529
Sex Ratios and Sexual Maturity of Swordfish (Xiphias gladius L.) in the
Waters of Taiwan
Sheng-Ping Wang, Chi-Lu Sun* and Su-Zan Yeh
Institute of Oceanography, National Taiwan University, Taipei, Taiwan 106, R.O.C. (Accepted June 2, 2003)
Sheng-Ping Wang, Chi-Lu Sun and Su-Zan Yeh (2003) Sex ratios and sexual maturity of swordfish (Xiphias
gladius L.) in the waters of Taiwan. Zoological Studies 42(4): 529-539. Lower jaw fork length (LJFL) was mea-sured in 551 female and 386 male swordfish at Tungkang, Nanfangao, and Shinkang fish markets during September 1997 to July 2000, and ovaries were removed from 208 of the swordfish collected at the Shinkang fish market during July 1998 to June 2000 whose LJFLs ranged between 95 and 257 cm. The sex ratio (the proportion of females to the total number of females and males) increased as the LJFL increased beyond 150 cm (sex ratio = 1 x 10-4LJFL1.6601), and all fish with LJFLs of greater than 210 cm were females. The estimated mean body length at sexual maturity (L50) for females was 168.2 cm; the smallest mature female was 135 cm. Among mature females, 79% were in the developmental stage of ripening and 21% were in the resting stage; no individual was in the spawning or recently spawned stage. No hydrated oocytes were observed in any of the female gonad samples. These observations indicate that swordfish do not spawn in the waters of Taiwan. According to the relationship between developmental stage and the diameters of oocytes, 50% of individuals will be reproductively active at an oocyte diameter of 372.1 µ which can be used as an index for reproductive activity of swordfish in the waters of Taiwan. http://www.sinica.edu.tw/zool/zoolstud/42.4/529.pdf
Key words: Swordfish, Xiphias gladius, Maturity, Length at maturity, Sex ratio.
T
he swordfish, Xiphias gladius, is a pelagic migratory species which is found in the tropical and temperate waters of all oceans (Nakamura 1985). In the waters of Taiwan, swordfish are mainly harvested as a bycatch of the longline fish-eries; a few are taken by harpoon, gill net, and set net. For the past 10 yr, the annual landings of swordfish in Taiwan have fluctuated: landings decreased from 1490 metric tons (t) in 1989 to 674 t in 1990, they ranged between 600 and 1000 t dur-ing 1991 to 1996. In 1997, the landdur-ings increased to 1500 t and to 1771 t in 2000.The size and age at sexual maturity and the sex ratios are fundamental biological parameters used in stock assessments. Estimates of body size or age at sexual maturity are necessary para-meters for age- and size-structured models, such as the spawner biomass per recruit model (Gabriel et al. 1989), egg per recruit model (Foale and Day
1997), and other size- or age-structured models (Deriso et al. 1985, Quinn II et al. 1990).
Limited research has been published on the reproductive biology or sexual maturity of sword-fish from waters of the Pacific Ocean. In the west-ern Pacific, Yabe et al. (1959) estimated the length at sexual maturity, batch fecundity, spawning area, and spawning season. Using gonad index data, Kume and Joseph (1969) and Uosaki and Bayliff (1999) estimated the body size at sexual maturity for female swordfish and described the geographic distribution of mature swordfish in the eastern Pacific. Uchiyama and Shomura (1974) inferred the spawning season and spawning area based on the occurrence of ripe ovaries of female sword-fish in Hawaiian waters, and estimated the total fecundity for 8 female swordfish. In the North Pacific, Weber and Goldberg (1986) found that swordfish off California were reproductively
inac-529
*To whom correspondence and reprint requests should be addressed. Tel/Fax: 886-2-23629842. E-mail: chilu@ ccms.ntu.edu.tw
tive during Aug. to Nov. Sosa-Nishizaki (1990) esti-mated the length at sexual maturity for female swordfish throughout the North Pacific (cited in DeMartini et al. 2000). Young et al. (2000) de-scribed the spawning season, and estimated the batch fecundity and sex ratios of swordfish in the waters of eastern Australia. Based on histological analysis of microscopic slides, DeMartini et al. (2000) estimated the length at sexual maturity and determined the sex ratio, size composition, and temporal distribution of swordfish caught by the Hawaii-based pelagic longline fishery.
There has been no study on the reproductive biology of swordfish in the western Pacific since the work of Yabe et al. (1959). Therefore, the objective of this study was to estimate the length at sexual maturity and sex ratios for swordfish in the waters of Taiwan. In order to estimate maturi-ty, gonadal development was determined based on histological analysis in addition to the appear-ance of the gonads and estimates of a gonad index. The results of this study can be used as biological input parameters for further evaluation of the swordfish stock in the western North
Pacific Ocean.
