Processing Technique and Property Evaluations of Chitosan Dressings
Ching-Wen Lou
1, b, Zong-Han Wu
2and Jia-Horng Lin
2, 3, 4,a1Institute of Biomedical Engineering and Materials Science, Central Taiwan University of Science
and Technology, Taichung 40601, Taiwan, R.O.C.
2Laboratory of Fiber Application and Manufacturing, Department of Fiber and Composite Materials,
Feng Chia University, Taichung City 40724, Taiwan, R.O.C.
3School of Chinese Medicine, China Medical University, Taichung 40402, Taiwan, R.O.C. 4Department of Fashion Design, Asia University, Taichung 41354, Taiwan, R.O.C.
ajhlin@fcu.edu.tw, bcwlou@ctust.edu.tw
Keywords: chitosan, freeze-dried, dressings.
Abstract. This study uses acetic acid as the solvent to make two different deacetylated chitosans
into chitosan solutions (CS1 and CS2). A specified amount of chitosan solutions are evaporated at
55 ℃ for 30, 60, and 90 minutes, immersed in NaOH-NaHCO3(aq), and then freeze-dried to form
six membrane types, CS1-30, CS1-60, CS1-90 ,CS2-30, CS2-60 and CS2-90. Scanning electron microscopic (SEM) observation, a swelling ratio test, and an antibacterial assay are performed to evaluate the influence of differing evaporation times on the CS membranes. The test results show that membrane CS1 types have good properties. In particular, CS1-60 possesses an optimal swelling property and a maximum inhibition zone.
Introduction
Skin is an important physical barrier, which protects the human body from harm by external objects. When skin is damaged, it has to be taken care of properly. Clinical treatment for wounds often uses wound dressings for protection and healing, including hemostasis in first aid, bacterial infection, and wound healing acceleration [1]. Chitosan is a natural polymer that is commonly used to heal wounds. It is derived from chitin that exists in fungal cell walls and crustacean exoskeletons, and is relatively inexpensive and abundant. Moreover, chitosan has been proven to be biodegradable, biocompatible, non-antigenic, non-toxic, bioadhesive, antimicrobial, and bioactive. It also possesses hemostatic capacity [2-5]. In this study, chitosan is dissolved in acetic acid, from
which Chitosan-NH2 group can obtain -H+ to form -NH3+. Then, when chitosan contacts
NaOH-NaCO3 (aq), H+ of Chitosan-NH3+ is replaced and then reduced as -NH2, thereby yielding gel chitosan membranes [6-7]. This process results in inter-crosslinked chitosan polymer and thus a polymer network structure; however, this process does not cause chitosan to lose its intrinsic property. Finally, the gel chitosan membranes are freeze-dried to form porous chitosan membranes. Used as dressings, the chitosan membranes have a dense surface to protect the wounds and prevent bacterial penetration, and a porous interior to absorb exudate and blood from wounds. Cross-sections of the membranes are observed with an SEM for their porosity, and membranes are evaluated with a water absorption test to determine their swelling ratio. Finally, the influence of varying evaporation times on their anti-bacterial ability is examined.
Experimental Materials
CS1 (C3646, Sigma Chemical Co., USA) is 88% deacetylated. CS2 (Global Technology company, Taiwan, R.O.C.) is 85% deacetylated. Sodium hydroxide (NaOH) is purchased from
from Shimakyu's Pure Chemical Co. Ltd, Japan.
