doi:10.1093/jac/dkl006
Advance Access publication 27 January 2006
Early empirical glycopeptide therapy for patients with
methicillin-resistant Staphylococcus aureus bacteraemia:
impact on the outcome
Chi-Tai Fang
1,2†, Wen-Yi Shau
3†, Po-Ren Hsueh
1,4, Yee-Chun Chen
1, Jann-Tay Wang
1,
Chien-Ching Hung
1, Loreen Y. L. Huang
5and Shan-Chwen Chang
1*
1
Division of Infectious Diseases, Department of Internal Medicine, National Taiwan University Hospital, Taipei,
Taiwan;
2Department of Medical Research, National Taiwan University Hospital, Taipei, Taiwan;
3Graduate
Institute of Clinical Medicine, National Taiwan University College of Medicine, Taiwan;
4Department of Laboratory
Medicine, National Taiwan University Hospital, Taipei, Taiwan;
5Institute of Preventive Medicine, College of
Public Health, National Taiwan University, Taiwan
Received 12 November 2005; returned 20 November 2005; revised 25 December 2005; accepted 27 December 2005
Objectives: To evaluate whether appropriate early empirical glycopeptide therapy improves outcomes of patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia.
Methods: We retrospectively collected the data for all adult patients with confirmed MRSA bacteraemia diagnosed and treated at National Taiwan University Hospital during the period 1 April 1997–31 March 2001, and followed their survival up to three years. The main outcome measures were MRSA-related death and all-cause mortality.
Results: There were 77 MRSA-related deaths among 162 patients. There was no statistically significant difference in MRSA-related deaths between patients receiving glycopeptides before or within 48 h after blood culture (n = 43) (55%, 18/33, non-septic shock group; 90%, 9/10, septic shock group) or those whose glycopeptide therapy was begun more than 48 h after blood culture (n = 119) (37%, 40/107, non-septic shock group; 83%, 10/12, septic shock group) (P = 0.11 and 1.00, respectively). The outcome measure of all-cause mortality from 30 days to 3 years yields similar results. Multivariate logistic regression analysis and Cox analysis showed that the length of delay (daily increment) between blood culture sampling and start of glycopeptide therapy did not have a statistically significant impact on MRSA-related death or all-cause 30-day mortality after adjusting for the effect of other variables [adjusted odds ratio 0.99, 95% confidence interval (95% CI) 0.88–1.12; adjusted hazard ratio 0.87, 95% CI 0.74–1.02, respectively). Conclusions: The hypothesis that earlier empirical use of glycopeptide therapy reduces mortality in patients with hospital-acquired MRSA bacteraemia was not supported.
Keywords: vancomycin, teicoplanin, nosocomial infections, prognosis
Introduction
The incidence of hospital-acquired methicillin-resistant Staphylo-coccus aureus (MRSA) infection has increased dramatically in many countries,1–7 with fatality rates for patients who develop bacteraemia as high as 20–50%.8–11 Clinicians treating patients with hospital-acquired infections face the dilemma of whether to prescribe glycopeptides as part of an initial empirical regimen
before culture results become available. Because of the danger of worsening the problem of emerging vancomycin-resistant Gram-positive bacteria,12,13an aggressive approach in empirical use of glycopeptides needs to be justified by evidence of its effectiveness. Surprisingly, it remains unclear whether appropriate early empirical therapy with a glycopeptide actually improves outcomes of patients with hospital-acquired MRSA infection. Three obser-vational studies investigating the role of empirical glycopeptide
... *Correspondence address. Tel: +886-2-2312-3456 ext. 5401; Fax: +886-2-23971412; E-mail: sc4030@ha.mc.ntu.edu.tw.
†
These authors contributed equally to this work.
...
511
therapy in treatment of MRSA bacteraemia have been published, with contradictory results.14–16Although a randomized trial using placebo in a control group is the best way to reach an unbiased conclusion, such trials may not be ethical because empirical gly-copeptide therapy is already widely assumed to be life-saving.17,18 We hypothesized that use of a careful study design,19involving only MRSA patients with control of the sepsis severity and other risk factors for mortality, would allow an observational study to demonstrate the theoretical benefit of earlier glycopeptide therapy after follow-up for 3 years.
Methods
SettingThis study was conducted at National Taiwan University Hospital (Taipei, Taiwan). The hospital is a university-affiliated medical centre with a 2000 bed capacity that provides both primary and tertiary referral care. Vancomycin and teicoplanin, the two glycopeptides available at our institution, were not used for surgical prophylaxis during the study period except for the rare patients who had a history of anaphylaxis tob-lactams. However, there was no restriction on the therapeutic use of glycopeptides.
Study design
We retrospectively collected the data for all adult patients with con-firmed MRSA bacteraemia diagnosed and treated from 1 April 1997 to 31 March 2001. During this period, vancomycin and teicoplanin were the only two intravenous agents appropriate for treatment of MRSA bacteraemia; quinupristin/dalfopristin and linezolid were not available at that time. The survival status of patients included in this analysis was followed until 31 March 2004.