MATERIALS AND METHODS
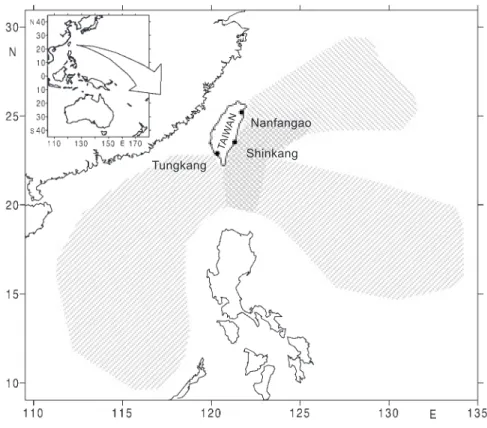
Gonad samples of swordfish were collected monthly at the Shinkang fish market (Fig. 1) during July 1998 to June 2000. The sex of each sample was identified based on the appearance of the gonads. Gonads were weighed and then pre-served in 10% buffered formalin for later histologi-cal analysis and measurement of oocytes. Monthly sex ratios were obtained from Tungkang, Nanfangao, and Shinkang fish markets (Fig. 1) from Sept. 1997 to July 2000. The length and weight including lower jaw fork length (LJFL; cm), from the tip of the lower jaw to the distal end of the central ray of the caudal fin; eye fork length (EFL; cm), from the posterior margin of the eye,s bony orbit to the distal end of the central ray of the cau-dal fin; and round weight (RW; kg), the total weight excluding the bill, were measured for each fish.
The sex ratios by month and length class (5-cm length intervals) were expressed as the proportion of females to total numbers of females and males:
Fig. 1. Fishing ports in Taiwan where gonad samples of swordfish and measurements for the sex ratio analysis were collected.
Fishing grounds of longline fisheries based at Tungkang. Fishing grounds of longline fisheries based at Nanfangao. Fishing grounds of longline fisheries based at Shinkang.
Tungkang TA IW AN Shinkang Nanfangao
sex ratio = number of females (1) number of females + number of males
.
A gonad index (GI) was calculated following the equation used by other studies (Hinton et al. 1997, Uosaki and Bayliff 1999, DeMartini et al. 2000):
Gl = ____In(GW)_______; (2) In(EFL)
where GW is the gonad weight in grams.
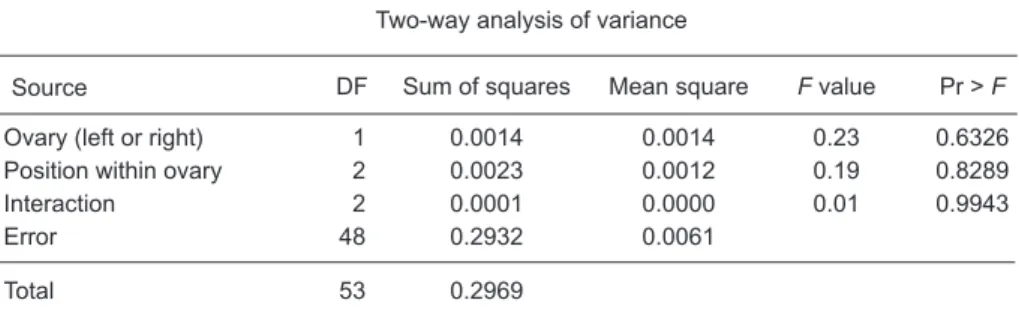
The sizes of right and left gonads often differ (Winters 1970, Wallace and Selman 1981), and DeMartini et al. (2000) indicated that the right ovaries of the swordfish were heavier than the left ovaries. In order to evaluate the synchronicity of egg development within and between ovary pairs, both left and right members of 3 pairs of ovaries were further divided into anterior, central, and pos-terior portions. Two-way analysis of variance was used to test possible differences in the numbers and diameters of oocytes between right and left gonads of the same individual.
Gonadal developmental stages were catego-rized based on the criteria of Murphy and Taylor (1990) as modified by DeMartini et al. (2000). Developmental stages of oocytes were classified into the (1) undeveloped stage, (2) developing stage, (3) maturing stage, (4) ripening stage, (5) spawning stage, (6) recently spawned stage, and (7) spent or resting stage. Individuals were desig-nated as mature if the most advanced oocytes were indicative of ≥ stage 4. Stages 4-6 are repro-ductively active stages, and stages1-3 and 7 are reproductively inactive stages (DeMartini et al. 2000).
Images of oocytes in histological preparations were obtained using an analog color camera (JVC model TK-C1380) mounted on a zoom dissecting microscope (Leica MZ6) at a magnification of 6.3-40x. The analog images were digitized when they were captured at a resolution of 307200 (640 x 480) pixels. The median of 25 random diameters provides a cost-efficient estimator of average maxi-mum oocyte size for multiple-spawning fishes (DeMartini et al. 2000). For each histological sec-tion, therefore, 25 oocytes were randomly selected from among those in the largest size class. The diameters of these oocytes were measured using the Image-Pro Plus image analysis program.
The relationship between oocyte size and the probability of reproductive activity was represented
by the logistic regression (DeMartini et al. 2000) as follows:
(3)
where OD is the diameter of oocyte (µ), p is the probability of reproductive activity, a is the inter-cept, and b is the slope.
