiazepines available in Taiwan was also collected.
The distribution of sex, age, medication use and comorbidities was expressed as a frequency (with percent). The categorical variables of the characteristics of the cases and controls were analyzed using the Chi-square test and the continuous variables were analyzed using the Student t-test. The univariable and multivariable unconditional logistic regression models were used to assess the association of acute pancreatitis with zopiclone use, shown as an odds ratio (OR) with 95% confidence interval (CI).
Variables found significantly in the univariable unconditional logistic regression model were further included in the multivariable unconditional logistic regression model. We further analyzed the interaction between active use of zopiclone and comorbidities including alcohol-related disease and biliary stone. All data processing and statistical analyses were performed with the SAS software version 9.2 (SAS Institute, Inc., Cary, North Carolina, USA). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the study population
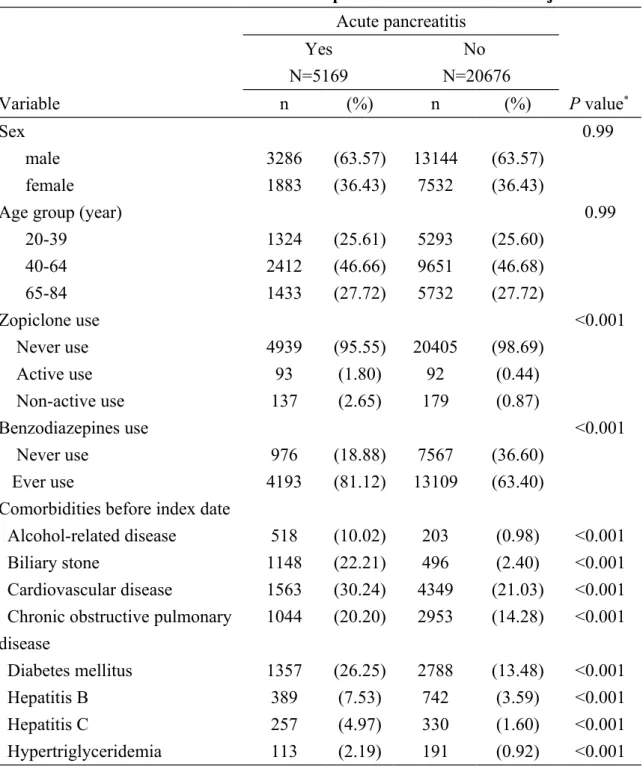
The study included 5169 cases of acute pancreatitis and 20676 controls with a similar age and sex distribution. Characteristics of the study subjects are demonstrated in Table 1. The mean ages (standard deviation) were 53.03 (16.47) years in cases and 52.97 (16.49) years in controls, without statistical significance (Student t-test, P value = 0.81). Among the cases, 230 subjects (4.45%) had ever used zopiclone and among the
controls, 271 subjects (1.31%) had ever used zopiclone, with statistical significance (Chi-square test, P value for < 0.001). The cases had higher proportions of ever use of benzodiazepines, alcohol-related disease, biliary stone, cardiovascular disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C and
hypertriglyceridemia than the controls did (Chi-square test, P value for < 0.001 for all variables).
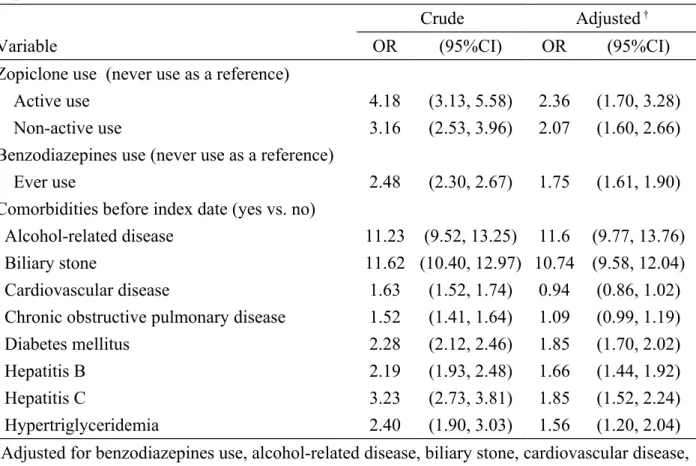
Odds ratio of acute pancreatitis associated with zopiclone use and comorbidities Table 2 demonstrates the OR of acute pancreatitis associated with zopiclone use and comorbidities. After adjusting for potential confounding variables, the multivariable unconditional logistic regression model demonstrated that the adjusted OR of acute pancreatitis was 2.36 for subjects with active use of zopiclone (95% CI 1.70, 3.28), with a reference to those with never use of zopiclone. The OR decreased to 2.07 for subjects with non-active use of zopiclone (95% CI 1.60, 2.66).