Patients were classified into the non-empirical glycopeptide group or the empirical glycopeptide group based on whether glycopeptide therapy was begun more than 48 h after blood culture sampling. MRSA-related deaths, all-cause mortality, and length of hospital stay, from the time of first MRSA-positive blood culture to discharge (or death) were compared between the two groups. We also used time interval as a continuous variable—from the first blood sampling that demonstrated MRSA bacteraemia to the start of glycopeptide therapy—as a potential explanatory variable in multivariate analysis. Microbiology
Culture, identification, and susceptibility testing of MRSA isolates were performed according to standard microbiological methods.20,21 BACTEC (Becton Dickinson, Spark, MD, USA) automated bacterial blood culture systems have been used since 1986 at our institution to facilitate rapid identification and reporting. Since January 2000, the clinical microbiology laboratory has routinely screened clinical MRSA isolates using brain–heart infusion agar supplemented with vancomycin 4 mg/L to detect S. aureus strains with reduced suscept-ibility to vancomycin.22
Inclusion criteria
The list of all adult patients (age‡ 16 years) in whom MRSA had been isolated from blood was obtained using the hospital computer database of clinical bacterial isolates. Because inclusion of patients without true MRSA bacteraemia would diminish the measured beneficial effect of empirical glycopeptide therapy, only patients with MRSA isolated from two blood cultures taken from two different peripheral sites were included in the analysis as confirmed MRSA bacteraemia cases. Similarly, patients with concomitant fungaemia or bacteraemia caused by other bacteria were excluded in order to avoid potential bias.
Clinical data
For each included case, demographic and clinical information was obtained from medical records. Survival data after hospital discharge were obtained from medical records in the outpatient department and the official death registration database23(Department of Health, Executive Yuan, Taiwan), which recorded the date of death of all Taiwanese citizens.
Severity of bacteraemia
Severity of MRSA bacteraemia on the day of positive blood culture sampling was assessed by severity of sepsis syndrome24and APACHE II scores.25To enhance applicability of the APACHE II scoring system to the present patient group, a modification allowed zero points to be assigned to the items ‘PaO2’ and ‘pH’ if the attending physicians did
not perform arterial blood gas analysis due to absence of cyanosis or respiratory distress.
Severity of underlying diseases
Severity of underlying disease before the onset of hospital-acquired MRSA infection was classified by McCabe–Jackson criteria.26 Specifically, leukaemia or lymphoma refractory to chemotherapy and advanced-stage cancer that made patients bedridden despite avail-able treatment were all classified as rapidly fatal. Uraemia requiring dialysis, decompensated liver cirrhosis, congestive heart failure, chronic respiratory failure requiring mechanical ventilatory assistance, aplastic anaemia and malignant diseases that cannot be cured but did not match the above-stated criteria for rapidly fatal diseases were classified as ultimately fatal. Other conditions were classified as non-fatal.
MRSA-related mortality
Death was considered to be related to MRSA infection if one or more of the following criteria15were present: blood cultures were positive
for MRSA at the time of death; death occurred before resolution of signs and symptoms of MRSA infection; death occurred not more than 14 days after onset of MRSA bacteraemia without another explanation for cause of death.
Statistical analysis
The Kaplan–Meier survival curve was used to analyse survival probability after the day of the first blood culture positive for MRSA. Log-rank test, Fisher’s exact test and Mann–Whitney test were used to compare survival curves, binary variables and continuous variables, respectively. Multivariate analysis was conducted using either Cox’s proportional hazards model, if the proportional assump-tion was applicable, or logistic regression model. The statistical soft-ware used for computation was S-PLUS 2000 for Windows (MathSoft Inc., MA, USA). Two-tailed P values of <0.05 were considered to be statistically significant.
Results
Characteristics of patients
From 1 April 1997 to 31 March 2001, a total of 162 adult patients with confirmed MRSA bacteraemia—but without concomitant fungaemia or bacteraemia caused by other bacteria—were diagnosed and treated. All 162 cases involved hospital-acquired infection (Table 1). The average delay (days) in start of glycopeptide therapy was 0 days (median, range: 0–1 days) in
empirical glycopeptide therapy group (n = 43) versus 3 days (median, range: 2–29 days) in the non-empirical glycopeptide group (n = 119) (P < 0.01). Patients in the empirical glycopeptide group were twice as likely to have septic shock at time of blood culture sampling as patients in the non-empirical glycopeptide group (23% versus 10%; P = 0.04). There was no significant difference in age, gender, sites of MRSA infection, and types and severity of underlying diseases between the two groups (Table 1).
Empirical antimicrobial therapy
Of the 162 patients, all but three received empirical anti-microbial therapy after blood culture sampling. The chosen agents, alone or in combination, included variousb-lactams and aminogly-cosides, vancomycin, teicoplanin, ciprofloxacin, trimethoprim/ sulfamethoxazole, clindamycin, metronidazole, and erythromycin. Among the 43 patients in the empirical glycopeptide group, 37 received vancomycin and 6 received teicoplanin in their Table 1. Characteristics of 162 patients with hospital-acquired MRSA bacteraemia
Number (%) Characteristics Empirical glycopeptide groupa(n = 43) Non-empirical glycopeptide group (n = 119) P value Delay (days) in start of glycopeptide therapy, median (range) 0 (0–1) 3 (2–29) <0.01**
Age, years median (range) 70 (35–92) 67 (18–99) 0.34
Age‡60 years 31 (72) 75 (63) 0.35 Gender, male 30 (70) 71 (60) 0.27 HIV seropositive 0 (0) 2 (2) 1.00 Neutropenia (<500/mm3) 4 (9) 9 (8) 0.75 Immunosuppressive therapyb 10 (23) 31 (26) 0.84 Alcoholism 2 (5) 4 (3) 0.66 Diabetes mellitus 10 (23) 35 (29) 0.55
Chronic heart diseasec 10 (23) 32 (27) 0.69
Chronic lung diseased 6 (14) 14 (12) 0.79
Uraemia requiring dialysis 11 (26) 34 (29) 0.84
Decompensated liver cirrhosise 9 (21) 11 (9) 0.06
Malignancies 13 (30) 33 (28) 0.84
McCabe classification
Rapidly fatal 7 (16) 14 (12) 0.44
Ultimately fatal 22 (51) 67 (56) 0.56
Non-fatal 14 (33) 38 (32) 1.00
APACHE II scoref, median (range) 19 (1–31) 17 (2–37) 0.23
APACHE II score‡15 31 (72) 80 (67) 0.70 Severity of SIRSf Septic shock 10 (23) 12 (10) 0.04** Severe sepsis 3 (7) 9 (8) 1.00 Sepsis 29 (67) 92 (77) 0.22 No sepsis 1 (2) 6 (5) 0.68
Site of MRSA infectiong
Endocarditis 2 (5) 5 (4) 1.00
Meningitis 0 (0) 1 (1) 1.00
Pneumonia 8 (19) 20 (17) 0.82
Catheter-related infection 20 (47) 52 (44) 0.86
Urinary tract infection 2 (5) 3 (3) 0.61
Soft tissue infection 13 (30) 23 (19) 0.20
Osteomyelitis 2 (5) 8 (7) 1.00
No obvious source 7 (16) 24 (20) 0.66
MRSA, methicillin-resistant S. aureus; HIV, human immunodeficiency virus; SIRS, systemic inflammatory response syndrome.
