行政院國家科學委員會專題研究計畫 成果報告
薑精油對肝癌細胞及正常肝細胞生理機能之影響
計畫類別: 個別型計畫 計畫編號: NSC91-2320-B-002-148- 執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學食品科技研究所 計畫主持人: 沈立言 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 2 月 10 日
行政院國家科學委員會專題研究計畫成果報告 薑精油對肝癌細胞及正常肝細胞生理機能之影響
Effect of ginger essential oil on the physiological functions of hepatoma cells and normal hepatocytes
計畫編號:NSC 91-2320-B-002-148 執行期限:91 年 8 月 1 日至 92 年 7 月 31 日 主持人:沈立言 國立台灣大學食品科技研究所 一、中文摘要 本 研 究 以 南 投 縣 所 栽 培 之 生 薑 (Zingiber officinale Roscoe)進行水蒸 氣蒸餾法所萃取之薑精油為實驗材料, 以 MTT assay 來探討不同濃度的薑精油 對於人類肝癌細胞株 Hep G2 與 Hep 3B 之生存力。並採用大白鼠初代肝細胞之 分離與培養為實驗模式,探討不同濃度 之薑精油對大白鼠初代肝細胞生存力、 抗氧化及解毒代謝能力之影響。實驗結 果顯示,薑精油平均萃取率為 0.16%。 經由 GC-MS 分析其精油成份含有高量 的萜烯類碳氫化合物,尤以 Geranial 含 量 高 達 25.2 % , Citronellol 次 之 約 為 18.7%。肝癌細胞株 Hep 3B 以高濃度 100~200μg/ml 薑精油處理 24 小時後細 胞生存抑制百分比皆顯著高於控制組(p <0.05),IC50約為 173μg/ml。而對於 肝癌細胞株 Hep G2 而言,50~200μ g/ml 薑精油則能顯著性抑制肝癌細胞 Hep G2 之生長,IC50為 94.2μg/ml。可 見薑精油對於肝癌細胞 Hep G2 之生存 抑制效果較 Hep 3B 佳。在薑精油低濃 度(50μg/ml)情況下,對於肝癌細胞 Hep G2 就有抑制 28.1%生存力,對初 代肝細胞則無顯著性影響,並且能夠降 低細胞之脂質過氧化。10μg/ml 濃度之 薑精油則可提高大白鼠初代肝細胞之抗 氧化及其解毒代謝系統能力。而當薑精 油濃度提升至 100μg/ml 時,對肝癌細 胞 Hep 3B、Hep G2 抑制效果達到 22.3 和 54.7%,對於正常初代肝細胞亦有 52.1%的傷害。 關鍵詞:薑精油、細胞生存力、抗氧 化、解毒作用、大白鼠初代肝細胞、人 類肝癌細胞株 Abstract
Ginger oil was extracted from Zingiber officinale Roscoe which was cultivated in Nanto county using the steam distillation method. The cell viability of hepatoma cell line Hep G2 and Hep 3B which were treated with various concentrations of ginger oil was investigated by MTT assay. In addition, the cell viability, antioxidation and detoxification systems of primary rat hepatocytes treated with various
concentrations of ginger oil were also investigated. The result showed that the average yield of extraction of fresh ginger was 0.16%. The major components of the ginger essential oil are geranial (25.2 %) and citronellol (18.7 %) in ginger essential oil according to the GC-MS analysis. In terms of the results of MTT assay,when the Hep 3B cells were treated with high concentration of ginger essential oil (100~200μg/ml) for 24 hours, the inhibition percentages of cell viability were significantly higher than that of the control (p<0.05). The IC50 of Hep 3B
was around 173μg/ml of ginger essential oil. Of Hep G2, treated with 50~200 μg/ml of ginger essential oil, inhibition percentages of cell viability were also significantly higher than that of the control (p<0.05). The IC50 of Hep G2
was about 94.2μg/ml of ginger essential oil. These results showed that the
inhibition effect of ginger essential oil on Hep G2 was much better than on Hep 3B. Under low concentration of ginger essential oil (50 µg/ml) treatment, there was 28.1 % inhibition of cell viability in Hep G2 cells, but no significant effect on primary rat hepatocytes, and also
decreasing the lipid peroxidation in hepatocytes. Ten µg/ml of ginger essential oil could increase the capabilities of antioxidation and detoxification systems in primary rat hepatocytes.While 100
µg/ml of ginger essential oil treatment not only could cause 22.3 and 54.7%
inhibition of cell viability in Hep 3B and Hep G2, respectively, but also cause 52.1 % inhibition of cell viability in primary rat hepatocytes.