Preparation of Wound Dressing
Chitosan membranes are prepared by immersion precipitation phase inversion using a casting process [6-7]. Acetic acid aqueous solution (1 wt%) is used as a solvent and NaOH (2
wt%)-NaHCO3 (0. 5 wt%) mixture is used as a non-solvent. CS1 powder with a weight of 1.0 g and CS2
powder with a weight of 2.0 g are separately added to acetic acid aqueous solution (1 wt%) to make 1 wt% chitosan solution (CS1) and 2 wt% chitosan solution(CS2). 2 ml of each CS1 and CS2 are
evaporated at 55 ℃ for 30, 60, and 90 minutes, respectively, immersed into NaOH-NaHCO3
aqueous solution for 6 hours, rinsed with deionized water repeatedly, and freeze-dried with a freeze dryer (FD-5A2P, HCS Co, Ltd., Taiwan, R.O.C.). The chitosan membranes prepared by evaporation for 30, 60, and 90 minutes are denoted as CS1-30, CS1-60, CS1-90, CS2-30, CS2-60, and CS2-90, respectively.
Tests
Scanning Electron Microscopic (SEM) Observation
Chitosan membranes are coated with gold with a thickness of approximate 300 nm in a vacuum conditioner, and then micrographs are taken at a voltage of 13 keV with an SEM (S3000N, Hitachi, Japan). Afterwards, the cross-sections of the samples are observed.
Swelling Ratio of Chitosan Membranes
Different chitosan membranes are evaluated with a swelling ratio test to determine their water
absorption ability. Samples are first weighed (W0) and then immersed in the deionized water at
room temperature for 10, 30, 60, and 240 minutes, respectively, after which samples are removed,
excess water is wiped from the surface, and they are weighed (Wt) again. The swelling ratio is
calculated using the following equation.
SR (%)=Wt−Wo
Wo ×100 ………(1),
where W0 is the weight of the sample before immersion, Wt is the weight of the sample after
immersion, and t is hours of immersion duration.
Antibacterial Assay
A qualitative antibacterial assay is performed by using Staphylococcus aureus (S. aureus), as specified in JIS1902-2002. The chitosan membrane has a diameter of 30 mm. A medium containing S. aureus is evenly coated onto a solid agar, and then covered by a chitosan membrane at 37 ℃ for a 24-hour culture. The diameter of the inhibition zone is then measured. The number of samples is 3.
Results and Discussion
SEM Observation of Chitosan Membranes
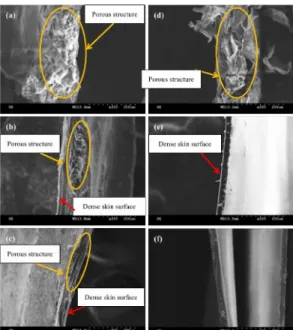
Figure 1 clearly shows that with an increase in the evaporation times, the cross-sections of
chitosan membranes change from a porous status to an ordinary membrane status. The longer the evaporation time, the greater the amount of water vapor that is emited. For both the CS1 series and
CS2 series, a 90-min evaporation results in a complete drying of chitosan before the NaOH-NaCO3
(aq) immersion and freeze-drying. As a result, there are no pores caused by the disappearing ice crystals, and the membranes appear in a membrane status. When the evaporation time is 30 or 60 minutes, only a small portion of water vapor escapes from the chitosan membranes. The immersion in NaOH-NaCO3 (aq) results in acid-base neutralization through the chitosan membranes, and freeze-drying then provides the CS1 series and the CS2 series with a dense surface and porous interior structure; however, their morphology still slightly differs from each other as a result of the
Figure 1. SEM images (200×) of the cross-sections of chitosan membranes of a) 30, b) CS1-60, c) CS1-90, d) CS2-30, e) CS2-CS1-60, and f) CS2-90.
Swelling Ratio of Chitosan Membranes
Figure 2. Swelling ratios of chitosan membranes as related to various evaporation times. Figure 2 distinctly shows that most chitosan membranes reach their water swelling equilibrium in 60 minutes, and their swelling ratios decrease afterwards. In particular, CS1-90 exhibits the maximum (354 %). The overall trend observed is that all chitosan membranes possess good water absorption ability, and as a result their swelling ratios are between 171 and 354 %. The shorter the evaporation time, the higher the water absorption of the chitosan membranes; the result of which is distinctly exemplified by the CS2 series. The porous interior structure of the chitosan membranes has a considerable influence on their water absorption ability. Also, an increase in the porosity of the chitosan membrane contributes to gaining capillary adsorbed water [7].
