Research Article
Risk Factors for the Progression of Mild Cognitive Impairment in
Different Types of Neurodegenerative Disorders
Pei-Hao Chen
,
1,2,3Shih-Jung Cheng,
1,2,4Hui-Chi Lin,
1Chuo-Yu Lee,
1,2,5and Chih-Ho Chou
6,71Department of Neurology, MacKay Memorial Hospital, Taipei, Taiwan 2Department of Medicine, Mackay Medical College, New Taipei City, Taiwan
3Graduate Institute of Mechanical and Electrical Engineering, National Taipei University of Technology, Taipei, Taiwan 4Department of Physical Therapy and Assistive Technology, National Yang-Ming University, Taipei, Taiwan
5Graduate Institute of Chemistry, Tamkang University, New Taipei City, Taiwan 6Department of Neurology, Chi-Mei Medical Center, Tainan, Taiwan
7Chia Nan University of Pharmacy and Science, Tainan, Taiwan
Correspondence should be addressed to Chih-Ho Chou; d940397@mail.chimei.org.tw
Received 2 February 2018; Revised 24 March 2018; Accepted 5 April 2018; Published 5 June 2018 Academic Editor: Woon-Man Kung
Copyright © 2018 Pei-Hao Chen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objective. Mild cognitive impairment (MCI) is a transitional state between normal aging and early dementia. It has a heterogeneous etiology and clinical course. This study aimed to examine the factors associated with the progression of MCI in different types of dementia disorders. Method. A retrospective, longitudinal, observational study of outpatients with MCI was conducted at a medical center in northern Taiwan. Patient medical records were reviewed, and risk factors were analyzed by multivariate analysis. Results. Among 279 patients with MCI, 163 (58.4%), 68 (24.4%), and 48 (17.2%) were diagnosed with Alzheimer’s disease, vascular cognitive impairment, and Lewy body diseases, respectively. During the observation period, 37.2% of patients progressed to dementia. Older age and a higher Clinical Dementia Rating Scale-Sum of Boxes were associated with the risk of progression. Hyperlipidemia was associated with a decreased risk. Converters were more likely to receive an antidementia prescription. Conclusion. Our study suggests the importance of comprehensive clinical profiling, risk factor assessment, and detailed drug history evaluations in improving our understanding and management of dementia subtypes.
1. Introduction
Dementia can result from several underlying diseases, including Alzheimer’s disease (AD), cerebrovascular disease, Lewy body diseases (LBD), frontotemporal dementia, and other less common disorders such as Huntington’s disease, supranuclear palsy, and others. Mild cognitive impairment (MCI) is considered an intermediate state between normal cognitive aging and very early dementia. Individuals diag-nosed with MCI may remain stable, return to normal
(14.4–55.6% of patients), or progress to dementia [1]. There
is extensive literature on MCI in AD but limited to other types of dementia. Many epidemiological studies have reported that the presence of vascular risk factors (i.e.,
hypertension, diabetes, cerebrovascular disease, and hyper-lipidemia) in midlife is associated with an increased risk of cognitive impairment and dementia, particularly AD and vascular dementia (VaD) [2, 3]. Previous studies have reported older age, lower education status, prestroke cogni-tive and functional status, and history of diseases as risk fac-tors for poststroke dementia [4]. However, cardiovascular risk factors in the midlife increase the risk of dementia, but their roles in the late-life are less clear [5]. Apart from vascu-lar risk factors, other clinical conditions have been reported to show associations with MCI and dementia, including chronic obstructive pulmonary disease [6], chronic heart disease [7], cirrhosis [8], and chronic kidney disease [9]. By contrast, there has been a limited focus on whether chronic
Volume 2018, Article ID 6929732, 8 pages https://doi.org/10.1155/2018/6929732
comorbid illnesses exhibit differential associations for differ-ent types of demdiffer-entia. Cognitive impairmdiffer-ent, seen with both delirium and dementia, has been associated with polyphar-macy. Inappropriate use of benzodiazepine (BZD) in the elderly is also a major public health problem. The conse-quences of inappropriate BZD use include falls, delirium, other cognitive dysfunction, acute respiratory failure, car accidents, dependence, and withdrawal symptoms [10]. Studies on associations between sedative hypnotics and
cog-nitive decline in elderly patients have yielded mixedfindings
[11]. Previous studies cannot yet determine whether the observed epidemiological association is a causal effect or the result of unmeasured confounding variables.