Key words: ginger essential oil, cell viability, antioxidation, detoxification, primary rat hepatocytes, human hepatoma cell lines
二、緣由與目的
薑(Zingiber officinale Roscoe)在 中國自古栽培為藥用及蔬菜用(胡, 1978),而其以水蒸氣蒸餾法所萃取之 薑精油更廣泛應用於食品、化妝品、藥 品等工業上(Lawrence, 1984)。薑在 台灣菜餚與中國傳統藥膳保健食品之食 物療法中亦扮演著相當重要的角色。現 今科學性的文獻報告中亦顯示,薑的確 具有許多不錯的生理機能,例如可以降 低噁心嘔吐的症狀(Yamahara et al., 1989; Bone et al., 1990; Arfeen et al., 1995; Meyer et al., 1995; Visalyaputra et al., 1998; Aikins Murphy, 1998; Ernst and Pittler, 2000; Vutyavanich et al., 2001; Fugh-Berman and Kronenberg 2003,)、 提升腸胃功能(Al-Yahya et al., 1989; Wu et al., 1990; Yamahara et al., 1990; Stewart et al., 1991; Platel and Srinivasan, 1996; Sharma and Gupta, 1998;
Micklefield et al., 1999; Pin et al., 2002)、對心血管疾病之預防有助益 (Srivastava, 1984, 1986, 1989;
Srinivasan and Sambaiah, 1991; Tanabe et al., 1993; Verma et al., 1993; Lumb, 1994; Bordia et al., 1997; Bhandari et al., 1998;
Fuhrman et al., 2000)、在預防癌症方 面亦具有潛力(Hashim et al., 1994; Soudamini et al., 1995; Katiyar et al., 1996; Vimala et al., 1999; Bode et al., 2001)。由於癌症是台灣十大死亡原因 之首,而肝癌又是癌症中名列第一的頭 號殺手。且肝臟是人體中重要的解毒代 謝器官,因此探討如何利用食物來預防 癌症、護肝達到養生保健的目的,的確 是非常重要的研究課題。故本研究採用 細胞模式,探討薑精油對肝癌細胞及正 常肝細胞生理機能的影響。以期尋找出 在何種條件下薑精油能發揮抑制肝癌細 胞的效果,並能提升正常肝細胞之解毒 代謝與抗氧化能力,達到人類防癌養生 保健之目的。 目前動物細胞或組織之培養技術已 廣泛的使用於各種有關生物醫學的領 域。其中已知肝臟是具有代謝控制、酵 素誘導、對激素反應及解毒抗氧化等特 殊生理功能的器官,而這些功能的執行 大部份都在肝的實質細胞 (parenchymal cell)中進行。以肝灌注 分離新鮮肝細胞配合細胞培養(cell culture)具有專一性、成本低、實驗條 件容易控制、免於其他內生性因子的影 響及大量樣品易取得等優點。因此,目 前此技術已廣泛被應用於多種研究中 (McQueen and Williams, 1987; Davila, 1991),包括肝細胞之毒性試驗、藥物 代謝、肝細胞的功能、控制因子間交互 作用,以及抗癌及抗突變等研究。所以 培養肝實質細胞,是一項重要的實驗技 術。自 Berry 和 Friend 於 1973 年發展 出利用膠原蛋白酶分離肝細胞之技術以 來,並經 Kreamer 等人於 1986 年修正 後,使得肝細胞培養技術更趨成熟,成 功率與產量均獲長足進步。 因 此 , 本 研 究 擬 以 薑 精 油 為 實 驗 材 料,作用於人類肝癌細胞株Hep G2與 Hep 3B,了解不同濃度薑精油對肝癌細 胞之抑制率。再利用肝灌注手術分離大 白鼠初代肝細胞配合細胞培養為實驗模 式,探討活體外不同濃度的薑精油對肝