aEmpirical glycopeptide group: patients began glycopeptide therapy before or within 48 h after blood culture sampling.
b
Cytotoxic chemotherapy, corticosteroid or ciclosporin.
cSymptomatic coronary artery disease or congestive heart failure.
d
Chronic obstructive pulmonary disease, bronchiectasis, or pneumoconiosis.
eWith physical evidence of portal hypertension.
f
At time of blood culture sampling.
g
Some patients had more than one infected site. **Statistically significant (P < 0.05).
empirical regimens. None of the agents used in the empirical regi-mens of the other 119 patients were active in vitro against MRSA. Definite antimicrobial therapy
By routine susceptibility test, all of the MRSA strains isolated from included patients were susceptible to both vancomycin and teicoplanin (see the Appendix for more details). The interval from blood culture sampling to receipt of results ranged from 2 to 7 days. After reporting of blood culture results, the 43 patients in the empirical glycopeptide group continued to receive their initial vancomycin or teicoplanin therapy, and 107 of the 119 patients in the non-empirical glycopeptide group started to receive vancomycin (104 patients) or teicoplanin (three patients). In the non-empirical glycopeptide group, 12 patients did not receive glycopeptide therapy, and 10 of them died before the blood culture report became available. The remaining two patients did not receive glycopeptide therapy for unspecified reasons.
During the course of treatment, 13 patients (four in the empirical glycopeptide group and nine in the non-empirical glycopeptide group) developed skin rash or leucopenia under vancomycin therapy, necessitating a change to teicoplanin. Among patients who survived for more than 7 days, duration of glycopeptide ther-apy ranged from 7 days to 6 weeks, depending on clinical response and whether endocarditis or osteomyelitis was present.
Timing and administration of glycopeptide therapy
Glycopeptide therapy was started before blood culture sampling in 11 patients due to fever and isolation of MRSA from non-blood sites such as sputum or wound. The interval between blood culture sampling and first dose of glycopeptide was 0–24 h in 16 patients, 24–48 h in 16 patients, 48–72 h in 35 patients, 72–96 h in 38 patients, 96–120 h in 19 patients, 120–144 h in six patients, 144–168 h in four patients and >168 h in five patients. The routine dosage and administration of vancomycin was either 15 mg/kg every 12 h or 7.5 mg/kg every 6 h, with adjustment for renal function, infused at a rate of 500 mg/h. The routine dosage and administration of teicoplanin was 12 mg/kg loading then 12 mg/kg/ day for endocarditis/septic arthritis or 6 mg/kg/day for other infec-tions, with adjustment for renal function, infused over 30 min.
During the study period, 21 patients who did not have septic shock (20 in the non-empirical glycopeptide group and one in the empirical glycopeptide group) were enrolled into a clinical trial and given an initial loading dose of 25 mg/kg vancomycin, infused at a rate of 500 mg/h, followed by the standard regimen. The delay in start of glycopeptide therapy was 3.5 days (median, range: 2– 5 days) in these 20 non-empirical-glycopeptide-group patients, and 1 day in the empirical-glycopeptide-group patient, respectively. Serum vancomycin levels
The median trough vancomycin levels were 10.1 (range: 8.4–45.6) mg/L among empirical glycopeptide therapy group versus 12.5 (range: 4.8–27.6) mg/L among non-empirical glycopeptide therapy group, without significant difference between two groups (P = 0.55). The median peak vancomycin levels were 20.1 (range: 11.1–58.6) mg/L versus 25.7 (range: 12.6–74.0) mg/L, without significant difference either (P = 0.93). After the loading dose, the 21 patients who were given 25 mg/kg vancomycin loading dose had a median 1 h post-loading serum vancomycin level of 26.2 (range: 5.8–51.3) mg/L.
