Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies are
encouraged to visit:
Immunochemical and molecular characterization of a novel cell line derived from the
brain of Trachinotus blochii (Teleostei, Perciformes): A
fish cell line with
oligodendrocyte progenitor cell and tanycyte characteristics
Chiu-Ming Wen
a,⁎
, Chun-Shun Wang
a, Tzu-Chuan Chin
a, Shih-Ting Cheng
a, Fan-Hua Nan
b,⁎
aDepartment of Life Sciences, National University of Kaohsiung, Kaohsiung, Taiwan b
Department of Aquaculture, National Taiwan Ocean University, Keelung, Taiwan
a b s t r a c t
a r t i c l e i n f o
Article history:
Received 26 October 2009
Received in revised form 3 February 2010 Accepted 10 February 2010
Available online 16 February 2010 Keywords:
DARPP-32 Ependyma Fish GFAP Neural stem cell Olig2 Oligodendrocyte Radial glia
Ependymal radial glial cells, also called tanycytes, are the predominant glialfibrillary acidic protein (GFAP)-and vimentin (VIM)-expressing cells infish ependyma. Radial glial cells have been proposed to be neural stem cells but their molecular expression is not well understood. Previous studies revealed thatfish neural progenitor and neural stem cells have A2B5, a marker for oligodendrocyte progenitor cells (OPCs). In this study, an A2B5+cell line, SPB, was isolated from the brain of the teleost Trachinotus blochii and characterized.
SPB cells usually grew as polygonal epithelial cells, but at high density, long processes were commonly observed. Using immunocytochemistry, SPB cells were shown to exhibit oligodendrocyte markers such as galactocerebroside and Olig2, and radial glial cell markers such as brain lipid-binding protein, GFAP, Sox2, and VIM. SPB cells were also observed to have DARPP-32, a marker for tanycytes in mammals, and primary cilia. RT-PCR additionally revealed expression of bone morphogenetic protein 4, connexin35, Noggin2, and proteolipid protein in SPB cells. Results of this study suggest that SPB cells are OPCs that can display tanycyte characteristics. Fish tanycytes can be neural stem cells suggesting that SPB cells are neural stem cells. SPB is thefirst fish cell line showing primary cilia and markers for both OPCs and tanycytes.
© 2010 Elsevier Inc. All rights reserved.
1. Introduction
In vitro studies have shown the presence of oligodendrocyte progenitor cell (OPC) markers A2B5, proteolipid protein (PLP), and DM20, as well as the presence of the astroglia marker, glialfibrillary acidic protein (GFAP), in teleost brain cells (Jeserich and Stratmann, 1992; Sivron et al., 1992, 1994; Wen et al., 2008a, 2009). Most of the proliferating cells in adult fish brain localize along the ependyma where neural stem and neural progenitor cells exist (Zupanc and Horschke, 1995; Ekström et al., 2001; Adolf et al., 2006; Chapouton et al., 2006; Grandel et al., 2006; Pellegrini et al., 2007; Lam et al., 2009). Ependymal radial glial cells or tanycytes are the most abundant GFAP- and vimentin (VIM)-expressing cells in the central nervous system (CNS) of adult teleosts (Kálmán, 1998; Arochena et al., 2004; Lazzari and Franceschini, 2004) suggesting that GFAP-expressing cells cultured in vitro are tanycytes.
Fish tanycytes have been reported to express aromatase B (AroB), brain lipid-binding protein (BLBP), NADPH-diaphorase, glutamine synthetase (GS), Nestin, S100 protein, Sox2, and VIM in addition to GFAP (Forlano et al., 2001; Adolf et al., 2006; Germanà et al., 2008; Lam et al., 2009; Tong et al., 2009; Wen et al., 2009). Fish tanycytes are similar to other ependymal cells, and exhibit epithelial character-istics, including the presence of keratins (8 and 18), desmosomes, and connexin-43 (Cx43) type gap junctions (Bodega et al., 1993, 1994, 1995; Bruni, 1998; Hernández et al., 1999; Wen et al., 2009); more-over, they may or may not be ciliated (Ma, 1993; Shioda et al., 1997; Bruni, 1998).