細胞之生存力、細胞形態變化、抗氧化 及解毒代謝系統之影響,以便有系統地 瞭解薑精油生理活性。 三、結果與討論 (一)薑精油之萃取與分析 以水蒸氣蒸餾法進行薑精油之萃 取,平均萃取率為 0.16%。 薑精油以 Hewlett Packard 5973 GC-MS 進行氣相層析質譜分析,分析圖譜 結果如圖一所示,共有 14 個主要吸收 波峰,其各代表成分如表一所示,薑精 油組成中以 Geranial 含量高達 25.2%, Citronellol 次之為 18.7%。這些成分多 為萜烯類碳氫化合物,也是薑精油香味 的主要來源。 (二)在肝癌細胞株 Hep G2 與 Hep 3B 生存力方面 肝癌細胞在薑精油處理 24 小時後 以 MTT assay 與倒立式位相差顯微鏡來 探討肝癌細胞生存力。在薑精油低濃度 50μg/ml 處理 24 小時後,肝癌細胞株 Hep 3B 生存力為 96.5%(p>0.05)。 當薑精油處理濃度達到 100μg/ml 時, 經由 Dunnett’s test 統計分析結果顯示, 與控制組比較下,則顯著地抑制 22.3% 肝 癌 細 胞 株 Hep 3B 之 生 存 力 ( p < 0.05 ) 。 而 高 濃 度 薑 精 油 175-200 μ g/ml 處 理 下 則 抑 制 50 % 肝 癌 細 胞 株 Hep 3B 的生存力,且形態上可明顯看 出肝癌細胞發生皺縮現象(Fig. 5D), 肝癌細胞株 Hep 3B 之 IC50約為 173μ g/ml(Fig. 2)。而在肝癌細胞株 Hep G2 方面,在低濃度 50μg/ml 薑精油處 理下已能夠顯著降低 28.1%肝癌細胞株 Hep G2 的生存力(p<0.05)。當薑精 油處理濃度達到 100μg/ml 以上時,肝 癌 細 胞 株 Hep G2 的 生 存 力 只 剩 26.8~45.3 % , 即 表 示 濃 度 達 到 100 μ g/ml 以上時可抑制 50%以上肝癌細胞 株 Hep G2 的生存力,觀察其形態也發 現肝癌細胞產生皺縮之現象(Fig. 6C, 6D ) , 其 IC50 為 94.2 μ g/ml ( Fig. 3 ) 。 由 以 上 結 果 可 知 , 薑 精 油 對 於 Hep G2 抑制生存力效果較 Hep 3B 佳, 而且在低濃度下即對 Hep G2 之生存力 具有抑制效果。 (三)大白鼠正常初代肝細胞方面 大白鼠初代肝細胞生存力: 大白鼠初代肝細胞在薑精油處理後 以 MTT assay 與倒立式位相差顯微鏡來 探討其生存力。由圖四之結果顯示,大 白鼠初代肝細胞經由 10μg/ml 薑精油 處理後,不但無抑制細胞之生存力,反 而有促進 16.2%生存力之效果。在細胞 形態方面,圖七之結果顯示經由薑精油 30μg/ml 處理 24 小時後,仍可清楚看 見大白鼠初代肝細胞的細胞膜及細胞核 相當完整(Fig. 7B),生長狀況與控制 組相似(Fig. 7A)。當薑精油的處理濃 度達到 70μg/ml 時,開始有抑制 28.4 %正常初代肝細胞生存力,隨著薑精油 處理濃度之增加初代肝細胞之生存力也 隨之下降。在薑精油濃度為 100μg/ml 以上時,正常初代肝細胞生存力僅剩 15.2∼52.3%,可見此濃度已對正常初 代肝細胞已有相當大的抑制生存力的作 用,同時細胞形態也已經漸漸有細胞膜 破損(Fig. 7D, 7E),在 200μg/ml 濃 度薑精油處理 24 小時後,細胞膜不但 破損,細胞萎縮,細胞核也消失,可見 肝細胞已受嚴重的傷害(Fig. 7F)。 脂質過氧化測定: 參考 Fraga 等人(1998)之方法。 利用脂質過氧化產物 malondialdehyde (MDA)在酸及高熱環境下,可與二 分子 thiobarbituric acid(TBA)縮合成 一粉紅色物質(TBA chromogen),在 excitation 515 及 emission 555 下,以螢 光分光光度計測定 MDA 濃度,即得 TBARS 值。結果顯示,正常大白鼠初 代肝細胞在薑精油處理組其 TBARS 質 皆低於控制組,即表示薑精油有降低脂 質過氧化之效果(Fig. 8)。此項目會 進一步深入探討。 抗氧化及解毒代謝之影響: 過氧化氫或有機過氧化物可藉由麩
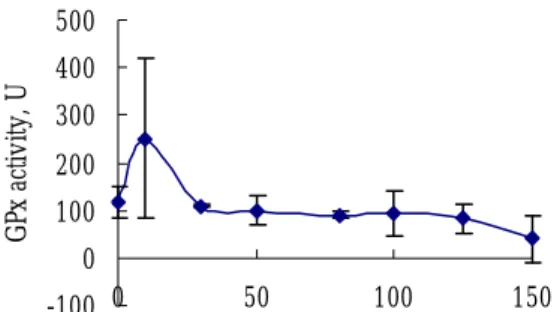