Antibacterial Efficacy of Chitosan Membranes
Table 1. Summary of the inhibition zones against S. aureus (n=3).
Diameter of Inhibition Zone (mm)
Sample Type Sample Type
CS1-30 30.9 mm CS2-30 35.4 mm
CS1-60 41.3 mm CS2-60 31.6 mm
CS1-90 32.5 mm CS2-90 N/A
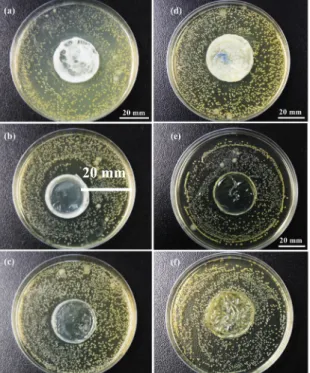
Figure 2 shows that both CS1 and CS2 series have antibacterial properties; meanwhile, Table 1
reports that CS1-60 exhibits an optimal inhibition zone (diameter: 41.3 mm). Chitosan is a cationic
polysaccharide, conveyed by the positively charged NH3+ groups of glucosamine, which may be a
fundamental factor contributing to its interaction with the negatively charged microbial cell surface, ultimately resulting in impairment of vital bacterial activities [8-9]. In addition, bacteria can evade and grow beneath CS2-90, the inhibition zone of which thus cannot be measured, or the
antibacterial efficacy of which is absent. Being evaporated for 90 minutes, the chitosan membranes are easily curled and wrinkled, and thus their insufficient obedience prevents the presence of an inhibition zone.
Figure 3. Bacterial inhibition of chitosan membranes of a) CS1-30, b) CS1-60, c) CS1-90, d) CS2-30, e) CS2-60, and f) CS2-90 against S. aureus as related to different evaporation times.
Conclusion
This study successfully produces chitosan membranes that can be used in dressings, and evaluates the properties of the resulting chitosan membranes. The results of SEM observation, a swelling ratio test, and an antibacterial assay show that CS1-60 possesses optimal chitosan membranes, which have great porosity, a high swelling ratio (332 %), and an optimum inhibition zone (diameter: 41.3 mm). In the future, this type of membrane could be combined with drug carriers or other natural polymers to form composite dressings. However the in vitro cytocompatibility needs to be examined.
Acknowledgement
The authors would especially like to thank National Science Council of the Taiwan, for financially supporting this research under Contract NSC 102-2622-166-002-cc2.
References
[1] C.L. Baum and J.A. Christopher: Dermatol. Surg. Vol. 31 (2005), p. 674.
[2] P.B. Malafaya, G.A. Silva and R.L.: Reis: Adv. Drug. Deliv. Rev. Vol. 59 (2007), p. 207. [3] M. Li, A.M. Dias, E.C. Leal, L. Carvalho, H.C. de Sousa and E. Carvalho: Acta. Biomater.
Vol. 10 (2013), p. 843.
[4] S. Huang and X. Fu: J. Controlled Release Vol. 142 (2010), p. 149. [5] Q. Gan and T. Wang, Colloid. Surface. B. Vol. 59 (2007), p. 24.
[6] F.L. Mi, S.S. Shyu, Y.B .Wu, S.T. Lee, J.Y. Shyong, R.N. Huang: Biomaterial Vol. 22 (2001), p. 165.
[7] W. S. Wan Ngah, C. S. Endud and R. Mayanar: React. Funct. Polym. Vol. 50 (2002), p. 181. [8] J.M. Yang, and H.T. Lin: J. Membrane. Sci. Vol. 243 (2004), p. 1.
[9] I. M. Helander, E.-L. Nurmiaho-Lassila, R. Ahvenainen, J. Rhoades and S. Roller: Int. J. Food
20 mm