Journal of Colloid and Interface Science 232, 207–209 (2000)
doi:10.1006/jcis.2000.7180, available online at http://www.idealibrary.com on
NOTE
Adsorption Equilibrium of Polyethylene Glycol in the Copper
Electroplating Solution on Activated Carbon
Polyethylene glycol (PEG) used as a brightening and
stabiliza-tion agent at the concentrastabiliza-tion of 30 mg dm
−3is a major organic
additive in the copper electroplating solution. Activated carbon,
Calgon Filtrasob 400, is used as the adsorbent to remove the PEG
from the used electroplating solution in order to broaden the
ap-peal of recycling it. The equilibrium of adsorption is attained within
14 days. The effect of the temperature on the amount of PEG
ad-sorbed on the activated carbon is insignificant for the temperatures
ranged from 288 to 313 K. The adsorption isotherm of PEG
con-forms to the Langmuir isotherm, q
e= Q
LK
LC
e/(1
+ K
LC
e), with a
high correlation coefficient of 0.9979. The large values of the
mono-layer adsorption capacity, Q
L, of 303 mg g
−1and the equilibrium
constant, K
L, of 0.273 dm
3mg
−1show a great adsorption potential
of PEG on the activated carbon. A high removal efficiency would
be expected at such a low original concentration of PEG. From the
results mentioned above, it is feasible to use activated carbon for
removing PEG from the electroplating solution, thereby achieving
the appeal of recycling.
°C2000 Academic PressKey Words: adsorption; polyethylene glycol; activated carbon;
electroplating solution; recycling.
INTRODUCTION
Polyethylene glycol (PEG) is widely applied in many industries, such as metal-forming, cosmetics, food, and polishing. It is a neutral surfactant and may be used as a brightening additive in copper electroplating solution. Activated carbon was employed to successfully remove the organic impurities from the electroplating solution and effectively remove certain essential components in the aqueous solution (1). Adsorption of PEG with different molecular weights (MWs) in aqueous solution on activated carbon reported by Zhao et al. (2), indicated large adsorption capacities of PEG on the activated carbon. The Freundlich isotherm, qe= kFCe1/n, constants kF(adsoption capacity, [mg g−1/(mg dm−3)1/n]) and n (heterogeneity factor) of PEG reported by Zhao et al. (2), were 10 and 2.2 for MW of 300 and 100, 6.6 for MW of 600 and 110, 6.7 for MW of 1000 and 150, 8.2 for MW of 2000 and 210, 13.4 for MW of 20,000 and 210, and 13.4 for MW of 200,000. However, the results of the above-mentioned investigations were not applicable for the removal of PEG from the electroplating solution by activated carbon adsorption. The electroplating solution is normally a complex mixture, including the salts of metal ions being plated, electrolytes, and various organic additives (3). The organic additives are added in relatively low concen-tration in order to modify the morphology, structure, and properties of deposit, but the brighteners are commonly used in relatively high concentration as com-pared to other organics. After electroplating, the wasted electroplating solution still contains valuable metal ions of high concentration and salts, while ineffec-tive organic addiineffec-tives of low concentration. The traditional treatment of wasted
electroplating solution is to form metal flocs by coagulation with chemical co-agulants and then the flocs are precipitated so as to match the effluent standard. However, the coagulation–precipitation treatment makes the reuse of effluent as electroplating solution impossible. In order to reduce raw materials, industrial wastewater, manufacturing costs, and regulation compliance, the activated car-bon may be used to adsorb the organic additives from the used electroplating solution with subsequent addition of new organic additives to regenerate the solution as a useful electroplating solution.
This paper presents the adsorption equilibrium of PEG, which has the greatest molecular weight among the organic additives in the electroplating solution (4). In addition, considering the application of the isotherm in the practical treatments, this study also examines the impact of temperature changes due to the season variation. The purpose of this paper is to investigate the feasibility of using the activated carbon to remove the organic additives from the electroplating bath to broaden the appeal of recycling it. The obtained parameters of the isotherm are useful for the proper design of adsorption treatment systems.