Outcome and prognostic factors
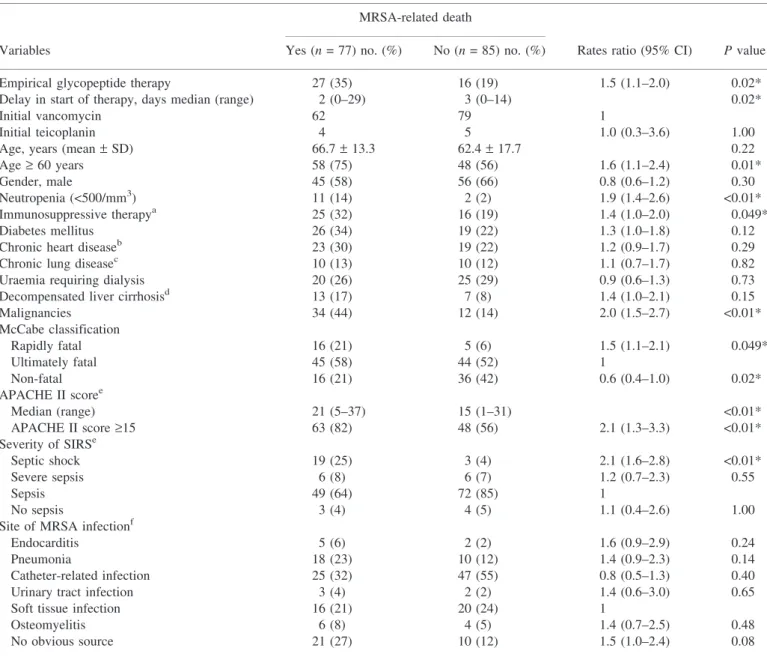
The all-cause mortality rate was 36% (59/162) on day 30, with a gradual increase to 75% (122/162) at the end of the 3 year follow-up. The death of 77 patients (48%) was related to MRSA infection. In the univariate analysis (Table 2), predictors of MRSA-related death included septic shock at time of blood culture sampling (P < 0.01), APACHE II scores ‡ 15 (P < 0.01), rapidly fatal underlying disease (P = 0.049), malignancy (P < 0.01), neutropenia (P < 0.01), immunosuppressive therapy (P = 0.049), and age ‡ 60 years (P = 0.01). In contrast, non-fatal underlying condition was a protective factor against MRSA-related death (P = 0.02). Patients who were enrolled into the vancomycin loading trial had fewer MRSA-related deaths (6/21, 29%) than other patients treated with vancomycin (56/120, 47%), but the difference was not statistically significant (P = 0.24).
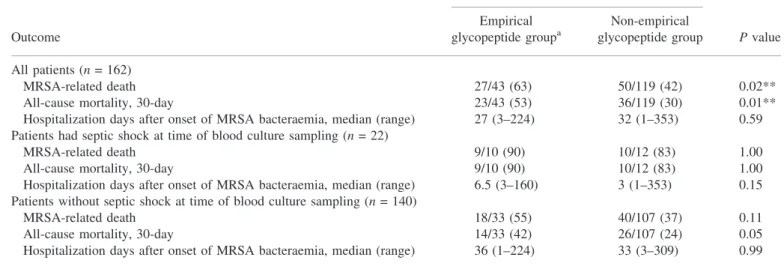
Effect of empirical glycopeptide therapy
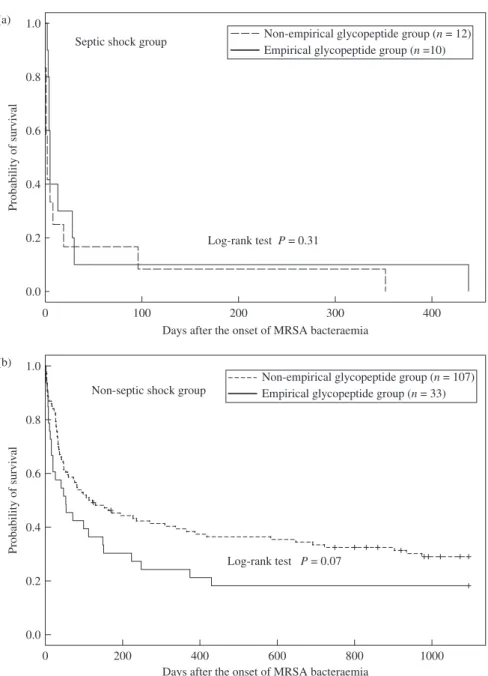
The proportion of patients who died within 30 days in the empirical glycopeptide group was significantly higher than that in the non-empirical glycopeptide group (23/43, 53% versus 36/119, 30%, P = 0.01) (Table 3). After stratification by septic shock at time of blood culture sampling, which was more common in empirical glycopeptide group, there was no significant difference between the empirical glycopeptide and non-empirical glyco-peptide groups in rates of MRSA-related death, all-cause 30 day mortality or length of hospitalization after onset of MRSA bacteraemia (Table 3). The 3 year Kaplan–Meier survival curves, stratified by septic shock at time of blood culture sampling, are shown in Figure 1. There was no significant difference between empirical glycopeptide and non-empirical glycopeptide groups (P = 0.31, septic shock group; P = 0.07, non-septic shock group). In multivariate analysis for MRSA-related death and all-cause 30 day mortality (Table 4), a longer delay (measured in days) in the start of glycopeptide therapy was not a significant predictor of mortality after adjusting for the effects of other variables [adjusted odds ratio (OR) 0.99, 95% confidence interval (95% CI) 0.88–1.12, P = 0.92, adjusted hazard ratio 0.87, 95% CI 0.74–1.02, P = 0.08, respectively, Table 4]. In contrast, septic shock at time of blood culture, age‡ 60 years and neutropenia were shown to be signi-ficant independent predictors of MRSA-related death or all-cause 30 day mortality (Table 4). Analysis for long-term outcome yielded three significant independent predictors for all-cause 1 year mor-tality: septic shock at time of blood culture (OR 14.2, 95% CI 1.5– 131.0, P = 0.02), age ‡ 60 years (OR 5.6, 95% CI 2.3–46.9, P < 0.01) and malignancy (OR 7.3, 95% CI 1.4–27.5, P = 0.02). Non-fatal underlying condition was an independent protect-ive factor (OR 0.42, 95% CI 0.18–0.99, P = 0.046). Age‡ 60 years (OR 6.7, 95% CI 2.5–17.6, P < 0.01) was the only independent predictor for all-cause 3 year mortality. Non-fatal underlying condition was again an independent protective factor (OR 0.28, 95% CI 0.11–0.72, P < 0.01). A longer delay in the start of gly-copeptide therapy was not a significant predictor of 1 or 3 year mortality after adjusting for the effects of other variables (adjusted OR 0.99, 95% CI 0.87–1.13, P = 0.90 and 0.96, 95% CI 0.84–1.10, P = 0.57, respectively).