胱甘肽(GSH)與麩胱甘肽過氧化酶 (GSH peroxidase)催化代謝形成氧化 態麩胱甘肽(GSSG),之後氧化態麩 胱甘肽再利用麩胱甘肽還原酶(GSH reductase ) 還 原 成 還 原 態 麩 胱 甘 肽 (GSH),在細胞內不斷代謝利用,維 持抗氧化系統保護細胞,避免氧化性傷 害。而當外來異物質侵入細胞時,麩胱 甘肽亦參與其代謝。外來異物會在麩胱 甘肽硫轉移酶(GST)催化下與麩胱甘 肽 反 應 形 成 結 合 物 ( GST S-conjugates)達到解毒之目的。實驗結 果顯示,在低濃度薑精油(10μg/ml) 處理初代肝細胞時,能增進麩胱甘肽還 原 酶 之 活 性 ( Fig. 9 ) , GSH peroxidase 的結果與麩胱甘肽還原酶結 果相似(Fig. 10)。可見薑精油能增進 正常初代肝細胞抗氧化系統之活性,保 護肝細胞避免受到氧化性傷害。在解毒 代謝方面,低濃度薑精油處理(10μ g/ml)下,能增進初代肝細胞解毒代謝 酵素麩胱甘肽硫轉移酶(GST)之活性 (Fig. 11),與初代肝細胞生存力之結 果相呼應。 (四)結論 由多種萜烯類碳氫化合物組成的薑 精油在低濃度(50μg/ml)下,對於肝 癌細胞 Hep G2 即有抑制 28.1%生存力 之效果,且對大白鼠初代肝細胞無不良 效果。此外,10μg/ml 濃度薑精油能夠 降低其脂質過氧化,提高抗氧化及解毒 代謝系統能力。而當薑精油濃度提升至 100μg/ml 時,對肝癌細胞株 Hep 3B、 Hep G2 生存力之抑制效果達到 22.3 和 54.7%,對於正常初代肝細胞亦有 52.1 %之抑制作用。 四、計畫成果自評 目前本研究室已完成不同薑精油濃 度對於人 類肝癌 細胞 株Hep 3B、Hep G2與大白鼠正常初代肝細胞之生存力 影響,並已完成不同濃度之薑精油對大 白鼠正常初代肝細胞之脂質過氧化、肝 細胞內麩胱甘肽及其相關酵素活性分 析。 五、參考文獻 胡南輝。1978。薑。出自「莖菜栽 培」,豐年社叢書,pp. 196-204。 林宗旦、林宗平、林景彬。1996。中藥 藥理學,華香園出版社,pp. 33-37。 鄧景衡。1984。台灣土人參。農業周 刊,10:16-20。 李世滄、陳榮洲。1993。中國醫藥食補 養生大辭典,旺文社股份有限公 司,pp. 127, 138, 142, 146, 150。 葉佳聖、沈立言、李宗貴、蔡順仁。 1997a. 補骨脂水萃取物及補骨脂 酚對葡萄糖/葡萄糖氧化酵素誘發 氧化緊迫之大白鼠初代肝細胞之 保護作用。中華民國營養學會雜 誌 22:89-102。 葉佳聖、沈立言、蔡順仁。1997b. 補骨 脂酚對大白鼠初代肝細胞之生存 力、脂質過氧化、麩胱甘肽濃度 與其相關代謝酵素活性之影響。 食品科學 24:293-305。
Agarwal M, Walia S, Dhingra S,
Khambay BP. 2001. Insect growth inhibition, antifeedant and
antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Management Science 57:289-300.
Aikins MP. 1998. Alternative therapies for nausea and vomiting of pregnancy. Obstetrics & Gynecology 91:149-155.
Arfeen A, Owen H, Plummer JL, Ilsley AH, Sorby-Adams RA, Doecke CJ. 1995. A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting. Anaesthesia & Intensive Care 23:449-452.
Banerjee S, Sharma R, Kale RK, Rao AR. 1994. Influence of certain essential oils on carcinogen-metabolizing enzymes and acid-soluble
sulfhydryls in mouse liver. Nutrition & Cancer 21:263-269.