MATERIALS AND METHODS
Materials
An activated carbon (Calgon, F-400) of mesh 12 to 40 is used as the adsorbent and washed by distilled water to remove the crushed carbon. It is dried at 383 K in a vacuum oven (CKN-20, Cherng Huei Co. Ltd., Taiwan) for at least 24 h before testing. The physical properties of F-400 determined by nitrogen gas adsorption at 77 K, using an automatic adsorption instrument, namely ASAP 2000, are as follows: BET surface area of 1026 m2/g, total pore volume of 0.61 cm3/g, average pore diameter of 17.65 ˚A. The average true density analyzed by AccuPyc 1330 VI. 01., is 2.18 g/cm3. It is noted that the BET specific surface area determined by the adsorption of nitrogen may not equal to the actual area occupied by the large molecules of PEG. However, nitrogen is a standard adsorbate commonly used to measure the specific surface area of adsorbent, and a larger BET surface area of adsorbent indicates a high adsorption potential. Thus, this study employs the standard methods for obtaining the physical properties of the activated carbon related to adsorption. The chemical properties of activated carbon, F-400, may be found in the work of Chiang et al. (5), which reported the following: ash of 7.25 wt%, functional groups (in m eq. /100 g) of carboxyl, lactone, phenolic, and carbonyl acidic types of 6, 4, 2, 12, and of basic type of 50, and pH 8.32. The major factors for characterizing the adsorptivity of carbon adsorbents include surface area, nonpolarity, pore size distribution, and surface oxide groups.
The inorganic compositions of the electroplating solution include H2SO4 of 60 g dm−3, CuSO4·5H2O of 200 g dm−3, and HCl of 30 mg dm−3. The only organic component in the electroplating solution is PEG (Merck, Catalog No. 807491), of which molecular weight (MW) is with average value of 6000, ranging from 5000 to 7000. The pH value of the electroplating solution is about 0.25. The recipe is according to that of Fang (4).
Methods
For attaining the adsorption equilibrium, bath solutions of 0.1 dm3with con-centrations ranged from 30 to 1000 mg dm−3of PEG are mixed with 0.05 g of
207 0021-9797/00 $35.00
Copyright°C2000 by Academic Press All rights of reproduction in any form reserved.
208
NOTE adsorbent and shaken for 14 days at 288, 298, 303, 313 K, respectively. Then the solution is filtrated through a 0.45-µm membrane filter. The concentration of filtrate is determined by a TOC analyzer (O.I.C. M-700). The correlation of TOC with PEG is that 0.6 mg dm−3 TOC is equivalent to 1 mg dm−3 PEG. Since it is preferable to use the weight concentration units for investigating the removal efficiency in wastewater treatments, the units of g dm−3and mg g−1 for the liquid and solid phases instead of0 (i. e., the excess number of moles of polymer per unit area of the interface) are used in this study.RESULTS AND DISCUSSION
Adsorption Equilibrium
The time variations of the amount of PEG adsorbed on the activated carbon (q) at 298 K are shown in Fig. 1. The purpose of Fig. 1 is to elucidate the times needed for cases with different initial concentrations to reach adsorption equilibrium. The tests of other temperatures indicate the same results although they are not shown in Fig. 1. The value of q does not change more than 2% after 12 days, especially for the case of the low initial concentration (Co). As a result, all samples are taken on the 14th day for determining adsorption isotherm. The finding that the time for reaching equilibrium for the case with low Cois less than that with high Cois of interest. This implies that the adsorption rate with a low Cois fast. It is noted that PEG, of which the molecular weights range from 5000 to 7000, belongs to polydisperse system. The time to reach equilibrium for the polydisperse system might be considerably longer than that for a similar monodisperse system. It is due to the gradual displacement of lower molecular fractions by those of higher molecular weight (6). If the monopolymer is used in this system, it would reduce the requirement of time to reach equilibrium.
Effect of Temperature
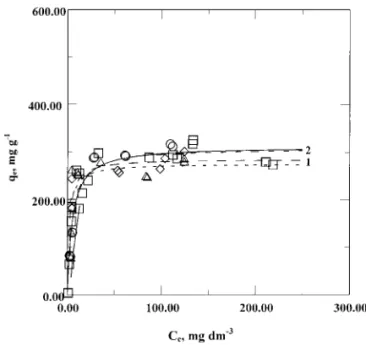
As is widely agreed, the adsorption is a spontaneous exothermic process (7), indicating that the amount of PEG adsorbed on the activated carbon is supposed to increase with the decrease of temperature. From the previous reference (4), the electroplating temperature is usually at 298 K to obtain the best product. With the consideration of the effect of seasons, four temperatures are conducted for the experiments. The Langmuir isotherms and experimental data are shown in Fig. 2. The results indicate that the amount of PEG adsorbed on the activated carbon is not significantly influenced by temperatures in the range of 288 to 313 K. Thus, for the practical operation of the wastewater treatment in 288 to
FIG. 1. Changes of amount of PEG adsorbed by activated carbon F-400 (q) in electroplating solution with time (t ).e; Experimental data of 298 K at Co= 450 (H) and 35 (L) mg dm−3. Co, initial concentration of PEG.