Discussion
Our results did not support the hypothesis that earlier empirical use of glycopeptide therapy reduces mortality in patients
with hospital-acquired MRSA bacteraemia. Kim et al.16also found no significant difference in MRSA-related mortality between patients who received an appropriate (30%, 9/30) and an inappropriate (39%, 38/97) empirical regimen (P = 0.36). Similarly, Roghmann14 found no statistically significant dif-ference in mortality from inappropriately empirically treated MRSA bacteraemia and mortality from appropriately empirically treated MSSA bacteraemia (relative risk 0.82, 95% CI 0.36–1.88) during a period of hospital policy restricting
empirical vancomycin use. These results, however, should be interpreted with caution. Although ours, Kim’s, and Roghmann’s data did not support the existence of a beneficial effect of empirical glycopeptide therapy on patient outcomes, the same data did not refute it, either. Lack of significant difference between empirical glycopeptide group and non-empirical glycopeptide group implies an inconclusive result caused by the limited sample size, rather than no effect of empirical glycopeptide therapy.
Table 2. Univariate analysis for risk factors of MRSA-related death
MRSA-related death
Variables Yes (n = 77) no. (%) No (n = 85) no. (%) Rates ratio (95% CI) P value
Empirical glycopeptide therapy 27 (35) 16 (19) 1.5 (1.1–2.0) 0.02*
Delay in start of therapy, days median (range) 2 (0–29) 3 (0–14) 0.02*
Initial vancomycin 62 79 1
Initial teicoplanin 4 5 1.0 (0.3–3.6) 1.00
Age, years (mean– SD) 66.7– 13.3 62.4– 17.7 0.22
Age‡ 60 years 58 (75) 48 (56) 1.6 (1.1–2.4) 0.01*
Gender, male 45 (58) 56 (66) 0.8 (0.6–1.2) 0.30
Neutropenia (<500/mm3) 11 (14) 2 (2) 1.9 (1.4–2.6) <0.01*
Immunosuppressive therapya 25 (32) 16 (19) 1.4 (1.0–2.0) 0.049*
Diabetes mellitus 26 (34) 19 (22) 1.3 (1.0–1.8) 0.12
Chronic heart diseaseb 23 (30) 19 (22) 1.2 (0.9–1.7) 0.29
Chronic lung diseasec 10 (13) 10 (12) 1.1 (0.7–1.7) 0.82
Uraemia requiring dialysis 20 (26) 25 (29) 0.9 (0.6–1.3) 0.73
Decompensated liver cirrhosisd 13 (17) 7 (8) 1.4 (1.0–2.1) 0.15
Malignancies 34 (44) 12 (14) 2.0 (1.5–2.7) <0.01* McCabe classification Rapidly fatal 16 (21) 5 (6) 1.5 (1.1–2.1) 0.049* Ultimately fatal 45 (58) 44 (52) 1 Non-fatal 16 (21) 36 (42) 0.6 (0.4–1.0) 0.02* APACHE II scoree Median (range) 21 (5–37) 15 (1–31) <0.01* APACHE II score‡15 63 (82) 48 (56) 2.1 (1.3–3.3) <0.01* Severity of SIRSe Septic shock 19 (25) 3 (4) 2.1 (1.6–2.8) <0.01* Severe sepsis 6 (8) 6 (7) 1.2 (0.7–2.3) 0.55 Sepsis 49 (64) 72 (85) 1 No sepsis 3 (4) 4 (5) 1.1 (0.4–2.6) 1.00
Site of MRSA infectionf
Endocarditis 5 (6) 2 (2) 1.6 (0.9–2.9) 0.24
Pneumonia 18 (23) 10 (12) 1.4 (0.9–2.3) 0.14
Catheter-related infection 25 (32) 47 (55) 0.8 (0.5–1.3) 0.40
Urinary tract infection 3 (4) 2 (2) 1.4 (0.6–3.0) 0.65
Soft tissue infection 16 (21) 20 (24) 1
Osteomyelitis 6 (8) 4 (5) 1.4 (0.7–2.5) 0.48
No obvious source 21 (27) 10 (12) 1.5 (1.0–2.4) 0.08
MRSA, methicillin-resistant S. aureus; CI, confidence interval; SIRS, systemic inflammatory response syndrome. Empirical glycopeptide group: patients began glycopeptide therapy before or within 48 h after blood culture sampling.
aCytotoxic chemotherapy, corticosteroid or ciclosporin.
b
Symptomatic coronary artery disease or congestive heart failure.
cChronic obstructive pulmonary disease, bronchiectasis, or pneumoconiosis.
d
With physical evidence of portal hypertension.
eAt time of blood culture sampling.
f
Some patients had more than one infected site. *Statistically significant (P < 0.05).
An important limitation in previous studies14,16as well as ours is that the information was retrospectively obtained and therefore the measurement of time interval between blood culture sampling and the start of glycopeptide therapy allowed analysis only by days. Instead of days, a more appropriate evaluation should have been performed in hours. A delay of more than several hours could be critical in terms of influencing the outcomes of these patients.