It is important to understand which clinical and medical factors might be associated with cognitive decline. Identify-ing these risk factors that hasten the onset of dementia is crucial for timely medical intervention and predicting prog-noses. Improved knowledge of comorbidities in patients with MCI would facilitate the development of preventive strate-gies aimed at slowing rapid clinical and functional deteriora-tion. This study aimed to examine the association between common clinical and neuropsychological factors in later life at the MCI stage and the risk of converting to dementia, with a particular focus on specific dementia disorders.
1.1. Specific
1.1.1. Study Design. This was a retrospective, longitudinal, observational study that used an unselected sample to test for associations between comorbidities in patients with MCI and the rate of cognitive decline or dementia.
1.1.2. Setting. Data used for this present study were obtained from a dementia care database from January 2014 to June 2017.
1.1.3. Participants. Patients were enrolled and studied at the neurological department of the MacKay Memorial Hospital (Taiwan). We recruited patients by reviewing electronic medical records and documenting information related to clinical measurements, diagnoses, comorbidities, neuroim-aging reports, biochemical tests, and neuropsychological
assessments at thefirst visit. The medical records were
ree-valuated by a dementia expert who was not involved in the assessments of the patients. The rate of decline in cognition was measured based on changes in the Mini-Mental Status Exam (MMSE) scores [12] and Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) [13]. A previous study reported that the conversion to dementia from MCI stages could be predicted by MMSE changes over time instead of single measurements [14]. This approach is useful for the detection of AD and other dementias in people with MCI. The Clinical Dementia Rating Scale (CDR) was ini-tially designed to stage clinical dementia in older persons [15]. It yields both a global score and CDR-SB score. The global score is used to stage dementia severity, whereas the CDR-SB score is a more detailed quantitative version of that scale [16]. Progression of MCI to dementia
(con-verter) was defined as a change in the overall CDR score
from 0.5 to ≥1.0. The inclusion criteria were as follows:
(1) cognitive complaints not normal for the age of the indi-vidual, (2) no dementia (cut-off MMSE score > 23 in
per-sons with at least 6 years of education or>13 in persons
with less than 6 years of education and CDR = 0.5), (3) cog-nitive decline, and (4) essentially normal functional
activi-ties in everyday life at the first visit [17]. All participants
were evaluated from thefirst visit and followed up at
regu-lar intervals for at least one year until a clinical diagnosis of
specific dementia (converter) or MCI (nonconverter) was
reached. We included patients who had received regular ambulatory care for 12 months or longer. The intervals between two cognitive assessments were greater than six months. Exclusion criteria were as follows: (1) inability to establish a definite diagnosis for converters at the end of the study; (2) clinical suspicion of frontotemporal demen-tia, corticobasal degeneration, progressive supranuclear palsy, or other rare types of dementia; and (3) mixed dementia or mixed neurodegenerative diagnoses. In cases of atypical clinical manifestations, one optimal diagnosis for each patient was made under the consensus of neurol-ogists. We excluded patients with uncertain dementia
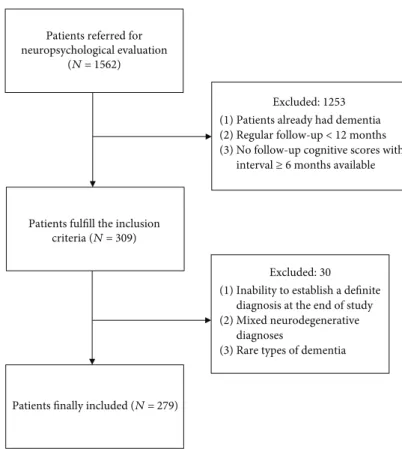
syn-dromes. Figure 1 provides a flowchart of the inclusion/
exclusion criteria.
1.1.4. Classification of Dementia Syndromes. Diagnoses of dementia syndromes were confirmed by two study clinicians who reviewed clinical, neuropsychological, and brain imag-ing data and biochemical tests. Comprehensive neuropsy-chological testing was performed by an experienced clinical
psychologist. Dementia subtypes were classified using
inter-national consensus clinical criteria. Dementia due to AD
was diagnosed in patients who fulfilled the National Institute
of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Associa-tion criteria for probable AD [18]. Dementia or MCI due to vascular disease was diagnosed in patients who fulfilled the American Heart Association/American Stroke Association Statement on Vascular Contributions to Cognitive Impair-ment and DeImpair-mentia criteria [19]. LBD is an umbrella term
for two closely related clinical diagnoses: Parkinson’s disease
dementia (PDD) and dementia with Lewy bodies (DLB). The third report of the DLB Consortium was used for probable DLB diagnoses [20]. The diagnosis of PDD was made based on recommendations of the movement disorder society task force [21]. The onset age of dementia was defined as the date on which the clinical symptoms and neuropsychological tests first allowed the diagnosis to be made. For nonconverters, MCI due to AD was diagnosed based on the National Insti-tute on Aging and the Alzheimer’s Association work group criteria [22]. Neuroimaging criteria for vascular cognitive disorders were based on the 2014 International Society for Vascular Behavioral and Cognitive Disorders (VASCOG) statement [23]. Diagnostic criteria for MCI due to Parkin-son’s disease or DLB are based on international consensus clinical criteria [24, 25].