FIG. 2. Adsorption of PEG in electroplating solution on activated carbon F-400 at different temperatures. 1· · ·, ----, – -, 2 —: simulations of Langmuir isotherms at different temperatures of 288, 298, 303, and 313 K;n, e, h, s, experimental data of 288, 298, 303, and 313 K , respectively. qe, Ce, adsorbate concentrations in solid and liquid phases at equilibrium, respectively.
313 K (e.g., without constant temperature control when treating the wastewater), it is safe to ignore the effect of temperature and use the parameters obtained from 298 K to design the treatment systems. However, for engineering consideration, the experimental data of all four temperatures in the range of 288 to 313 K are treated as a whole set of 298 K as discussed below.
Adsorption Isotherm
Over the past decades, a considerable number of studies have been made on the polymer adsorption theory, providing a description of the polymer concentration and possible conformation in the interfacial region (8). Although, in view of the practical application in the wastewater treatment and of the convenient prediction of the amount of the activated carbon needed to meet the requirement removal efficiency, the Langmuir isotherm is usually the first choice for testing its validity, several isotherms (Langmuir, Freundlich, Redlich-Peterson, Temkin) have been tested and compared with their applicabilities in this study. As discussed below, the Langmuir isotherm gives the best fit and is thus presented hereafter.
The Langmuir isotherm is as follows:
qe= QLKLCe
1+ KLCe, [1]
where, qeand Ceare the adsorbate concentrations in solid and liquid phases at equilibrium, respectively. QLand KLare Langmuir isotherm constants. QL represents the monolayer adsorption capacity. The Langmuir isotherm is appli-cable to represent the homogeneous adsorption. It assumes that the adsorption takes place on an energetically uniform surface and is valid only to monolayer. To obtain an explict relation between the equilibrium concentration (Ce) and the amount of PEG adsorbed on activated carbon (qe), Eq. [1] is rearranged:
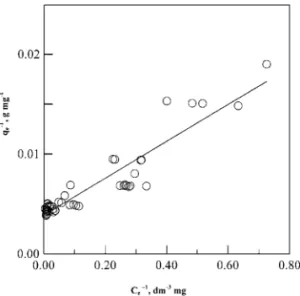
1 qe = 1 QLKL 1 Ce + 1 QL. [2]
According to Eq. [2], a plot of 1/qevs 1/Ceis shown in Fig. 3, which is fitted by the least-square regression analysis. The values of QL, KL, and correlation coefficient (r2) are 303 mg g−1, 0.273 dm3mg−1, and 0.9979, respectively. The good applicability of Langmuir isotherm to experimental data indicates that the adsorption of PEG on the microporous activated carbon, Calgon F-400, might be
NOTE
209
FIG. 3. Langmuir isotherm (-) of PEG in electroplating solution on activated carbon, F-400.s, Experimental data of all four temperatures of 288, 298, 303, and 313 K. qe, Ce, same as in Fig. 2.
limited to monolayer. The high adsorption capacity, QL, of 303 mg g−1shows a great adsorption potential of PEG on the activated carbon. The concentration of PEG in the electroplating solution is usually at 30 mg dm−3 (4), and the concentration after treatment would locate on the high adsorption region of the isotherm (i.e., on the steep profile as seen in Fig. 2). It means that a good removal efficiency of PEG would be expected when using the activated carbon as the adsorbent. In addition, such a low original concentration would reduce the demand of time for reaching equilibrium when using the batch treatment system as indicated in Fig. 1. It is interesting to note that the molecular weight (MW) of adsorbate PEG is as high as 5000 to 7000. The usual description of conformations of the adsorbate at an adsorbing interface is in terms of three types of subchains: trains, which have all their segments in contact with the adsorbent; loops, which have no contacts with the surface but connect two trains; and tails which are unadsorbed chain ends (8). The feasible adsorption of PEG on the activated carbon (as illustrated in Figs. 2 and 3) with the average pore diameter as small as 17.65 ˚A indicates that the molecule with high MW may enter the pore with its front of smallest size facing the pore. As a result, the unadsorbed end of PEG would also enter the pores to contact with the pore surfaces and be adsorbed on the surface of the pores. Similarly feasible adsorption of PEG of MW as high as 200,000 from aqueous solution on montmorillonite clays has been also reported by Zhao et al. (2).