Subsamples taken from fixed gonads were washed, dehydrated in alcohol and xylene, and in-filtrated with paraffin. Histological sections of 5-7 µ were cut with a Leica 2055 rotary microtome and stained with Harris, hematoxylin and eosin coun-terstain (Hunter and Macewicz 1985). Microscopic slides were examined with a Nikon SE compound microscope at a magnification of 40-400x.
The length at which 50% of all individuals were sexually mature (L50), was estimated from the proportion of mature individuals in each 5-cm length class and the fitted logistic curve (King 1995) as follows:
P = 1 (4)
1+exp[r×(L-L50)] ;
where P is the proportion of mature individuals within a length class, r is the slope of the curve, and L50is the length (LJFL) at 50% sexual maturi-ty. L50 and r were estimated using the nonlinear least square procedure (Gauss-Newton method, NLIN of SAS Institute, 1990).
In order to compare our estimates of body length at sexual maturity with those estimated by other authors, the equation LJFL = 7.7911 + 1.0647 EFL (Sun et al. 2002) was used to convert the different measurements of body length.
RESULTS
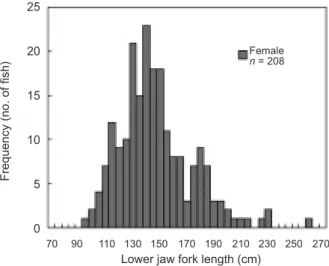
The LJFL of 551 females and 386 males was measured at the Tungkang, Nanfangao, and Shinkang fish markets for the sex ratio analysis. The range of the LJFL was 83 to 290 cm for females and 78 to 206 cm for males, with both clustered between 95 and 170 cm (Fig. 2).
The total number of female samples was greater than that of males. The estimated sex ratio for all samples was 0.59 which significantly differed (χ2 = 28.67; p < 0.01) from the expected 0.5 (Table 2).
The proportion of females was higher than males in each month. Sex ratios significantly
dif-fered from 0.5 during the period from Feb. to July (Table 2).
The sex ratio fluctuated from 0.4 to 0.7 (mean, 0.55; standard error, 0.074) without a sig-nificant pattern at a LJFL of less than 150 cm. The sex ratio increased for LJFLs greater than 150 cm, and all samples were females (i.e., a sex ratio of 1) at LJFLs larger than 210 cm (Fig. 3). The relation-ship between the sex ratio and LJFL over the
range from 150 to 210 cm was given by Sex ratio = 1 x 10-4LJFL1.6601
(r2= 0.8304; n = 13, 5-cm classes).
At the Shinkang fish market, 208 female swordfish gonads were collected (Table 3). The
Table 1. Two-way analysis of variance for the effect of sampling locations of
ovaries on the (A) diameters and (B) numbers of oocytes larger than 150 µ for swordfish in Taiwanese waters
A. Effect of ovary location on oocyte diameter
Source
Two-way analysis of variance
DF Sum of squares Mean square F value Pr > F
Ovary (left or right) 1 0.0014 0.0014 0.23 0.6326
Position within ovary 2 0.0023 0.0012 0.19 0.8289
Interaction 2 0.0001 0.0000 0.01 0.9943
Error 48 0.2932 0.0061
Total 53 0.2969
Source
Two-way analysis of variance
DF Sum of squares Mean square F value Pr > F Ovary (left or right) 1 40398.6852 40398.6852 0.27 0.6036
Position within ovary 2 363.5926 181.7963 0.00 0.9988
Interaction 2 115838.4815 57919.2407 0.39 0.6780
Error 48 7096266.4444 147838.8843
Total 53 7252867.2037
B. Effect of ovary location on oocyte numbers
Table 2. Numbers of female and male swordfish
collected from Taiwanese waters and the chi-square values for a 0.5 sex ratio in each month
Sample sizes
Month ♀ ♂ Sex ratio χ2 value p value
Jan. 48 42 0.53 0.40 0.5271 Feb. 21 10 0.68 3.90 0.0482* Mar. 27 14 0.66 4.12 0.0423* Apr. 93 63 0.60 5.77 0.0163* May 88 52 0.63 9.26 0.0023** June 59 38 0.61 4.55 0.0330* July 50 32 0.61 3.95 0.0468* Aug. 46 41 0.53 0.29 0.5919 Sept. 37 30 0.55 0.73 0.3924 Oct. 23 15 0.61 1.68 0.1944 Nov. 25 22 0.53 0.19 0.6617 Dec. 34 27 0.56 0.80 0.3701 Total 551 386 0.59 28.67 < 0.01** *p < 0.05; **p < 0.01.
Fig. 2. Size frequency distribution (5-cm intervals) of swordfish
collected at the Tungkang, Nanfangao, and Shinkang fish mar-kets, from September 1997 to June 2000.