Because observational studies, including the present one, cannot yield a conclusive result, it appears that placebo-controlled ran-domized clinical trials are indeed required to resolve this question. A small to moderate benefit of a therapeutic intervention usually requires randomized clinical trials to demonstrate this, because differences in patient characteristics between the groups in an observational study might bias results. The physicians had reasons to choose treatment, and these reasons are likely to influence
outcomes. The reasons are not necessarily reflected in the variables we have gathered, and their influence is not necessarily completely corrected by multivariable analysis. Pre-defined inclusion criteria and adequate allocation concealment in a randomized clinical trial could indeed solve such issues. Before an evidence-based practice guideline can be formulated, a balanced view on this issue may be necessary. Use of institution-specific prediction models27for the probability of MRSA as the aetiology of hospital-acquired infec-tions may help clinicians to decide whether to start glycopeptide antibiotics empirically. On the other hand, routine use of glyco-peptide antibiotics as part of empirical therapy in patients with a low possibility of MRSA infection should be discouraged.
A meta-analysis of 14 randomized trials involving 2413 patients had shown no benefit of adding glycopeptides to initial empirical antimicrobial regimen for febrile neutropenic patients.28 To be Table 3. Effect of empirical glycopeptide therapy, stratified by septic shock at presentation
Number of deaths (% of group)
Outcome
Empirical glycopeptide groupa
Non-empirical
glycopeptide group P value All patients (n = 162)
MRSA-related death 27/43 (63) 50/119 (42) 0.02**
All-cause mortality, 30-day 23/43 (53) 36/119 (30) 0.01**
Hospitalization days after onset of MRSA bacteraemia, median (range) 27 (3–224) 32 (1–353) 0.59 Patients had septic shock at time of blood culture sampling (n = 22)
MRSA-related death 9/10 (90) 10/12 (83) 1.00
All-cause mortality, 30-day 9/10 (90) 10/12 (83) 1.00
Hospitalization days after onset of MRSA bacteraemia, median (range) 6.5 (3–160) 3 (1–353) 0.15 Patients without septic shock at time of blood culture sampling (n = 140)
MRSA-related death 18/33 (55) 40/107 (37) 0.11
All-cause mortality, 30-day 14/33 (42) 26/107 (24) 0.05
Hospitalization days after onset of MRSA bacteraemia, median (range) 36 (1–224) 33 (3–309) 0.99
aEmpirical glycopeptide group: patients began glycopeptide therapy before or within 48 h after blood culture sampling.
**Statistically significant (P < 0.05).
Table 4. Multivariate analysis for risk factors of MRSA-related death and 30-day all-cause mortality
MRSA-related death All-cause 30-day mortality All-cause 30-day mortality Variables Odds ratioa P value Odds ratioa P value Hazard ratiob P value Delay in start of therapy (daily increment) 0.99 (0.88–1.12) 0.92 0.83 (0.66–1.04) 0.10 0.87 (0.74–1.02) 0.08 Age‡ 60 years 2.90 (1.25–6.75) 0.01* 2.13 (0.88–5.15) 0.09 1.49 (0.80–2.76) 0.21 Neutropenia (<500/mm3) 3.87 (0.65–22.91) 0.14 4.44 (0.90–21.83) 0.07 3.15 (1.30–7.64) 0.01* Malignancies 2.65 (0.92–7.60) 0.07 1.54 (0.52–4.60) 0.44 1.58 (0.78–3.20) 0.21 Immunosuppressive therapy 1.22 (0.45–3.32) 0.70 1.56 (0.55–4.39) 0.40 1.55 (0.73–3.29) 0.26 McCabe classification non-fatal 0.60 (0.25–1.41) 0.24 1.03 (0.40–2.64) 0.95 1.29 (0.64–2.61) 0.47 rapidly fatal 2.27 (0.54–9.63) 0.27 2.10 (0.52–8.47) 0.30 1.38 (0.59–3.23) 0.47 Septic shock at time of blood
culture sampling
9.31 (2.35–36.8) <0.01* 13.29 (3.50–50.6) <0.01* 6.72 (3.62–12.5) <0.01*
a
Logistic regression analysis.
bCox proportional hazard analysis.
life-saving in critically ill patients, empirically used agents must have a potent antimicrobial activity against the causal microorgan-ism(s). Nevertheless, vancomycin has been consistently shown to be a much less effective antistaphylococcal drug than the semisyn-thetic penicillins in many in vitro, in vivo and clinical studies.29–32 Failure to identify a statistically significant benefit of empirical glycopeptide therapy in our and Kim’s studies, both involving only MRSA patients, may be explained by the unsatisfactory antimi-crobial activity of vancomycin against MRSA.29–32 In contrast, Lodise et al.15studied 103 patients with MRSA and 64 patients with MSSA, and they were able to identify a statistically significant beneficial effect (mortality 44.7% versus 86.7%, P = 0.006) of appropriate empirical therapy (either semisynthetic penicillins or vancomycin) among patients with APACHE II scores‡ 15.5 and a high-risk source infection, but not in patients with lower risk.
The inferior efficacy of vancomycin against S. aureus can be further reduced by an elevation of MICs to 1–2 mg/L,33which is still well within the susceptibility range. A survey of MRSA strains at our hospital during 1995–1996 showed a vancomycin MIC50of
0.5 mg/L and a MIC90of 1 mg/L.34A second survey during 1998–
1999 showed an increased vancomycin MIC50of 1 mg/L and an
increased MIC90of 2 mg/L (see the Appendix).35This factor could
be another reason that empirical glycopeptide therapy cannot be shown to be beneficial in our study.