1.1.5. Comorbidities and Medication History. Comorbidities, including diabetes mellitus, hypertension, hypercholesterol-emia, heart disease (history of coronary artery disease,
atrial fibrillations, heart failure, or valvular heart disease), gastrointestinal disorder, chronic kidney disease, liver func-tion impairment, anemia, and depression, were documented by reviewing the medical records and laboratory documents in the hospital. Medication history of using antidementia agents (cholinesterase inhibitors or memantine) and hyp-notic agents, including BZD or nonbenzodiazepine hyphyp-notic agents (“Z drugs”) during observational periods, were iden-tified from the medical records and/or medical history
interviews. Taking five or more regular medications
pre-scribed >30 days at the time of diagnosis was defined as
polypharmacy in our study.
1.1.6. Statistical Methods. We used a stepwise multiple logis-tic regression model to analyze the data. Each categorical
var-iable was examined using χ2 tests. The Student t-test was
used to analyze continuous variables. Variables with ap value
≤ 0.1 were included in the multiple regression model. The
effects of each variable were represented by odds ratios and
corresponding 95% confidence intervals (CIs), which were
calculated based on the exponential coefficient of the multi-ple logistic regression model. We also analyzed these factors in different etiologic subgroups. All statistical analyses were performed using R version 3.4.2 (2017-09-28).
1.1.7. Ethical Review. This present study was reviewed and approved by the MacKay Memorial Hospital Institutional Review Board (number 18MMHIS037).
2. Results
We recruited 279 patients with MCI from the database. A total of 163 patients were diagnosed with AD (female 64.4%), 68 had vascular cognitive impairment (female 30.9%), and 48 had Lewy body diseases (LBD) (female 45.8%). The distributions of age did not differ significantly among these groups. In this patient cohort, 58.7%, 31.8%, and 46.5% had hypertension, diabetes, and hypercholesterol-emia, respectively, while 19.2% diagnosed as having depres-sive disorders. Additionally, 37.4% of our MCI patients progressed to dementia with a mean follow-up period of
27.09± 15.09 months. The proportion of conversion in the
three dementia syndromes was 39.9% in AD, 38.2% in
VaD, and 27.1% in LBD (p = 0 2683) (Figure 2). Univariate
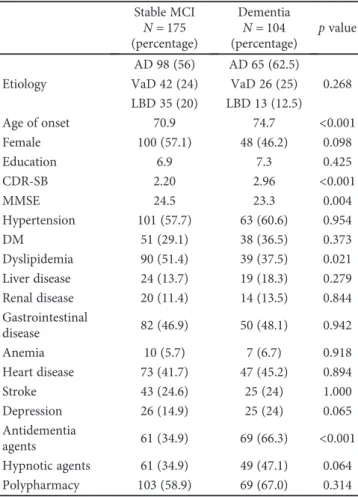
tests showed that the ages of onset, CDR-SB, and baseline MMSE were significant between stable MCI and converters (Table 1). Converters were more likely to receive
antidemen-tia agents (66.3% versus 34.9%,p < 0 001). The MMSE scores
could be divided into subscores for orientation, memory, calculation, language, and perceptual motor function. The subscores of orientation were significantly different between MCI converters and nonconverters.
A multiple logistic regression model revealed that older
age at onset, female sex, and a greater CDR-SB were signi
fi-cantly associated with a higher risk of converting from MCI to dementia. Receiving antidementia agents was strongly associated with a higher probability of this conversion Patients referred for
neuropsychological evaluation
(N = 1562)
Patients fulfill the inclusion
criteria (N = 309)
Excluded: 1253 (1) Patients already had dementia (2) Regular follow-up < 12 months (3) No follow-up cognitive scores with
interval ≥ 6 months available
Patients finally included (N = 279)
Excluded: 30 (1) Inability to establish a definite
diagnosis at the end of study (2) Mixed neurodegenerative
diagnoses
(3) Rare types of dementia
(Table 2). Hyperlipidemia was associated with a decreased risk of conversion. The interaction between hyperlipidemia
and etiologies was nonsignificant. We also evaluated baseline
MMSE scores (substituting CDR-SB in the model), and the
results of the other risk factors were unaltered. We tested four interactions between etiologies and each one of DM, hyper-tension, stroke, and heart disease, respectively, in the
multi-ple logistic regression model, and all were nonsignificant.