70 90 110 130 150 170 190 210 230 250 270 290
Lower jaw fork length (cm)
45 40 35 30 25 20 15 10 5 0
Frequency (no. of fish)
Female n = 551 Male n = 386
range of LJFL was 95 to 257 cm (Fig. 4). The mean monthly GI values of female swordfish fluc-tuated around a GI of 1 (Fig. 5). Except for the period from Mar. to June, mean monthly GI values were less than 1. For individual GI estimates of all female samples collected in the waters of Taiwan, only 2 (at 1.508 and 1.382, respectively) were greater than the reproductively active index, a GI of 1.375, proposed by Hinton et al. (1997).
Results of the two-way analyses of variance did not indicate significant differences in the num-bers or diameters of oocytes among the 3 loca-tions within the ovary or between the right and left
ovaries (p > 0.05; Table 1). For convenience of sampling, the posterior portion was selected for histological analysis, counts, and measurements of oocytes in this study.
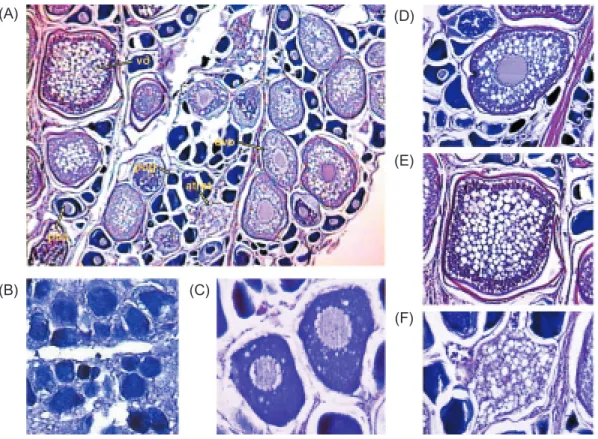
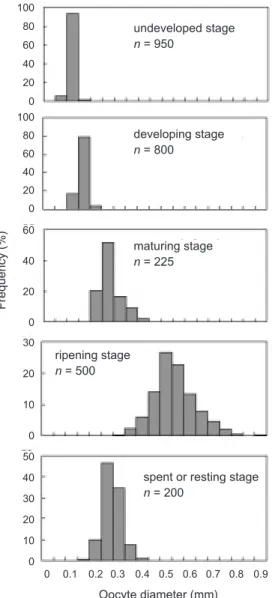
All histological sections of gonad samples were examined (Fig. 6), and the sexual maturity stages were determined based on developmental stages. Diameters of oocytes were measured for each ovarian developmental stage. Mean diame-ters were 70.4 (range, 35-122) µ for the undeve-loped stage; 117.4 (60-187) µ for the developing stage; 235.0 (157-363) µ for the maturing stage;
Fig. 4. Size frequency distribution (5-cm intervals) of swordfish
(with gonad samples) collected at the Shinkang fish market, from September 1997 to June 2000.
Fig. 3. Relationship between sex ratio and lower jaw fork
length (LJFL, 5-cm classes) for swordfish collected from the Tungkang, Nanfangao, and Shinkang fish markets, from September 1997 to June 2000.
Sex ratio
Lower jaw fork length (cm)
Table 3. Monthly numbers of female gonad samples and the length range of
swordfish collected at the Shinkang fish market of Taiwan, from July 1998 to June 2000
1998 1999 2000
Sample LJFL (cm) Sample LJFL (cm) Sample LJFL (cm)
size range size range size range Total
Jan. 3 111-127 9 139-173 12 Feb. 7 110-177 2 143-149 9 Mar. 7 137-229 4 144-167 11 Apr. 11 109-195 27 99-198 38 May 9 95-184 15 99-257 24 June 8 112-179 13 104-187 21 July 9 95-186 13 125-180 22 Aug. 20 95-230 8 117-155 28 Sept. 5 111-171 11 106-194 16 Oct. 2 108-197 3 116-182 5 Nov. 2 125-161 16 107-212 18 Dec. 2 136-165 2 154-170 4 Total 47 108 83 208
Frequency (no. of fish)
25 20 15 10 5 0 70 90 110 130 150 170 190 210 230 250 270
Lower jaw fork length (cm)
Female
511 (275-853) µ for the ripening stage; and 244.8 (142-382) µ for the spent or resting stage (Fig. 7).
The logistic regression of reproductive activity on the diameter of oocytes (OD) was
= -23.259+0.0625.OD (r2= 0.9923; n = 17, 50-µ classes).
Accordingly, 50% of oocytes were active when the oocyte diameter was about 372 µ, 90% of oocytes were active when the oocyte diameter was 407 µ, and 99% of oocytes were active when the oocyte diameter was 445 µ.
Of the total 208 female samples, 47 were designated as mature (developmental stage ≥ stage 4). The smallest mature female had a LJFL of 135 cm. Of the 47 mature females, 37 were in the stage of ripening, 10 were resting, and none was spawning or recently spawned. No hydrated oocytes were observed in any gonad samples including the 2 whose GI values were greater than 1.375.