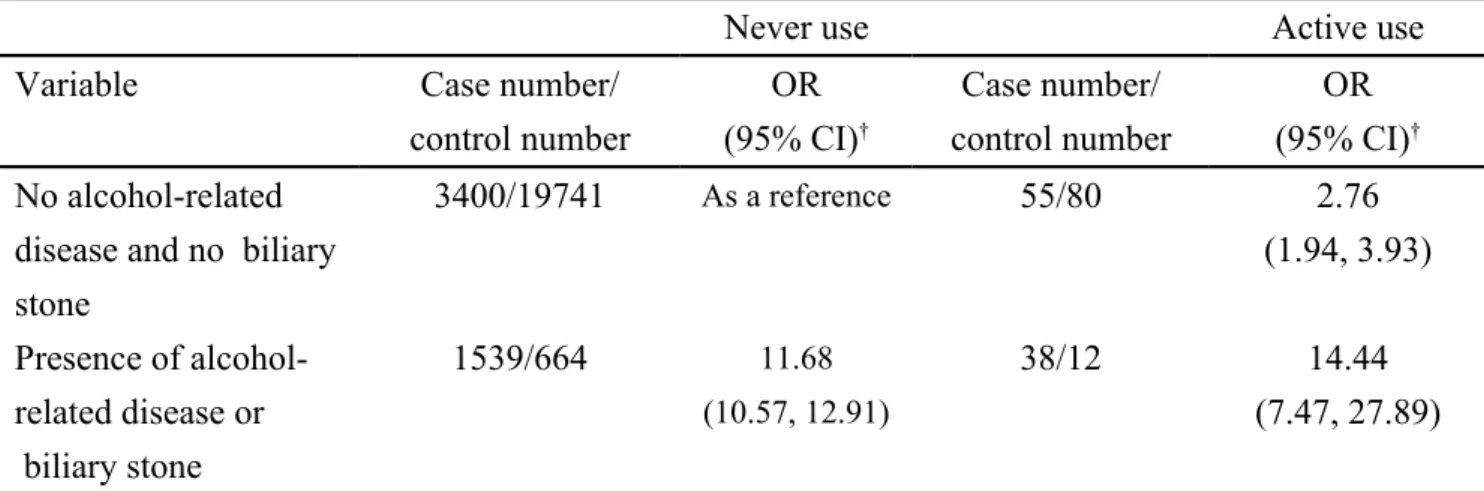
Odds ratio of acute pancreatitis estimated by interaction effect between active use of zopiclone and alcohol-related disease or biliary stone
Table 3 demonstrates the OR of acute pancreatitis estimated by interaction effect between active use of zopiclone and alcohol-related disease or biliary stone. In further analysis, as a reference of subjects with never use of zopiclone and without alcohol-related disease and biliary stone, the adjusted OR of acute pancreatitis was 2.76 in those with active use of zopiclone and without alcohol-related disease and biliary stone (95% CI 1.94, 3.93). The adjusted OR increased to 14.44 in those with active use of
zopiclone and with alcohol-related disease or biliary stone (95% CI 7.47, 27.89). This indicates that there is a strong interaction effect between active use of zopiclone and alcohol-related disease or biliary stone on risk of acute pancreatitis (P value for interaction = 0.002).
In this population-based case-control study, we found that active use of zopiclone was significantly associated with increased odds of acute pancreatitis after adjusting for potential confounding variables (adjusted OR 2.36). In further analysis, we found that people with active use of zopiclone alone had an adjusted OR of 2.76 in association with acute pancreatitis, even in absence of alcohol-related disease and biliary stone (Table 3). This indicates that zopiclone may have a unique effect on the pancreas, independent of comorbidities studied. To date, since there have been no formal case reports about zopiclone-associated acute pancreatitis in the literature, that is why we only used the United States FDA report to highlight this issue. However, the real pathogenesis of zopiclone-associated acute pancreatitis cannot be completely
determined by the U.S. FDA report and this observational study. After reviewing the relevant literature, we think the potential mechanism could be partially explained by the use of zopiclone, which could be associated with biliary stone and might further
contribute to acute pancreatitis. Although no formal case report about zopiclone-related biliary stone is available in the literature, the U.S. FDA had reported that some patients using zopiclone with side effects had bile duct stone. Furthermore, the odds ratio of acute pancreatitis was markedly high among people actively using zopiclone and with presence of alcohol-related disease or biliary stone (adjusted OR 14.44, Table 3). This indicates that clinicians should keep in mind about the risk of acute pancreatitis among people comorbid with alcohol-related disease or biliary stone when prescribing
zopiclone. In addition, we think the positive association between non-active use of zopiclone and acute pancreatitis may be explained by the confounding effect. Zopiclone is a short-acting hypnotic agent with an elimination half-life of approximately 5 to 6 hours and no accumulation exists on repeated use. Theoretically, there should be no residual effect coming from zopiclone if people stopped using zopiclone for one month
or longer. Therefore, any episode of acute pancreatitis occurring in people not actively using zopiclone should not be attributable to zopiclone effect, but instead can be attributable to other etiologies, such as alcohol-related disease, biliary stone or hypertriglyceridemia. However, patients with non-active use of zopiclone could be those with poor compliance and they might just take zopiclone as needed, but we could not prove it. These patients actually should belong to the active use group. If it
happened, it can partially explain why the positive association between non-active use of zopiclone and acute pancreatitis.