These interactions were not reported. Selection of risk factors in the model was based on the previous studies and afore-mentioned statistical criteria.
We performed a subgroup analysis from the MCI cohort according to the etiology. Age of onset was a significant risk factor in AD and VaD. Female was associated with an increased risk in AD. CDR-SB was associated with higher risk in AD and VaD. Receiving antidementia agents was sig-nificantly associated with conversion in AD. The trend was
mostly consistent with the finding in the main cohort
(Table 3). The LBD and AD subgroups had very similar risk
factor profiles of conversion from MCI to dementia.
3. Discussion
MCI is heterogeneous in its etiology and clinical course. Older age and higher CDR-SB are major important markers for the identification of patients at a higher risk of progres-sion. In our present study, we found that CDR-SB is highly indicative both in the whole cohort and different subgroup analyses. Because of the scoring rule, a CDR global score of 0.5 was unable to detect the cognitive categories that were
affected (i.e., memory, orientation, judgment/problem
solv-ing, community affairs, hobbies, and personal care). In
con-trast, CDR-SB includes all categories, and the sensitivity might differ from the CDR global score.
Cholesterol has been recently shown to be important for synaptic transmission, and many neurodegenerative diseases, including AD and PD, are characterized by impaired choles-terol turnover in the brain [26]. A population-based prospec-tive cohort study from Denmark showed that subjects with objective cognitive impairment who were most likely to prog-ress were older, physically inactive, had a higher level of total cholesterol, and had a history of depression [27]. Reviews and meta-analyses revealed that midlife high total serum
0 20 40 60 80 100 120 140 160 180 Clinical diagnosis
Numbers of converters and stable MCI patients
Converters Stable MCI
AD VAD LBD
Figure 2: Proportions of conversion to dementia in patients with different clinical diagnoses. AD: Alzheimer’s disease; VaD: vascular dementia; LBD: Lewy body diseases.
Table 1: Univariate analysis of clinical characteristics in 279 MCI patients who remained stationary course or progressed to dementia.
Stable MCI Dementia
p value N = 175 (percentage) N = 104 (percentage) Etiology AD 98 (56) AD 65 (62.5) 0.268 VaD 42 (24) VaD 26 (25) LBD 35 (20) LBD 13 (12.5) Age of onset 70.9 74.7 <0.001 Female 100 (57.1) 48 (46.2) 0.098 Education 6.9 7.3 0.425 CDR-SB 2.20 2.96 <0.001 MMSE 24.5 23.3 0.004 Hypertension 101 (57.7) 63 (60.6) 0.954 DM 51 (29.1) 38 (36.5) 0.373 Dyslipidemia 90 (51.4) 39 (37.5) 0.021 Liver disease 24 (13.7) 19 (18.3) 0.279 Renal disease 20 (11.4) 14 (13.5) 0.844 Gastrointestinal disease 82 (46.9) 50 (48.1) 0.942 Anemia 10 (5.7) 7 (6.7) 0.918 Heart disease 73 (41.7) 47 (45.2) 0.894 Stroke 43 (24.6) 25 (24) 1.000 Depression 26 (14.9) 25 (24) 0.065 Antidementia agents 61 (34.9) 69 (66.3) <0.001 Hypnotic agents 61 (34.9) 49 (47.1) 0.064 Polypharmacy 103 (58.9) 69 (67.0) 0.314
AD: Alzheimer’s disease; VaD: vascular dementia; LBD: Lewy body diseases; MMSE: Mini-Mental State Examination; CDR-SB: Clinical Dementia Rating-Sum of Boxes; DM: diabetes mellitus. Remark: antidementia agents: acetylcholinesterase inhibitors and memantine. Hypnotic agents: benzodiazepines and nonbenzodiazepine hypnotics.
Table 2: Logistic regression model of variables associated with conversion to dementia in the MCI cohort.