The proportion of mature females for each
length class (5-cm intervals) was fitted to the logis-tic curve to estimate the L50:
Fig. 6. Histological sections of ovaries of swordfish (A); (B) undeveloped stage; (primitive oogonia; pog); (C) developing stage
(previtel-logenic oocytes; poc); (D) maturing stage (early vitel(previtel-logenic; evo); (E) ripening stage (vitel(previtel-logenic; vo); (F) spent or resting stage (atret-ic; atret).
Fig. 5. Monthly variations in mean gonad index of female
swordfish collected from the Shinkang fish market, from July 1998 to June 2000. The dotted line presents a GI of 1.375; the vertical bar is the standard error for each month.
Month GI (A) (B) (C) (D) (E) (F)
P = 1
1 + exp [-0.1392×(L-168.16)] (r2= 0.9863; n = 28, 5-cm classes).
The 95% confidence interval (C.I.) for the L50was 168.2 ± 1.61 cm (Fig. 8), corresponding to an age at maturity of about 5 yr (Sun et al. 2002).
DISCUSSION
Estimates of sex ratios for swordfish in previ-ous studies were higher than that of this study, except for DeMartini et al. (2000). The sex ratio of swordfish caught by the Hawaii-based longline
fishery was 0.53 (DeMartini et al. 2000). The ratio of females to males was 2.94: 1 (for a sex ratio of 0.75) for swordfish caught off the coast of southern California (Weber and Goldberg 1986); 2.3: 1 (for a sex ratio of 0.70) for swordfish caught by the Canadian fishery in the western North Atlantic (Stone and Porter 1997); and 2.25: 1 (for a sex ratio of 0.69) for swordfish in the eastern Australian AFZ (Australian Fishing Zone) (Young et al. 2000). In this study, 65% of samples had a LJFL of less than 170 cm, and the sex ratio was 1.06: 1 (312 females and 294 males; for a sex ratio of 0.51). According to the relationship between the sex ratio and length in this study, the sex ratio fluc-tuated around 0.5 for lengths less than 170 cm LJFL (Fig. 3) and did not significantly differ from 1 : 1 (χ2 = 0.53; p = 0.46). Since most samples of swordfish caught in the waters of Taiwan had LJFLs of less than 170 cm (Fig. 2), it is not surpris-ing that the sex ratio of swordfish in the waters of Taiwan was close to 0.5.
The relationship between the sex ratio and body size can provide effective information to reconstruct the sex composition from catch data. Similar results were also reported in other studies (Suzuki and Miyabe 1990, Arocha and Lee 1996, Stone and Porter 1997, DeMartini et al. 2000). Stone and Porter (1997) used a linear regression function to describe the trend in the sex ratio vs. length for swordfish caught by the Canadian fish-ery in the western North Atlantic. DeMartini et al. (2000) modeled the relationship for swordfish caught by the Hawaii-based longline fishery by a power function, and suggested that the influence of different gear types or catchability on estimates
Fig. 8. Relationship between mature percentage and lower jaw
fork length (5-cm classes) for female swordfish in the waters of Taiwan.
Fig. 7. Frequency distribution of oocyte diameter in different
mature stages for female swordfish in the waters of Taiwan. undeveloped stage n = 950 developing stage n = 800 maturing stage n = 225
spent or resting stage n = 200 ripening stage n = 500 100 80 60 40 20 0 100 80 60 40 20 0 60 40 20 0 30 20 10 0 50 40 30 20 10 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 Oocyte diameter (mm) Frequency (%) P = 1 1 + exp [-0.1392×(L-168.16)] r2= 0.9863 0 50 100 150 200 250 300
Lower jaw fork length (cm)
100 90 80 70 60 50 40 30 20 10 0 Percent of maturity
of the sex ratio should be considered. However, catches of swordfish in the waters of Taiwan were almost entirely made by longline fisheries. Therefore, the relationship between the sex ratio and length estimated in this study should be useful in establishing the swordfish sex composition from the swordfish caught in the waters of Taiwan.
The estimate of length or age at L50 is an important parameter for fish stock assessments. In this study, the L50for female swordfish caught in the waters of Taiwan was estimated based on
his-tological analysis. The estimate of length at sexual maturity has been reported by other methods in previous reproductive research on swordfish (Table 4). Yabe et al. (1959) estimated the body size at sexual maturity to be 150-170 cm for EFL (or 168-189 cm for LJFL according to the relation-ship between LJFL and EFL in Sun et al. (2002)) for female swordfish in the western Pacific Ocean by analyzing the relationship between gonad weight and body length. Kume and Joseph (1969) assumed that eastern Pacific female swordfish
Body size at sexual maturity (LJFL, cm)
♀ ♂
Table 4. Estimates of body size at sexual maturity reported by various research on the reproductive biology
of swordfish
Investigator(s)
Area Analytical method
Western Pacific Ocean Yabe et al. (1959) Length-ovary weight relation 168-189a
-Eastern Pacific Ocean Kume and Joseph (1969) GI analysis 189a
-North Pacific Sosa-Nishizaki (1990) GI analysis 178a
-Straits of Florida and Taylor and Murphy (1992) Histological analysis 182 112
adjacent waters
Mediterranean Sea de la Serna et al. (1996) GI and histological analysis 142
-Western Atlantic Ocean Arocha and Lee (1996) Observation of ovary with yolked or 179 129 hydrated oocyte and testis with milt
Central North Pacific Ocean DeMartini et al. (2000) Histological analysis 161.8 117.3
Waters east of Taiwan This study Histological analysis 168.2 -b
aEFL in the original paper was converted into LJFL using the relationship between LJFL and EFL (Sun et al. 2002). bMaturities of male samples were determined in this study, but the L
50of males could not be estimated because of insufficient speci-mens.