Some important limitations should be discussed in this present study. First, the
underlying causes of acute pancreatitis could not be found in this study due to a natural limitation of the database. That is why only 32.23% of patients with acute pancreatitis had prior history of alcohol-related disease and/or biliary stone (Table 1). The
prevalence of alcohol-related disease and biliary stone among patients with acute pancreatitis seems to be underestimated. Similarly, we did not know whether any case of acute pancreatitis could be potentially attributable to zopiclone use or not. Second, due to the same limitation, we did not known how many patients in groups of mild acute pancreatitis and in severe acute pancreatitis had pulmonary insufficiency, renal insufficiency, cardio-circulatory dysfunctions or clinical sepsis. Therefore, we could not assess the relationship between zopiclone use and severity of acute pancreatitis. It indicates a further research direction. Third, due to the same limitation, we could not capture the use of other remedy of sleeping aids, such as herbal medication. Fourth, there was no information on whether people really took zopiclone. Instead, we used the zopiclone prescription as an exposure definition. Whether or not people actively using zopiclone have other undetected factors to be related to acute pancreatitis cannot be totally determined in this observational study. Therefore, increased risk of acute
pancreatitis among people actively using zopiclone can only be regarded as relative not absolute.
The strength of our study is using a population-based case-control design with a great statistical power. This study serves as an updated discussion on the association between zopiclone use and the risk of acute pancreatitis. Based on our findings, we also suggest clinicians can report the cases of zopiclone-associated acute pancreatitis in the future. We conclude that people actively using zopiclone are associated with increased risk of acute pancreatitis. Clinicians should consider acute pancreatitis risk when prescribing zopiclone, particularly comorbid with alcohol-related disease or biliary stone. Further systematic studies or case reports are required to establish the definite role of zopiclone on the risk of acute pancreatitis.
Acknowledgement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation in Taichung in Taiwan, Taiwan Brain Disease Foundation in Taipei in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Specific author contributions
Shih-Wei Lai substantially contributed to the conception of this article. He planned and conducted the study. He initiated the draft of the article and critically revised the article. Hsueh-Chou Lai and Cheng-Li Lin conducted the data analysis and critically revised the article.
Kuan-Fu Liao planned and conducted the study. He participated in the data interpretation and also critically revised the article.
Conflict of Interest Statement
REFERENCES
1. Noble S, Langtry HD, Lamb HM. Zopiclone. An update of its pharmacology, clinical efficacy and tolerability in the treatment of insomnia. Drugs 1998; 55: 277-302.
2. Hajak G. A comparative assessment of the risks and benefits of zopiclone: a review of 15 years' clinical experience. Drug Saf 1999; 21: 457-69.
3. Allain H, Delahaye C, Le Coz F et al. Postmarketing surveillance of zopiclone in insomnia: analysis of 20,513 cases. Sleep 1991; 14: 408-13.
4. eHealthMe study from FDA and social media reports. Review: could zopiclone cause acute pancreatitis? http://www.ehealthme.com/print/ds17446113 [cited in 2014 September].
5. Sandzen B, Rosenmuller M, Haapamaki MM et al. First attack of acute pancreatitis in Sweden 1988 - 2003: incidence, aetiological classification, procedures and mortality - a register study. BMC Gastroenterol 2009; 9: 18. 6. Roberts SE, Thorne K, Evans PA et al. Mortality following acute pancreatitis:
social deprivation, hospital size and time of admission: record linkage study.
BMC Gastroenterol 2014; 14: 153.
7. Chen CH, Dai CY, Hou NJ et al. Etiology, severity and recurrence of acute pancreatitis in southern taiwan. J Formos Med Assoc 2006; 105: 550-5.
8. Lai SW, Muo CH, Liao KF et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011; 106: 1697-704.
9. Ksiadzyna D. Drug-induced acute pancreatitis related to medications commonly used in gastroenterology. Eur J Intern Med 2011; 22: 20-5.
10. Georgiev V. (S)-Zopiclone Sepracor. Current Opinion In Investigational Drugs
(London, England: 2000) 2001; 2: 271-3.