OR 2.50% 97.50% p value (Intercept) 0.0014 1.00E− 04 0.029 <0.001 VaD 2.082 0.910 4.909 0.089 LBD 0.536 0.225 1.223 0.146 Age 1.071 1.032 1.113 <0.001 Gender 0.540 0.293 0.980 0.044 CDR-SB 1.553 1.221 2.008 <0.001 Hyperlipidemia 0.554 0.310 0.980 0.044 Depression 1.624 0.730 3.623 0.233 Antidementia agents 5.162 2.663 10.522 <0.001 Hypnotic agents 1.346 0.719 2.517 0.352
VaD: vascular dementia; LBD: Lewy body diseases; CDR-SB: Clinical Dementia Rating-Sum of Boxes. Remark: antidementia agents: acetylcholinesterase inhibitors and memantine. Hypnotic agents: benzodiazepines and nonbenzodiazepine hypnotic agents.
cholesterol was associated with an increased risk of MCI, AD, and cognitive decline in late-life; however, high cholesterol in late-life was not associated with MCI, AD, VaD, any demen-tia, or cognitive decline [28]. Patients with late-life
cardiovas-cular factors, including body mass index, atrialfibrillation,
hypertension, hyperlipidemia, and diabetes, were more likely to have a diagnosis of VaD than the normal populations. However, there were no associations between AD and DLB with hypertension, hyperlipidemia, or diabetes [5]. In our study, we found that hypercholesterolemia was not a risk fac-tor but had a nonsignificant protective effect on the progres-sion of MCI. More than 70% of our patients used statins during the study period. However, we were not sure based on the prescription pattern, whether these patients received treatment from other hospitals or clinics. A previous study
concluded that statins may slow the rate of cognitive decline and delay the onset of AD and all-cause dementia in cogni-tively healthy elderly individuals, whereas individuals with MCI may not have comparable cognitive protection from these agents [29]. A meta-analysis of two large trials,
includ-ing a total of 26,340 patients aged≥ 40 years with
cardiovas-cular risk factors, reported that statins administered in later life do not prevent cognitive decline or dementia [30].
In Taiwan, cholinesterase inhibitors and memantine can be provided by the health insurance system under the follow-ing rules. AD or PDD was diagnosed by a neurologist or psychiatrist using the clinical diagnostic criteria. They had the latest MMSE scores of 10–26 and did not have contrain-dications. The authority required annual reevaluations of the initial prescription, and the reimbursement would be
Table 3: Logistic regression model of variables associated with conversion to dementia in the AD (a), LBD (b), and VaD (c) cases of the MCI cohort. (a) Subgroup of AD OR 2.50% 97.50% p value (Intercept) 0.008 1.00E− 04 0.309 0.013 Age 1.058 1.010 1.112 0.021 Gender 0.424 0.202 0.874 0.021 CDR-SB 1.395 1.030 1.913 0.034 Hyperlipidemia 0.761 0.364 1.569 0.462 Depression 1.124 0.419 2.985 0.814 Antidementia agents 3.411 1.572 7.789 0.003 Hypnotic agents 1.800 0.808 4.051 0.151 (b) Subgroup of LBD OR 2.5% 97.50% p value (Intercept) 0 0 0.529 0.067 Age 1.186 1.021 1.446 0.051 Gender 0.143 0.012 1.009 0.080 CDR-SB 1.079 0.486 2.358 0.845 Dyslipidemia 0.321 0.045 1.825 0.218 Depression 7.221 0.720 106.364 0.109 Antidementia agents 66.025 7.839 1340.956 0.001 Hypnotic agents 0.410 0.057 2.3819 0.337 (c) Subgroup of VaD OR 2.50% 97.5% p value (Intercept) 0.000 0.000 0.068 0.009 Age 1.106 1.030 1.203 0.010 Gender 0.563 0.129 2.233 0.421 CDR-SB 2.172 1.229 4.309 0.015 Hyperlipidemia 0.459 0.131 1.550 0.211 Depression 8.786 1.059 101.545 0.057 Hypnotic agents 0.820 0.216 2.934 0.762
AD: Alzheimer’s disease; VaD: vascular dementia; LBD: Lewy body diseases; CDR-SB: Clinical Dementia Rating-Sum of Boxes. Remark: hypnotic agents indicate benzodiazepines and nonbenzodiazepine hypnotic agents. Antidementia agents were not reimbursed for VaD patients in Taiwan health insurance system. They were not analyzed in the model due to sparsity.
discontinued in case there is too much deterioration in MMSE scores or CDR. In our study, 45.5% of patients received antidementia agents during the observation period. Prescribing these medicines was associated with the likeli-hood of converting to dementia. However, those who received antidementia treatments also had higher CDR-SB
(significant) and lower baseline MMSE scores (not
signifi-cant). A possible explanation is that the physicians more likely prescribed antidementia agents to improve the cogni-tive functions in patients who they found were more severe at baseline. The results did not imply that the clinical courses in MCI patients were changed by these treatments.