Berry MN and Friend DS. 1973. High yield production of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Biol Chem. 59:722-734.
Bhandari U, Sharma JN, Zafar R. 1998. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J
Ethnopharmacol 16:167-171. Bode AM, Ma WY, Surh YJ, Dong Z.
2001. Inhibition of epidermal growth factor-induced cell
transformation and activator protein 1 activation by ginger. Cancer Res 61:850-853.
Bone ME, Wilkinson DJ, Young JR, Mcneil J, Charlton S. 1990. Ginger root- a new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major
gynaecological surgery. Anaesthesia 45:669-671.
Bordia A, Verma SK, SrivastavsKC. 1997. Effect of ginger (Zingiber officinale Rosc.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostagland Leuk Essen Fatty 56:379-384.
Chen HC, Chang MD, Chang TJ. 1985. Antibacterial properties of some spice plants before and after heat treatment. Chinese Journal of Microbiology & Immunology 18:190-195.
Davila JC, Rodriguez M, Acosta D. 1991. Toxicological studied of gossypol in primary culture of postnatal rat hepatocytes. In Vitro Toxicol 4:161-172.
Ernst E and Pittler MH. 2000. Efficacy of ginger for nausea and vomiting: a systemic review of randomized clinical trails. Brit J Anaesth 84(3):367-371.
Freshney RI. 1994. In “Culture of Animal Cells: a manual of basic technique”
chapter 1, 2 and 16. Wiley-Liss Inc. New York.
Fugh-Berman A, Kronenberg F. 2003. Complementary and alternative medicine (CAM) in reproductive-age women: a review of randomized controlled trials . Reprod Toxicol. 17(2):137-152.
Fuhrman B, Rosenblat M, Hayek T, Coleman R, Aviram M. 2000. Ginger extract consumption reduces plasma cholesterol inhibits LDL oxidation and attenuates
development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr 130:1124-1131.
Gugnani HC and Ezenwanze EC. 1985. Antibacterial activity of extracts of ginger and African oil bean. J Communicable Disease 17:233-236. Hashim S, Aboobaker VS, Madhubala R,
Bhattacharya RK, Rao AR. 1994. Modulatory effects of essential oils form spices on the formation of DNA adduct by aflatoxin B1 in vitro. Nutr Cancer 21:169-175. Howard RB, Christensen AK, Gibbs FA,
Pesch LA. 1967. The enzymatic preparation of isolated, intact parenchymal cells from rat liver. J Cell Biol 35:675-684.
Janes ME, Nannapaneni R, Johnson MG. 1999. Identification and
characterization od two bacteriocin-producing bacteria isolated from garlic and ginger root. J Food Protect 62:899-904.
Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MTS, Pitot HC. 1986. Use of a low-speed, isodensity percoll centrifugation method to increase the viability of isolated hepatocyte preparations. In Vitro Cell Develop Biol 22:201-211. Langmead L and Rampton DS. 2001.
Review article: herbal treatment in gastrointestinal and liver disease- benefits and dangers. Aliment
Lawrence BM. 1984. Major tropical spices-ginger (Zingiber officinale Rosc.). Perfumer & Flavorist 9:1-40. Lii CK, Wang ST, Chen HW, Sheen LY.
1996. Glutathione and glutathione-related enzyme activities of of male and female rat hepatocytes under various culture conditions. Arch Toxicol 70:822-829.
Lumb AB. 1994. Effect of dried ginger on human platelet function. Thromb Haemost 71:110-111.
Katiyar SK, Agarwal R, Mukhtar H. 1996. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale
rhizome. Cancer Res 56:1023-1030. McQueen CA and Williams GM. 1987.
Toxicology studies in cultured hepatocytes from various species. Life Sci 18:1139-1144.
Meyer K, Schwartz J, Crater D, Keyes B. 1995. Zingiber officinale (ginger) used to prevent 8-Mop associated nausea. Dermatoloty Nursing 7:242-244.
Micklefield GH, Redeker Y, Meister V, Jung O, Greving I, May B. 1999. Effects of ginger on gastroduodenal motility. Int J Clin Pharm
Therapeutics. 37:341-346. Pin M, Yoshio K, Atsushi I, Hiroshi S,
Susumu K, Takashi N. 2002. The herbal medicine Dai-kenchu-to and one of its active components ﹝6﹞-shogaol increase intestinal blood flow in rats. Life Sci 70:2061-2070. Platel K and Srinivasan K. 1996.
Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Nutr 47:55-59. Reddy AC and Lokesh BR. 1992. Studies
in spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem 11:117-124.
Sambaiah K and Srinivasan K. 1989. Influence of spices and principles on hepatic mixed function
oxygenase system in rat. Indian J Biochem Biophys. 26:254-258. Seglen PO. 1972. Preparation of rat liver
cells. II Effects of ions and
chelators on tissue dispersion. Exp Cell Res 76:25-30.
Sharma SS and Gupta YK. 1998. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale). J Ethnopharmacol 62:49-55.
Sheen LY, Lii CK, Sheu SF, Meng RHC, Tsai SJ. 1996. Effect of the active principle of garlic-diallyl sulfide- on the cell viability, detoxification capability and antioxidantion system of primary rat hepatocytes. Food Chem Toxicol 34:971-978. Sheen LY, Sheu SF, Tsai SJ, Meng RHC,
Lii CK. 1999a. Effect of garlic active principle of garlic – diallyl sulfide – on the cell viability, lipid peroxidation, glutathione
concentration and its related enzyme actives in primary rat hepatocytes. Amer J Chin Med 27:95-105.
Sheen LY, Wu CC, Lii CK, Tsai SJ. 1999b. Metabolites of diallyl disulfide and diallyl sulfide in primary rat
hepatocytes. Food Chem Toxicol 37:1139-1146.
Sheen LY, Wu CC, Lii CK, Tsai SJ. 2001. Effect of diallyl disulfide and diallyl sulfide, the active principles of garlic, on the alflatoxin B1-induced DNA damage in primary rat
hepatocytes. Toxicol Lett 122:45-52. Soudamini KK, Unnikrishnan MC,
Sukumaran K, Kuttan R. 1995. Mutagenicity and anti-mutagenicity of selected spices. Indian J Physiol & Pharmacol 39:347-353.
Srinivasan K and Sambaiah K. 1991. The effect of spices on cholesterol 7 alpha-hydroxylase activity and on serm and hepatic cholesterol levels in the rat. Int J Vit & Nutr Res 61:364-369.
extracts of onion, garlic and ginger on platelet aggregation and
metabolism of arachidonic acid in blood vascular system: in vitro study. Prostaglandin Leuk Essent Fatty 13:227-235.
Srivastava KC. 1986. Isolation and effects of some ginger components of platelet aggregation and eicosanoid biosynthesis. Prostaglandin Leuk Essent Fatty 35:183-185.
Stewart JJ, Wood MJ, Wood CD, Mims ME. 1991. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology 42:111-120.
Tanabe M, Chen YD, Saito K, Kano Y. 1993. Cholestrol biosynthesis inhibitory component form Zingiber officinale Roscoe. Chem Pharm Bull Tokyo 41:710-713.
Tantaoui-Elaraki A and Beraoud L. 1994. Inhibition of growth and aflatoxin production in Aspergillus
parasiticus by essential oil of selected plant materials. J Environ Pathol Toxicol & Oncol 13:67-72. Vimala S, Norhanom AW, Yadav M. 1999.
Anti-tumor promoter activity in Malaysian ginger rhizobia used in traditional medicine. Bri J Cancer 80:110-116.
Visalysputra S, Petchpaisit N,
Somcharoen K, Choavaratana R. 1998. The efficacy of ginger root in the prevention of postoperative nausea and vomiting after outpatient
gynaecological laparoscopy. Anaesthesia 53:506-510.
Vutyavanich T, Kraisarin T, Ruangsri R. 2001. Ginger for nausea and
vomiting in pregnancy: randomized, double-masked, placebo-controlled trail. Obstet Gynecol 97:577-582. Wattenberg LW. 1985. Chemoprevention
of cancer. Cancer Res 45:1-8. Weinstein IB. 1981. The scientific basis
for carcinogen detection and primary cancer prevention. Cancer 47:1133-1141.
al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS, Tariq M. 1989. Gastroprotective activity of ginger Zingiber officinale Rosc. in albino rats. Amer J Chin Med 17:51-56.
Yamahara J, Huang QR, Li YH, Xu L, Fujimura H. 1990. Gastrointestinal mobility enhancing effect of ginger and its active constituents. Chem Pharm Bull Tokyo 38:430-431. Yamahara J, Rong HQ, Naitoh Y, Kitani T,
Fujimura H. 1989. Inhibition of cytotoxic drug-induced vomiting in suncus by a ginger constituent. J Ethnopharmacol 27:353-355. Yin MC and Cheng WS. 1998. Inhibition
of Aspergillus niger and Aspergillus flavus by some herb and spices. J Food Protect 61:123-125.