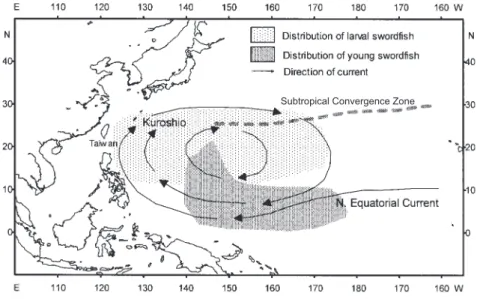
Fig. 9. The distribution of larval and young swordfish, Subtropical Convergence Zone, and currents in the North Pacific Ocean. The
distributions of larval and young swordfish were described by Yabe et al. (1959) and Nishikawa and Ueyanagi (1974).
were about to spawn when the gonad index was equal to or greater than 3, and the body length of EFL was about 170 cm (about 189 cm for LJFL). Sosa-Nishizaki,s (1990) estimate of length at sexu-al maturity at about 160 cm for EFL (about 178 cm LJFL) for“most individuals”was also inferred from a length-based GI (DeMartini et al. 2000). In the Straits of Florida and adjacent waters, Taylor and Murphy (1992) using histological evaluations estimated an L50of 182 cm for the LJFL of females and 112 cm for the LJFL of males. In the Mediterranean Sea, de la Serna et al. (1996) esti-mated an L50 of 142 cm for the LJFL of female swordfish by analyzing gonad indices by length and class and validated it with a histological method. In the western Atlantic Ocean, Arocha and Lee (1996) estimated that the L50was 179 cm for the LJFL of females and 129 cm for the LJFL of males based on the observation of ovaries with yolked or hydrated oocytes for females and testes with milt for males. DeMartini et al. (2000) estimat-ed the L50 values for female and male swordfish caught by the Hawaii-based longline fishery based on histological information; their estimates were 143.6 cm for the EFL of females and 102.0 cm for the EFL of males (about 161.8 cm for the LJFL of females and 117.3 cm for the LJFL of males). The estimated length at sexual maturity for female swordfish in this study was similar to those in the western and central Pacific Ocean but differed from those in other areas. The difference between the Pacific and the Atlantic might be a result of geographical isolation and stock structuring (Chow et al. 1997, Reeb et al. 2000). Among regions of the Pacific, the estimated variation in the length at sexual maturity might instead reflect different envi-ronmental conditions.
Histological analysis of the developmental stages of oocytes is the most accurate method of determining sexual maturity (West 1990), but the preparation of histological sections is expensive and time-consuming. An alternative method to determine sexual maturity or reproductive activity is to estimate the GI. However, the GI is often not a reliable measure for distinguishing between mature but reproductively inactive females and immature females during nonspawning seasons (West 1990, Mejuto and Garcia 1997). In this study, 35 of the total of 37 ripening samples (stage 4) with a GI below the reproductively active index (a GI of 1.375) estimated by Hinton et al. (1997) were determined to be active based on histological observation. Thus, the reproductively active index for swordfish captured in the Straits of Florida
(Hinton et al. 1997) is not completely suitable for swordfish in the waters of Taiwan. In addition to the histological analysis and GI, oocyte diameter was used as a criterion for reproductive activity. In order to determine reproductive activity, female samples were classified using the criterion of an oocyte diameter of 372.1 µ (p = 50%). Similar results were obtained using histological observa-tion and the criterion of oocyte diameter. Only one of the 37 ripening samples was determined to be inactive. Therefore, the“reproductively active oocyte diameter”predicted in this study should be more accurate than the reproductively active GI estimated by Hinton et al. (1997) for determining the reproductive activity for swordfish in the waters of Taiwan.