Risks of cognitive decline and delirium were known to be associated with polypharmacy. In a prospective cohort study
of 294 elderly patients, 22% takingfive or fewer medications
were found to have impaired cognition when compared with
33% of patients taking 6–9 medications and 54% in patients
taking 10 or more medications [31]. A previous study of patients with cognitive impairment found that 70.4% were on multiple medications and 42% took BZD [32]. In our
study, 61.5% of patients took more thanfive types of
medica-tions and almost 40% of patients were prescribed sedative/ hypnotic drugs for at least 30 days during the study. In a large cohort of postmenopausal women, all types of antidepressant
use and different levels of depression severity were associated
with subsequent cognitive impairment [33]. By contrast, MCI has reported being a risk factor for depressive and anx-iety disorders, suggesting common pathological pathways for cognitive and psychiatric outcomes [34]. A recently pub-lished article found that executive dysfunction in elderly peo-ple with depression may be associated with the age effect [35]. The reciprocal effects of depression and MCI were inconclu-sive. Our data revealed a nonsignificant trend (p = 0 06) sup-porting a link between depression and cognitive decline.
The effects of hypnotic agents and risk of dementia
remained a subject of debate. A recent study based on
lon-gitudinal data found a slight association, but no dose-effect,
in elderly nondemented individuals [36]. We found that hypnotic agents were very commonly used in depressed patients in our cohort. Neither depression nor hypnotics use was associated with conversion from MCI to dementia.
Thesefindings are consistent with a recent study performed
in Taiwan [37].
3.1. Limitations. In our study, the diagnosis of dementia
syn-dromes was justified by two clinicians who were experienced
in dementia care. Comprehensive neuropsychological testing was performed by an experienced clinical psychologist. We excluded MCI of uncertain etiology and mixed neurodegen-erative diagnoses. Lack of pathological confirmation was a weakness of our study, similar to the majority of the previ-ously published articles. The primary clinical etiologic diag-nosis remained stable in more than 90% of our patients. Previous results from a multicenter longitudinal database suggested that hospital-based studies were more accurate in categorizing dementia subtypes because they involve a multidisciplinary diagnostic approach and an adequate diag-nostic infrastructure compared with community-based stud-ies [38]. Due to the Personal Data Protection Act of 2010 of
Taiwan, medication history may not be perfectly completed. The small case numbers limited our capacity to analyze whether there were any differences among different demen-tia syndromes.
4. Conclusions
Our retrospective, longitudinal, observational study showed the distribution of dementia subtypes from MCI and described the clinical progression, comorbidity, and medica-tion history associated with dementia subtypes. We believe
that comprehensive clinical profiling, assessments of risk
fac-tors, and detailed drug history evaluations are helpful to bet-ter understand and manage dementia subtypes. Our study suggests the importance of early detection of individuals with MCI. In the future, prospective studies should establish which of these factors are the most influential to aid the development of treatment and prevention strategies. Poly-pharmacy and hypnotic sedatives should be avoided if they are not essential, especially in elderly patients with MCI.
Data Availability
The data used to support the findings of this study are
available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
[1] R. C. Petersen, O. Lopez, M. J. Armstrong et al., “Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementa-tion subcommittee of the American Academy of Neurology,” Neurology, vol. 90, no. 3, pp. 126–135, 2018.
[2] M. Baumgart, H. M. Snyder, M. C. Carrillo, S. Fazio, H. Kim, and H. Johns, “Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective,” Alzheimer’s & Dementia, vol. 11, no. 6, pp. 718–726, 2015.
[3] Y. D. Reijmer, E. Berg, J. M. Dekker et al.,“Development of vascular risk factors over 15 years in relation to cognition: the Hoorn study,” Journal of the American Geriatrics Society, vol. 60, no. 8, pp. 1426–1433, 2012.
[4] G.-C. Hu and Y.-M. Chen,“Post-stroke dementia: epidemiol-ogy, mechanisms and management,” International Journal of Gerontology, vol. 11, no. 4, pp. 210–214, 2017.
[5] B. N. Dugger, M. Malek-Ahmadi, S. E. Monsell et al., “A cross-sectional analysis of late-life cardiovascular factors and their relation to clinically defined neurodegenerative diseases,” Alzheimer Disease & Associated Disorders, vol. 30, no. 3, pp. 223–229, 2016.