Fig. 1. Gas chromatogram of ginger essential oil.
Table 1. The composition of ginger essential oil
Peak number
component M. W. Conc. (%) CAS Reg. No.
1 Myrcene 136 4.67 123-35-3 2 Limonene 136 2.60 138-86-3 3 β-phellandrene 136 8.70 555-10-2 4 1,8- cineole 154 10.33 470-86-2 5 Terpinolene 136 1.45 586-62-9 6 2-heptanol+ 6-methyl-5-hepten-2-one - 2.61 543-49-7 110-93-0 7 citronellal+ α-copaene - 2.27 106-23-0 3856-25-5 8 Linalool 154 3.65 78-70-6 9 Neral 152 2.74 106-26-3 10 Zingiberene 204 3.23 495-60-3 11 Geranial 154 25.24 141-27-5 12 β-sesquiphellandrene + ar-curcumene + geranyl acetate - 6.69 20307-83-9 644-30-4 105-87-3 13 Citronellol 156 18.69 106-22-9 14 Geraniol 154 0.99 106-24-1 5.00 10.00 15.00 20.00 25.00 30.00 0 500000 1000000 1500000 2000000 2500000 3000000 3500000 4000000 4500000 5000000 Time--> Abundance TIC: 920128S3.D 2 4 6 8 3 11 14 1 9 5 7 10 13 12
0 20 40 60 80 100 120 0 50 100 150 200 v ia b ility ( % o f c o n tr o l)
Conc. of ginger oil (μg/ml)
Fig. 2. Effect of various concentrations of ginger oil on the cell viability of hepatoma cell line Hep 3B after 24 hrs treatment. Values are expressed as means±SD from 3 data.* Significant difference (p<0.05) by Dunnett’s test .
0 20 40 60 80 100 120 0 50 100 150 200 v ia b ility ( % o f co n tr o l)
Conc. of ginger oil (μg/ml)
Fig. 3. Effect of various concentrations of ginger oil on the cell viability of hepatoma cell line Hep G2 after 24 hrs treatment. Values are expressed as means±SD from 3 data.* Significant difference (p<0.05) by Dunnett’s test.
* * * * * * * * * * *
D
0 50 100 150 200 0 50 100 150 200 v ia b ility ( % o f c o nt ro l)Conc. of ginger oil (μg/ml)
Fig. 4. Effect of various concentrations of ginger oil on the cell viability of primary rat hepatocytes after 24 hrs treatment by MTT assay. * Significant difference (p<0.05) by Dunnett’s test.