For MRSA strains with an elevated vancomycin MIC, pharma-cokinetic factors may potentially influence the efficacy of therapy. Glycopeptides have poor penetration into lung tissues.36 Our patients with MRSA pneumonia did have a greater risk for mortality (Table 2), although this was not significant due to small sample sizes. Furthermore, standard vancomycin doses may
0 200 400 600 800 1000
Days after the onset of MRSA bacteraemia 0.0 0.2 0.4 0.6 0.8 1.0 Probability of survi v al
Non-septic shock group
Log-rank test P = 0.07
0 100 200 300 400
Days after the onset of MRSA bacteraemia 0.0 0.2 0.4 0.6 0.8 1.0 (a) (b) Probability of survi v al
Septic shock group
Log-rank test P = 0.31
Non-empirical glycopeptide group (n = 12) Empirical glycopeptide group (n =10)
Non-empirical glycopeptide group (n = 107) Empirical glycopeptide group (n = 33)
Figure 1. Kaplan–Meier survival curves of patients with MRSA bacteraemia, empirical glycopeptide therapy group versus non-empirical glycopeptide therapy
group. The patients were further classified by: (a) presence of septic shock at the time of blood culture sampling; (b) no septic shock at the time of blood culture sampling.
be subtherapeutic in critically ill patients during the first 24–48 h due to altered volume of distribution and other pharmacokinetic parameters in patients with sepsis syndrome.37 This could also reduce the impact of earlier empirical vancomycin therapy for MRSA strains with a vancomycin MIC of 1–2 mg/L.
It should be acknowledged that culture sampling itself might have been delayed in some cases due to difficulties in suspecting the presence of MRSA bacteraemia. The timing of diagnosis and treatment might not be early enough even in the empirical glycopeptide group. We cannot rule out the possibility that aggressive treatment with a glycopeptide at an earlier stage of MRSA infections might be significantly beneficial. Efforts should be directed toward early recognition and rapid diagnosis of MRSA infections instead of merely relying on empirical glycopeptide therapy. Employment of more rapid molecular diagnostic methods such as mecA probes38 may allow earlier treatment for MRSA infections after culture samples are drawn, and avoid unnecessary glycopeptides therapy for those do not have MRSA infections at the same time.
We conclude that the effect of earlier empirical use of glyco-peptide therapy on reducing mortality in patients with hospital-acquired MRSA bacteraemia was not supported. Before we can confidently endorse a guideline supporting an aggressive approach with empirical glycopeptide use, randomized trials are required to provide solid evidence of its effectiveness in reduction of MRSA-related morbidity and mortality.
Acknowledgements
This study was supported by grant NTUH-88-M105 from the National Taiwan University Hospital, Taipei, Taiwan.
Transparency declarations
None to declare. Dr Wen-Yi Shau is now an employee of GlaxoSmithKline.
References
1. Boyce JM, Cookson B, Christiansen K et al. Methicillin-resistant Staphylococcus aureus. Lancet Infect Dis 2005; 5: 653–63.
2. Duckworth G. Controlling methicillin resistant Staphylococcus aureus: time to return to more stringent methods of control in the
United Kingdom?BMJ 2003; 327: 1177–8.
3. NINSS report on surgical site infection and hospital-acquired
bacteremia.Commun Dis Rep CDR Wkly 2000; 10: 213–6.
4. Pittet D, Wenzel RP. Nosocomial bloodstream infections: secular
trends in rates, mortality, and contribution to total hospital deaths.Arch
Intern Med 1995; 155: 1177–84.
5. Edmond MB, Wallace SE, McClish DK et al. Nosocomial bloodstream infections in United States hospitals: a 3-year analysis. Clin Infect Dis 1999; 29: 239–44.
6. Chen ML, Chang SC, Pan HJ et al. Longitudinal analysis of methicillin-resistant Staphylococcus aureus isolates at a teaching
hospital in Taiwan.J Formos Med Assoc 1999; 98: 426–32.
7. Hsueh PR, Teng LJ, Chen WH et al. Increasing prevalence of
methicillin-resistant Staphylococcus aureus causing nosocomial
infec-tions at a university hospital in Taiwan from 1986 to 2001.Antimicrob
Agents Chemother 2004; 48: 1361–4.
8. Cosgrove SE, Sakoulas G, Perencevich EN et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36: 53–9.
9. Blot SI, Vandewoude KH, Hoste EA et al. Outcome and attributable mortality in critically ill patients with bacteremia involving
methicillin-susceptible and methicillin-resistantStaphylococcus aureus. Arch Intern
Med 2002; 162: 2229–35.
10. Harbarth S, Rutschmann O, Sudre P et al. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch Intern Med 1998; 158: 182–9.
11. Romero-Vivas J, Rubio M, Fernandez C et al. Mortality associated
with nosocomial bacteremia due to methicillin-resistantStaphylococcus
aureus. Clin Infect Dis 1995; 21: 1417–23.
12. Centers for Disease Control and Prevention. Preventing the spread of vancomycin resistance, recommendations of the Hospital
Infection Control Practices Advisory Committee (HICPAC). MMWR
1995; 44 (RR12): 1–13.
13. Amyes SGB. Treatment of staphylococcal infection: prescriptions
must be part of a package that includes infection control.BMJ 2005;
330: 976–7.
14. Roghmann MC. Predicting methicillin resistance and the effect of
inadequate empiric therapy on survival in patients withStaphylococcus
aureus bacteremia. Arch Intern Med 2000; 160: 1001–4.
15. Lodise TP, McKinnon PS, Swiderski L et al. Outcomes analysis of
delayed antibiotic treatment for hospital-acquiredStaphylococcus aureus
bacteremia.Clin Infect Dis 2003; 36: 1418–23.