[6] K. Kakkera, K. P. Padala, M. Kodali, and P. R. Padala, “Associ-ation of chronic obstructive pulmonary disease with mild cognitive impairment and dementia,” Current Opinion in Pulmonary Medicine, vol. 24, no. 2, pp. 173–178, 2017. [7] J. W. Sacre, J. Ball, C. Wong et al.,“Mild cognitive impairment
with chronic heart disease,” European Heart Journal - Cardio-vascular Imaging, vol. 19, no. 3, pp. 285–292, 2018.
[8] T. B. Chen, S. Y. Yiao, Y. Sun et al., “Comorbidity and dementia: a nationwide survey in Taiwan,” PLoS One, vol. 12, no. 4, article e0175475, 2017.
[9] A. R. Zammit, M. J. Katz, M. Bitzer, and R. B. Lipton, “Cogni-tive impairment and dementia in older adults with chronic kidney disease: a review,” Alzheimer Disease & Associated Disorders, vol. 30, no. 4, pp. 357–366, 2016.
[10] G. Airagnes, A. Pelissolo, M. Lavallée, M. Flament, and F. Limosin,“Benzodiazepine misuse in the elderly: risk factors, consequences, and management,” Current Psychiatry Reports, vol. 18, no. 10, p. 89, 2016.
[11] J. D. Picton, A. B. Marino, and K. L. Nealy,“Benzodiazepine use and cognitive decline in the elderly,” American Journal of Health-System Pharmacy, vol. 75, no. 1, pp. e6–e12, 2018. [12] M. F. Folstein, S. E. Folstein, and P. R. McHugh,
““Mini-men-tal state”. A practical method for grading the cognitive state of patients for the clinician,” Journal of Psychiatric Research, vol. 12, no. 3, pp. 189–198, 1975.
[13] S. E. O'Bryant, S. C. Waring, C. M. Cullum et al., “Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study,” Archives of Neurology, vol. 65, no. 8, pp. 1091–1095, 2008.
[14] I. Arevalo-Rodriguez, N. Smailagic, M. Roqué i Figuls et al., “Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI),” Cochrane Database of Systematic Reviews, no. 3, article Cd010783, 2015.
[15] C. P. Hughes, L. Berg, W. L. Danziger, L. A. Coben, and R. L. Martin,“A new clinical scale for the staging of dementia,” The British Journal of Psychiatry, vol. 140, no. 6, pp. 566–572, 1982.
[16] C. A. Lynch, C. Walsh, A. Blanco et al.,“The clinical dementia rating sum of box score in mild dementia,” Dementia and Geriatric Cognitive Disorders, vol. 21, no. 1, pp. 40–43, 2006. [17] R. C. Petersen,“Mild cognitive impairment,” CONTINUUM:
Lifelong Learning in Neurology, vol. 22, no. 2, Dementia, pp. 404–418, 2016.
[18] G. M. McKhann, D. S. Knopman, H. Chertkow et al.,“The diag-nosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,” Alzheimer’s & Dementia, vol. 7, no. 3, pp. 263–269, 2011. [19] P. B. Gorelick, A. Scuteri, S. E. Black et al., “Vascular
contributions to cognitive impairment and dementia: a state-ment for healthcare professionals from the American Heart Association/American Stroke Association,” Stroke, vol. 42, no. 9, pp. 2672–2713, 2011.
[20] I. G. McKeith, D. W. Dickson, J. Lowe et al.,“Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium,” Neurology, vol. 65, no. 12, pp. 1863– 1872, 2005.
[21] B. Dubois, D. Burn, C. Goetz et al.,“Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force,” Movement Disorders, vol. 22, no. 16, pp. 2314–2324, 2007.
[22] M. S. Albert, S. T. DeKosky, D. Dickson et al.,“The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on
Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease,” Alzheimer’s & Dementia, vol. 7, no. 3, pp. 270–279, 2011.
[23] P. Sachdev, R. Kalaria, J. O'Brien et al.,“Diagnostic criteria for vascular cognitive disorders: a VASCOG statement,” Alzheimer Disease & Associated Disorders, vol. 28, no. 3, pp. 206–218, 2014.
[24] I. Litvan, J. G. Goldman, A. I. Tröster et al.,“Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Move-ment Disorder Society Task Force guidelines,” Movement Disorders, vol. 27, no. 3, pp. 349–356, 2012.
[25] American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, iGroup Press Co., LTd., Washington, DC, USA, 5th edition, 2013.
[26] A. M. Petrov, M. R. Kasimov, and A. L. Zefirov, “Brain choles-terol metabolism and its defects: linkage to neurodegenerative diseases and synaptic dysfunction,” Acta Naturae, vol. 8, no. 1, pp. 58–73, 2016.