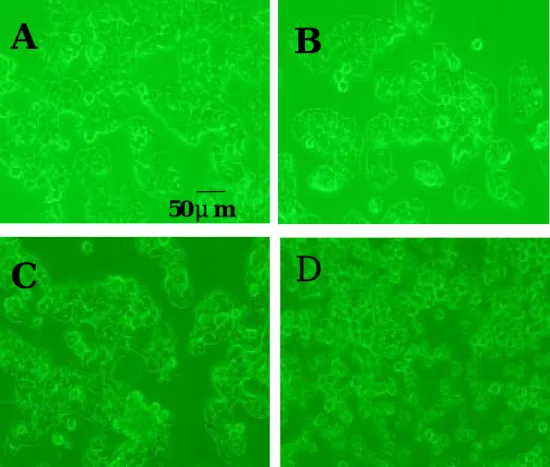
Fig. 5. Effect of various concentrations of ginger oil on the morphology of hepatoma cell line Hep 3B after 24 hrs treatment. A=control, B=50μg/ml, C=100μg/ml, D=175μg/ml. (100X) ** * * * *
50μm
A
C
B
D
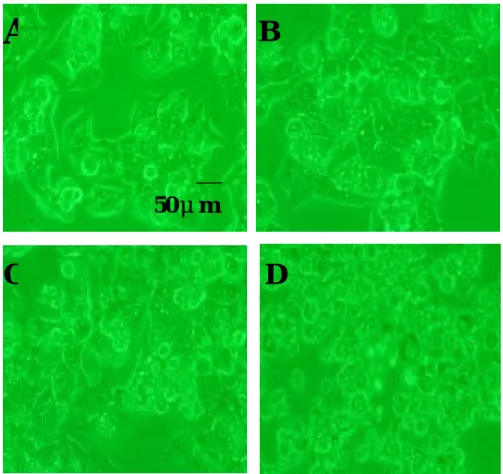
Fig. 6. Effect of various concentrations of ginger oil on the morphology of hepatoma cell line Hep G2 after 24 hrs treatment. A=control, B=30 μg/ml, C=100μg/ml, D=150μg/ml. (200X)
A
B
C
D
E. 150 μg/ml
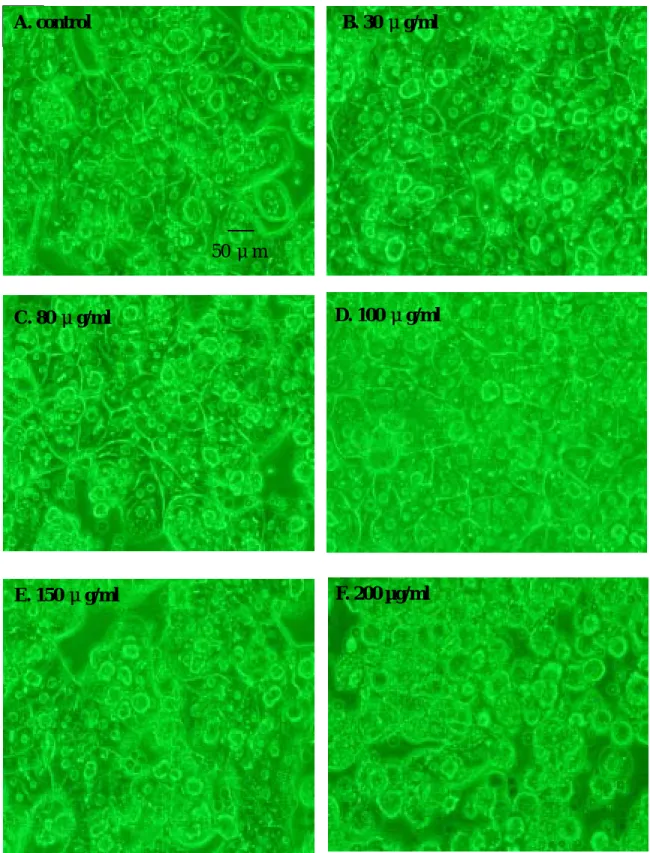
Fig. 7. Effect of various concentrations of ginger oil on the morphology of primary rat hepatocytes after 24 hrs treatment. (200X)
A. control
50 μm
C. 80 μg/ml D. 100 μg/ml
F. 200 µg/ml B. 30 μg/ml
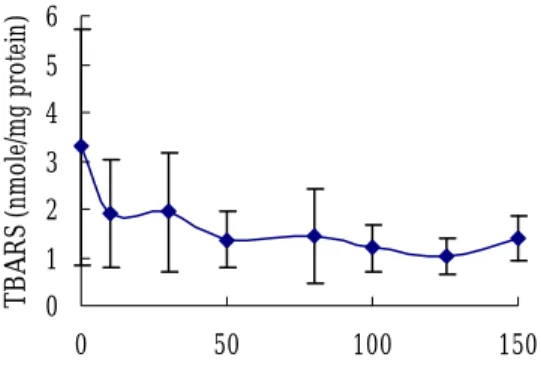
0 1 2 3 4 5 6 0 50 100 150 T B AR S ( nm ole/m g p ro te in )
Conc. of ginger oil (μg/ml)
Fig. 8. Effect of various concentrations of ginger oil on thiobarbituric acid-reactive substances (TBARS) production in primary rat hepatocytes after 24 hrs treatment. 0 50 100 150 200 0 50 100 150 G R d a ct ivi ty , U
Conc. of ginger oil (μg/ml)
Fig. 9. Changes of GSH reductase activity in primary rat hepatocytes treated with various concentrations of ginger oil for 24 hrs.
-100 0 100 200 300 400 500 0 50 100 150 G Px a ct ivi ty , U
Conc. of ginger oil (μg/ml)
Fig. 10. Changes of GSH peroxidase activity in primary rat hepatocytes treated with various treatment concentrations of the ginger oil for 24 hrs. 0 500 1000 1500 2000 0 50 100 150 GS T ac tiv ity , U
Conc. of ginger oil (μg/ml)
Fig. 11. Changes of GSH S-transferase activity in primary rat hepatocytes treated with various treatment concentrations of the ginger oil for 24 hrs.