16. Kim SH, Park WB, Lee KD et al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2004; 54: 489–97.
17. Cordova SP, Heath CH, McGechie DB et al. Methicillin-resistant Staphylococcus aureus bacteraemia in Western Australian teaching hospitals, 1997–1999: risk factors, outcomes and implications for
management.J Hosp Infect 2004; 56: 22–8.
18. Coello R, Glynn JR, Gaspar C et al. Risk factors for developing
clinical infection with methicillin-resistant Staphylococcus aureus
(MRSA) amongst hospital patients initially only colonized with MRSA. J Hosp Infect 1997; 37: 39–46.
19. Leibovici L, Samra Z, Konigsberger H et al. Long-term survival
following bacteremia or fungemia.JAMA 1995; 274: 807–12.
20. Kloos WE, Bannerman TL. Staphylococcus and Micrococcus. In:
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, eds.Manual
of Clinical Microbiology. 7th edn. Washington, D.C.: ASM Press, 1999; 264–82.
21. National Committee for Clinical Laboratory Standard. Performance Standards for Antimicrobial Disk Susceptibility Tests-4th edition: Approved Standard M2-A6. NCCLS, Villanova, PA, USA, 1990.
22. Tenover FC, Lancaster MV, Hill BC, et al. Characterization of staphylococci with reduced susceptibilities to vancomycin and other
glycopeptides.J Clin Microbiol 1998; 36: 1020–7.
23. Hsieh GY, Chen PC, Wang JD. Verification and correction of error for death registration data of the Department of Health R.O.C. between
1980 and 1997.Taiwan J Public Health 2002; 21: 329–38.
24. Knaus WA, Draper EA, Wagner DP et al. APACHE II: A severity of
disease classification system.Crit Care Med 1985; 13: 818–29.
25. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–910.
26. McCabe WR, Jackson GG. Gram-negative bacteremia. Arch Inter Med 1962; 110: 847–64.
27. Lodise TP Jr, McKinnon PS, Rybak M. Prediction model to
identify patients with Staphylococcus aureus bacteremia at risk for
methicillin resistance.Infect Control Hosp Epidemiol 2003; 24: 655–61.
28. Vardakas KZ, Samonis G, Chrysanthopoulou SA et al. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic
patients: a meta-analysis of randomised controlled trials.Lancet Infect
29. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339: 520–32.
30. Gonzalez C, Rubio M, Romero-Vivas J et al. Bacteremic pneumonia
due toStaphylococcus aureus: a comparison of disease caused by
methicillin-resistant and methicillin-susceptible organisms. Clin Infect
Dis 1999; 29: 1171–7.
31. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin
or vancomycin plus rifampin in methicillin-resistant Staphylococcus
aureus endocarditis. Ann Intern Med 1991; 115: 674–80.
32. Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug abusers. Antimicrob Agents Chemother 1990; 34: 1227–31.
33. Sakoulas G, Moise-Broder PA, Schentag J et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of
methicillin-resistantStaphylococcus aureus bacteremia. J Clin Microbiol
2004; 42: 2398–402.
34. Chang SC, Fang CT, Chen CY et al. In vitro activity of quinupristin/ dalfopristin against common pathogenic bacteria isolated in Taiwan. Diagn Microbiol Infect Dis 1999; 33: 299–303.
35. Fang CT, Chang SC, Chen YC et al. In vitro activity of linezolid against clinical gram-positive bacterial isolates from Taiwan: an area
with a high prevalence of antibiotic resistance.Int J Antimicrob Agents
2001; 18: 267–70.
36. Cruciani M, Gatti G, Lazzarini L et al. Penetration of vancomycin into
human lung tissue.J Antimicrob Chemother 1996; 38: 865–9.
37. Soto J, Sacristan JA, Alsar MJ. Necessity of a loading dose
when using vancomycin in critically ill patients.J Antimicrob Chemother
1991; 27: 875.
38. Huletsky A, Giroux R, Rossbach V et al. New real-time PCR assay
for rapid detection of methicillin-resistant Staphylococcus aureus
directly from specimens containing a mixture of staphylococci.J Clin
Microbiol 2004; 42: 1875–84.
Appendix
Antimicrobial susceptibility of MRSA strains
By routine disc susceptibility test (Table 5), all of the MRSA strains isolated from included patients were susceptible to both
vancomycin and teicoplanin. Two (1%) strains were also suscept-ible to erythromycin, clindamycin, minocycline and trimethoprim/ sulfamethoxazole. Another four (2%) strains were resistant to macrolides but susceptible to clindamycin, minocycline and trimethoprim/sulfamethoxazole. Among the remaining 156 strains, 80 (49%) were resistant to all four tested b-lactam, non-glycopeptide antibiotics: erythromycin, clindamycin, minocycline and trimethoprim/sulfamethoxazole. The other strains were resistant to at least two of the above four agents.
Routine screening using brain–heart infusion agar supple-mented with vancomycin 4 mg/L did not detect any vancomycin-intermediate S. aureus strain. To survey the level of MICs of vancomycin to the MRSA strains isolated from patients involved in this study, we randomly selected 18 strains from the available 102 blood culture isolates and conducted MIC determination using the standard agar dilution method (National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Fourth Edition: Approved Standard M7-A4. NCCLS, Wayne, PA, USA, 1997). Among the 18 tested MRSA isolates, the vancomycin MICs were 1 mg/L in 16 strains and 0.5 mg/L in two strains.
Table 5. Routine disc susceptibility of MRSA strains involved in this study Susceptible no. (%) (n = 162) Vancomycin 162 (100) Teicoplanin 162 (100) Erythromycin 3 (2) Clindamycin 25 (15) Minocycline 62 (38) Trimethoprim/sulfamethoxazole 33 (20)