According to results of the histological evalua-tion of ovaries in this study, developmental stages for mature samples belonged to the ripening and resting stages, and no hydrated oocytes were observed among our samples. This observation indicates that swordfish do not spawn in the waters of Taiwan. It is unlikely that spawning swordfish are less vulnerable to longline fishing gear in Taiwan,s waters because they are regularly caught in other regions. Yabe et al. (1959) inferred that swordfish spawn throughout the wide area of the southern waters of the Subtropical Convergence Zone in the North Pacific Ocean (Fig. 9), based on information gained from the collection of ripe ovaries, larvae, and juveniles. Larvae of swordfish occur in waters with sea surface temperatures (SSTs) higher than 24
°
C (Nishikawa and Ueyanagi 1974). Historical SSTs (IGOSS 2001) in the spawning area of swordfish evaluated by Yabe et al. (1959) were always higher than this limiting temperature of 24°
C. In addition, observations that larval swordfish are widely distributed over the southern waters of the Subtropical Convergence Zone and are more concentrated around tropical areas (Nishikawa and Ueyanagi 1974) support this theory of the spawning area of swordfish in the North Pacific Ocean. Therefore, we hypothesize an eastward migration by mature swordfish in the waters of Taiwan to the southern waters of the Subtropical Convergence Zone during the spawn-ing season. Since larval swordfish are relatively abundant within the Subtropical Convergence Zone and juvenile swordfish are distributed near the tropics (Yabe et al. 1959, Nishikawa and Ueyanagi 1974), sea surface currents (Gross1993, Lalli and Parsons 1993) might transport larval swordfish southwards in the North Pacific (Fig. 9). Yabe et al. (1959) implied that pre-young swordfish(with body lengths of 100-300 mm) are distributed to the south of 30
°
N and move northward to high-er latitudes afthigh-er the post-young stage (with body lengths of 300-600 mm). Migration patterns of adult swordfish in the North Pacific Ocean are not well understood. It seems that swordfish move seasonally from the western North Pacific in the summer to the eastern North Pacific in autumn and winter (Sosa-Nishizaki and Shimizu 1991). How-ever, additional research, including tagging experi-ments, is necessary to further test these hypothe-ses of the migratory routes of swordfish. Regard-less, it is clear that swordfish do not spawn to any great extent in Taiwanese waters. Therefore inter-national cooperative research and management are necessary to maintain optimal harvest levels.Acknowledgments: We thank Dr. Kurt M.
Schaefer of the Inter-American Tropical Tuna Commission, Dr. Nancy C. H. Lo of the Southwest Fisheries Science Center, National Marine Fish-eries Service, and Mr. Peter Ward of the Bureau of Rural Sciences, Department of Agriculture Fisheries and Forestry, Australia, who reviewed the manuscript. We also thank the anonymous referees for their valuable comments. This study was partially financially supported by the Fisheries Administration, Council of Agriculture, Taiwan, through grant 89-AST-1.2-FID-02(07) to Chi-Lu Sun.
REFERENCES
Arocha F, DW Lee. 1996. Maturity at size, reproductive sea-sonality, spawning frequency, fecundity and sex ratio in the swordfish from the Northwest Atlantic. Int. Comm. Conserv. Atl. Tunas Coll. Vol. Sci. Pap 45: 350-357. Chow S, H Okamoto, Y Uozumi, Y Takeuchi. 1997. Genetic
stock structure of the swordfish (Xiphias gladius) inferred by PCR-RFLP analysis of the mitochondrial DNA control region. Mar. Biol. 127: 359-367.
de la Serna JM, JM Ortiz de Urbina, D Macias. 1996. Observations on sex-ratio, maturity and fecundity by length-class for swordfish (Xiphias gladius) captured with surface longline in the Western Mediterranean. Int. Comm. Conserv. Atl Tunas Coll. Vol. Sci. Pap 45: 115-139.
DeMartini EE, JH Uchiyama, HA Williams. 2000. Sexual matu-rity, sex ratio, and size composition of swordfish, Xiphias gladius, caught by the Hawaii-based pelagic longline fish-ery. Fish. Bull. 98: 489-506.
Deriso RB, TJ Quinn II, PR Neal. 1985. Catch-age analysis with auxiliary information. Can. J. Fish. Aquat. Sci. 42: 815-824.
Foale S, R Day. 1997. Stock assessment of trochus (Trochus niloticus) (Gastropoda: Trochidae) fisheries at West
Nggela, Solomon Islands. Fish. Res. 33: 1-16.
Gabriel WJ, MP Sissenwine, WJ Overholtz. 1989. Analysis of spawning stock biomass per recruit: an example for Geor-ges Bank haddock. N. Am. J. Fish. Mgmt. 9: 383-391. Gross MG. 1993. Currents. In MG Gross, ed. Oceanography,
a view of earth. 6th ed. Englewood Cliffs, NJ: Prentice-Hall Press, pp. 159-185.
Hinton MG, RG Taylor, MD Murphy. 1997. Use of gonad indices to estimate the status of reproductive activity of female swordfish, Xiphias gladius: a validated classifica-tion method. Fish. Bull. 95: 80-84.
Hunter JR, BJ Macewicz. 1985. Measurement of spawning fre-quency in multiple spawning fishes. In R Lasker, ed. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. US Dept. Comm., NOAA Tech. Rep. NMFS 36, pp. 79-94.
IGOSS. 2001. Integrated Global Ocean Services System Pro-ducts Bulletin. (available at http://ingrid.ldeo.columbia. edu/SOURCES/IGOSS/)
Lalli CM, TR Parsons. 1993. Surface currents. In CM Lalli, TR Parsons, eds. Biological oceanography: an introduction. Oxford, New York, Seoul, Tokyo: Pergamon Press, pp. 40-42.