[27] J. Skov Neergaard, K. Dragsbæk, C. Christiansen, M. Asser Karsdal, S. Brix, and K. Henriksen, “Objective cognitive impairment and progression to dementia in women: the prospective epidemiological risk factor study,” The Journal of Prevention of Alzheimer's Disease, vol. 4, no. 3, pp. 194–200, 2017.
[28] K. J. Anstey, K. Ashby-Mitchell, and R. Peters,“Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis,” Journal of Alzheimer's Disease, vol. 56, no. 1, pp. 215–228, 2017. [29] K. Bettermann, A. M. Arnold, J. Williamson et al., “Statins,
risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study,” Journal of Stroke & Cerebrovascular Diseases, vol. 21, no. 6, pp. 436–444, 2012.
[30] B. McGuinness, D. Craig, R. Bullock, P. Passmore, and Cochrane Dementia and Cognitive Improvement Group, “Statins for the prevention of dementia,” Cochrane Database of Systematic Reviews, no. 1, article Cd003160, 2016.
[31] J. Jyrkkä, H. Enlund, P. Lavikainen, R. Sulkava, and S. Hartikainen,“Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population,” Pharmacoepidemiology & Drug Safety, vol. 20, no. 5, pp. 514–522, 2011.
[32] A. Robles Bayon and F. Gude Sampedro, “Inappropriate treatments for patients with cognitive decline,” Neurología, vol. 29, no. 9, pp. 523–532, 2014.
[33] J. S. Goveas, P. E. Hogan, J. M. Kotchen et al.,“Depressive symptoms, antidepressant use, and future cognitive health in postmenopausal women: the Women’s Health Initiative Memory Study,” International Psychogeriatrics, vol. 24, no. 08, pp. 1252–1264, 2012.
[34] S. S. Mirza, M. A. Ikram, D. Bos, R. Mihaescu, A. Hofman, and H. Tiemeier,“Mild cognitive impairment and risk of depres-sion and anxiety: a population-based study,” Alzheimer’s & Dementia, vol. 13, no. 2, pp. 130–139, 2017.
[35] K.-C. Wang, P. K. Yip, Y. Y. Lu, and Z. T. Yeh,“Depression in older adults among community: the role of executive func-tion,” International Journal of Gerontology, vol. 11, no. 4, pp. 230–234, 2017.
[36] S. L. Gray, S. Dublin, O. Yu et al.,“Benzodiazepine use and risk of incident dementia or cognitive decline: prospective popula-tion based study,” BMJ, vol. 352, article i90, 2016.
[37] H. I. Shih, C. C. Lin, Y. F. Tu et al.,“An increased risk of revers-ible dementia may occur after zolpidem derivative use in the elderly population: a population-based case-control study,” Medicine, vol. 94, no. 17, p. e809, 2015.
[38] T. D. Koepsell, D. P. Gill, and B. Chen,“Stability of clinical eti-ologic diagnosis in dementia and mild cognitive impairment: results from a multicenter longitudinal database,” American Journal of Alzheimer's Disease & Other Dementias, vol. 28, no. 8, pp. 750–8, 2013.
Stem Cells
International
Hindawi www.hindawi.com Volume 2018 Hindawi www.hindawi.com Volume 2018 INFLAMMATIONEndocrinology
International Journal ofHindawi www.hindawi.com Volume 2018 Hindawi www.hindawi.com Volume 2018
Disease Markers
Hindawi www.hindawi.com Volume 2018 BioMed Research InternationalOncology
Journal of Hindawi www.hindawi.com Volume 2013 Hindawi www.hindawi.com Volume 2018 Oxidative Medicine and Cellular Longevity Hindawiwww.hindawi.com Volume 2018
PPAR Research
Hindawi Publishing Corporation
http://www.hindawi.com Volume 2013 Hindawi www.hindawi.com
The Scientific
World Journal
Volume 2018 Immunology Research Hindawi www.hindawi.com Volume 2018 Journal ofObesity
Journal of Hindawi www.hindawi.com Volume 2018 Hindawi www.hindawi.com Volume 2018 Computational and Mathematical Methods in Medicine Hindawi www.hindawi.com Volume 2018Behavioural
Neurology
Ophthalmology
Journal of Hindawi www.hindawi.com Volume 2018Diabetes Research
Journal ofHindawi
www.hindawi.com Volume 2018
Hindawi
www.hindawi.com Volume 2018 Research and Treatment
AIDS
Hindawi
www.hindawi.com Volume 2018
Gastroenterology Research and Practice
Hindawi www.hindawi.com Volume 2018