Hydrophobic attraction contributes the adsorption of nonpolar segments of PEG polymer chains on the hydrophobic surfaces of activated carbon. Such an attraction is of long range for polymer adsorption. At a very low coverage, the adsorbed PEG is like an isolated one and the interaction between the adsorbed PEG could be ignored. As the coverage increases, the lateral interaction between the adsorbed polymer molecules becomes significant (6). Further, the configu-ration of the adsorbed polymer may vary and may become the types of longer loops and tails (6). Thus, referring to the work of Vincent (6), the interaction of the adsorbed PEG may exist and become more important with increasing cover-age resulting in smaller area per molecule ( Am), a lower fraction of segments in trains (Fmt), and a greater thickness of the adsorbed polymer (δ). In the plateau region of the isotherm of Fig. 2, further adsorption is precluded. Here, Amand Fmtattain their minimum values, andδ its maximum value (6). To clarify this, the Temkin isotherm, which considers the effect of indirect adsorbate/adsorbate interaction on adsorption, is tested. The Temkin isotherm is
qe= RT
bT ln( ATCe) [3]
where, R is the gaseous constant, T is the absolute temperature in K, ATand bT are the constants for the Temkin isotherm. The values of isotherm parameters,
AT, bT, and r2are 12.79 dm mg−1, 60.84 J mol−1g mg−1, and 0.8594, respec-tively. The reasonable validity of Temkin isotherm thus indicates the interaction of the adsorbed PEG.
CONCLUSIONS
Using Langmuir isotherm to describe the adsorption of PEG from the elec-troplating solution on the activated carbon, F-400, shows a good applicability with high correlation coefficient. The amount of PEG adsorbed on the activated carbon is not significantly affected by the changes of temperatures ranged from 288 to 313 K. The high adsorption capacity, QL, of 303 mg g−1shows a great adsorption potential of PEG on the activated carbon. In addition, a good removal efficiency of PEG would be expected under such a low original concentration of PEG of 30 mg dm−3. As a result, it is feasible to use the activated carbon for the removal of PEG from the electroplating solution, achieving the appeal of recycling. The presence of other organics, metals, and inorganics in the waste streams might compete with PEG and affect the adsorption of PEG from the electroplating solution on the activated carbons. Further efforts would be useful in order to elucidate their impacts.
ACKNOWLEDGMENTS
The authors thank Professors S. L. Lo and S. C. Wu of Graduate Institute of Environmental Engineering of National Taiwan University (NTU) for their kind-ness of providing some experimental equipments, the National Science Council of Taiwan for the financial support under Grant NSC 89-2211-E-002-025, and the Powder Technology Laboratory of Chemical Engineering Department of NTU for the assistance in powder characterization.
REFERENCES
1. Smisek, M., and Cerny, S., “Activated Carbon.” Elsevier, New York, 1970. 2. Zhao, X., Urano, K., and Ogasawara, S., Colloid Polym. Sci. 267, 899 (1989). 3. Pletcher, D., “Industrial Electrochemistry.” Chapman & Hall, New York,
1982.
4. Fang, C. L., “General Concepts of Additives in the Electroplating Solution.” Finishing Science Pub., Taipei, Taiwan, 1996.
5. Chiang, Y. C., Chiang, P. C., and Chang, E. E., J. Environ. Sci. Health, A33(7), 1437 (1998).
6. Vincent, B., in “Chemistry and Technology of Water-Soluble Polymers” (C. A. Finch, Ed.), p. 215. Plenum Press, New York, 1983.
7. Jankowska, H., Swiatkowski, A., and Choma, J., “Active Carbon.” Ellis Horwood, New York, 1991.
8. Fleer, G. J., Cohen Stuart, M. A., Scheutjens, J. M. H. M., Cosgrove, T., and Vincent, B., “Polymer at Interfaces.” Chapman and Hall, London, 1993.
Chiung-Fen Chang∗ Ching-Yuan Chang∗,1 Wen-Tien Tsai† Shian-Chee Wu∗
∗Graduate Institute of Environmental Engineering
National Taiwan University Taipei 106, Taiwan
†Department of Environmental Engineering and Health
Chia Nan College of Pharmacy and Science Tainan 717, Taiwan
Received October 29, 1999; accepted August 28, 2000
1To whom correspondence should be addressed. E-mail: cychang3@ccms. ntu.edu.tw.