11. Eszopiclone: esopiclone, estorra, S-zopiclone, zopiclone--Sepracor. Drugs R D 2005; 6: 111-5.
12. Hsiao F-Y, Hsieh P-H, Gau C-S. Ten-year trend in prescriptions of z-hypnotics among the elderly: A nationwide, cross-sectional study in Taiwan. Journal of
Clinical Gerontology and Geriatrics 2013; 4: 37-41.
13. Database NHIR. Taiwan. http://nhird.nhri.org.tw/en/Backgroundhtml [cited in 2014 September].
14. Lai SW, Liao KF, Liao CC et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore)
2010; 89: 295-9.
15. Lai SW, Liao KF, Chen PC et al. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer 2012; 13: 143-8.
16. Musch B, Maillard F. Zopiclone, the third generation hypnotic: a clinical overview. Int Clin Psychopharmacol 1990; 5 Suppl 2: 147-58.
17. Lai SW, Lin CL, Liao KF, Lin CY. Amiodarone use and risk of acute pancreatitis: A population-based case-control study. Heart Rhythm 2015; 12: 163-6.
18. eHealthMe study from FDA and social media reports. Review: ccould zopiclone cause bile duct stone (choledocholithiasis)?
Table 1. Characteristics of cases with acute pancreatitis and control subjects Acute pancreatitis Yes N=5169 No N=20676 Variable n (%) n (%) P value* Sex 0.99 male 3286 (63.57) 13144 (63.57) female 1883 (36.43) 7532 (36.43)
Age group (year) 0.99
20-39 1324 (25.61) 5293 (25.60) 40-64 2412 (46.66) 9651 (46.68) 65-84 1433 (27.72) 5732 (27.72) Zopiclone use <0.001 Never use 4939 (95.55) 20405 (98.69) Active use 93 (1.80) 92 (0.44) Non-active use 137 (2.65) 179 (0.87) Benzodiazepines use <0.001 Never use 976 (18.88) 7567 (36.60) Ever use 4193 (81.12) 13109 (63.40)
Comorbidities before index date
Alcohol-related disease 518 (10.02) 203 (0.98) <0.001
Biliary stone 1148 (22.21) 496 (2.40) <0.001
Cardiovascular disease 1563 (30.24) 4349 (21.03) <0.001 Chronic obstructive pulmonary
disease 1044 (20.20) 2953 (14.28) <0.001 Diabetes mellitus 1357 (26.25) 2788 (13.48) <0.001 Hepatitis B 389 (7.53) 742 (3.59) <0.001 Hepatitis C 257 (4.97) 330 (1.60) <0.001 Hypertriglyceridemia 113 (2.19) 191 (0.92) <0.001
Data are presented as the number of subjects in each group, with percentages given in parentheses.
Table 2. Odds ratio and 95% confidence interval of acute pancreatitis associated with zopiclone use and comorbidities
Crude Adjusted †
Variable OR (95%CI) OR (95%CI)
Zopiclone use (never use as a reference)
Active use 4.18 (3.13, 5.58) 2.36 (1.70, 3.28)
Non-active use 3.16 (2.53, 3.96) 2.07 (1.60, 2.66)
Benzodiazepines use (never use as a reference)
Ever use 2.48 (2.30, 2.67) 1.75 (1.61, 1.90)
Comorbidities before index date (yes vs. no)
Alcohol-related disease 11.23 (9.52, 13.25) 11.6 (9.77, 13.76)
Biliary stone 11.62 (10.40, 12.97) 10.74 (9.58, 12.04)
Cardiovascular disease 1.63 (1.52, 1.74) 0.94 (0.86, 1.02)
Chronic obstructive pulmonary disease 1.52 (1.41, 1.64) 1.09 (0.99, 1.19)
Diabetes mellitus 2.28 (2.12, 2.46) 1.85 (1.70, 2.02)
Hepatitis B 2.19 (1.93, 2.48) 1.66 (1.44, 1.92)
Hepatitis C 3.23 (2.73, 3.81) 1.85 (1.52, 2.24)
Hypertriglyceridemia 2.40 (1.90, 3.03) 1.56 (1.20, 2.04)
†Adjusted for benzodiazepines use, alcohol-related disease, biliary stone, cardiovascular disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C and
Table 3. Odds ratio of acute pancreatitis by interaction effect between active use of zopiclone and alcohol-related disease or biliary stone
Never use Active use
Variable Case number/
control number OR (95% CI)† Case number/ control number OR (95% CI)† No alcohol-related
disease and no biliary stone 3400/19741 As a reference 55/80 2.76 (1.94, 3.93) Presence of alcohol-related disease or biliary stone 1539/664 11.68 (10.57, 12.91) 38/12 14.44 (7.47, 27.89) †Adjusted for benzodiazepines use, cardiovascular disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C and hypertriglyceridemia.
The interaction between active use of zopiclone and presence of alcohol-related disease or biliary stone was significant (P value for interaction = 0.002).