King M. 1995. Reproduction and recruitment. In M King, ed. Fisheries biology, assessment and management. Oxford, UK: Fishing News Books Press, pp. 151-165.
Kume S, J Joseph. 1969. Size composition and sexual maturi-ty of billfish caught by the Japanese longline fishery in the Pacific Ocean east of 130
°
W. Bull. Far Seas Fish. Res. Lab. 2: 115-162.Mejuto J, B Garcia. 1997. A preliminary analysis of gonadal indices of the swordfish (Xiphias gladius L.) in the Atlantic Ocean. Int. Comm. Conserv. Atl.Tunas Coll. Vol. Sci. Pap. 46: 336-344.
Murphy MD, RG Taylor. 1990. Reproduction, growth, and mor-tality of red drum Sciaenops ocellatus in Florida waters. Fish. Bull. 88: 531-542.
Nakamura I. 1985. FAO species catalogue. Vol. 5. Billfishes of the world. An annotated and illustrated catalogue of marlins, sailfishes, spearfishes and swordfishes known to date. FAO Fish. Synop., No. 125, Vol. 5. FAO, Rome. Nishikawa T, S Ueyanagi. 1974. The distribution of the larvae
of swordfish, Xiphias gladius, in the Indian and Pacific Oceans. In RS Shomura, F Williams, eds. Proceedings of the International Billfish Symposium, part 2. Review and contributed papers. US Dept. Comm., NOAA Tech. Rep. NMFS SSRF-675. U.S. Government Printing Office, Washington, D.C., pp. 261-264.
Quinn II TJ, R Fagen, J Zheng. 1990. Threshold management policies for exploited populations. Can. J. Fish. Aquat. Sci. 47: 2016-2029.
Reeb CA, L Arcangeli, BA Block. 2000. Structure and migra-tion corridors in Pacific populamigra-tions of the swordfish Xiphias gladius, as inferred through analyses of mitochon-drial DNA. Mar. Biol. 136: 1123-1131.
SAS Institute. 1990. SAS/STAT user,s guide, vers. 6. 4th ed. Cary, NC: SAS Institute, pp. 1135-1194.
Sosa-Nishizaki O, M Shimizu. 1991. Spatial and temporal CPUE trends and stock unit inferred from them for the Pacific swordfish caught by the Japanese tuna longline fishery. Bull. Nat. Res. Inst. Far Seas Fish. 28: 75-89. Stone HH, JM Porter. 1997. Development of a swordfish
sex-ratio-at-size relationship for catches from the Canadian fishery. Int. Comm. Conserv. Atl. Tunas Coll. Vol. Sci.
Pap. 46: 311-314.
Sun CL, SP Wang, SZ Yeh. 2002. Age and growth of the swordfish (Xiphias gladius L.) in the waters around Taiwan determined from anal-fin rays. Fish. Bull. 100: 822-835. Suzuki Z, N Miyabe. 1990. Heterogeneous sex ratio of Atlantic
swordfish and the implication to cohort analysis. Int. Comm. Conserv. Atl. Tunas Coll. Vol. Sci. Pap. 32: 377-386.
Taylor RG, MD Murphy. 1992. Reproductive biology of the swordfish Xiphias gladius in the Straits of Florida and adjacent waters. Fish. Bull. 90: 809-816.
Uchiyama JH, RS Shomura. 1974. Maturation and fecundity of swordfish, Xiphias gladius, from Hawaiian waters. In RS Shomura, F Williams, eds. Proceedings of the Inter-national Billfish Symposium, part 2. Review and con-tributed papers. US Dept. Comm., NOAA Tech. Rep. NMFS SSRF-675. U.S. Government Printing Office, Washington, D.C., pp. 142-148.
Uosaki K, WH Bayliff. 1999. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1988-1992. Inter-Am. Trop. Tuna Comm. Bull.
21: 275-488.
Wallace RA, K Selman. 1981. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 21: 325-345. Weber EC, SR Goldberg. 1986. The sex ratio and gonad
indices of swordfish, Xiphias gladius, caught off the coast of southern California in 1978. Fish. Bull. 84: 185-186. West G. 1990. Method of assessing ovarian development in
fishes: a review. Aust. J. Mar. Freshwater Res. 41: 199-222.
Winters GH. 1970. Biological changes in coastal capelin from the over-wintering to the spawning condition. J. Fish. Res. Board Can. 27: 2215-2224.
Yabe H, S Ueyanagi, S Kikawa, H Watanabe. 1959. Study on the life-history of the swordfish, Xiphias gladius Linnaeus. Rep. Nankai Reg. Fish. Res. Lab. 10: 107-150.
Young J, A Drake, T Carter, J Farley. 2000. Reproductive dynamics of broadbill swordfish (Xiphias gladius) in the eastern Australian AFZ--preliminary results. 13th Meeting of the Standing Committee on Tuna and Billfish, working paper, BBRG-12. July 5-12, 2000. Noumea, New Cale-donia. Oceanic Fisheries Programme, Secretariat of the Pacific Committee, Noumea, New Caledonia.