In rodents, four OPC types have been observed: glial-restricted precursors, motor neuron–oligodendrocyte precursors, oligodendro-cyte-type 2 astrocyte (O-2A) progenitors, and polydendrocytes (Liu and Rao, 2004). Typically, O-2A cells arise from A2B5-positive, platelet-derived growth factorα receptor (PDGFRα)-negative glial-restricted precursors, but some O-2A cells are derived from A2B5-negative and PDGFRα- and NG2 (AN2)-positive polydendrocytes (Baracskay et al., 2007). Glial-restricted precursors are derived from neuroepithelial cells (Rao and Mayer-Proschel, 1997) whereas polydendrocytes are gener-ated from GFAP-, VIM-, or PDGFRα-positive type B cells (Menn et al., 2006; Li and Grumet, 2007), which are generated from radial glial cells (Merkle et al., 2004). OPCs have been reported to exhibit the stem cell markers Nestin and Sox2, and may also exhibit oligodendrogenic
⁎ Corresponding authors. Wen is to be contacted at the Department of Life Sciences, National University of Kaohsiung, No. 700, Kaohsiung University Road, Nan-Tzu District, Kaohsiung 81148, Taiwan. Tel.: + 886 7 5919231; fax: + 886 7 5919404. Nan, Department of Aquaculture, National Taiwan Ocean University, No. 2, Beining Road, Jhongjheng District, Keelung 202, Taiwan. Tel.: +886 2 2462 2192x5231.
E-mail addresses:wenchiumin@nuk.edu.tw(C.-M. Wen),fhnan@mail.ntou.edu.tw (F.-H. Nan).
1095-6433/$– see front matter © 2010 Elsevier Inc. All rights reserved. doi:10.1016/j.cbpa.2010.02.003
Contents lists available atScienceDirect
Comparative Biochemistry and Physiology, Part A
j o u r n a l h o m e p a g e : w w w. e l s e v i e r. c o m / l o c a t e / c b p atranscription factors Olig1, Olig2, and Nkx2.2 (Liu et al., 2002; Liu and Rao, 2004; Jakovcevski et al., 2009). During oligodendrocyte maturation, the OPCs may also exhibit the oligodendrocyte precursor proteins O1 and O4, PLP, DM20, Sox8, Sox9, Sox10, galactocerebroside (GalC) and/or myelin basic protein; however, they may show a loss of Sox2, NG2, A2B5, and PDGFRα expression (Liu et al., 2002; Jakovcevski et al., 2009). In the teleost CNS, a subset of radial glial cells are either OPCs or they may generate OPCs (Park and Appel, 2003; Park et al., 2007; Kim et al., 2008a,b). As in rodents,fish OPCs also express oligodendrocyte transcription factors Nkx2.2, Olig1, Olig2, and Sox10 (Kucenas et al., 2008; Schebesta and Serluca, 2009; Zannino and Appel, 2009). Gen-eration of OPCs infish as in other vertebrates is up-regulated by the Hedgehog and Notch signal transduction pathways, whereas it is down-regulated by Wnt and bone morphogenetic protein (BMP) signal pathways (Park and Appel, 2003; Kim et al., 2008a,b). Recently, a continuous cell line (GBC4), established from grouper (Epinephelus coioides) brain, was reported to express markers for OPCs and tanycytes, suggesting that tanycytes can display OPC characteristics (Wen et al., 2009). However, compared to the amount of information available from mammal studies,fish OPCs and tanycytes are not well known. To increase knowledge of the characteristics offish OPCs and tanycytes, studies using continuous cell lines from adultfish CNS are useful.
In this study, an A2B5-expressing continuous cell line (SPB) was established from the brain of snubnose pompano (Trachinotus blochii) and was analyzed using immunocytochemistry with a panel of anti-bodies and RT-PCR to reveal molecular expressions. Our results demonstrate that SPB cells have both tanycyte- and OPC-specific characteristics. The presence of primary cilia, along with expression of DARPP-32, a rodent tanycyte marker (Hemmings and Greengard, 1986; Hökfelt et al., 1988), Olig2, an oligodendrocyte transcription factor, and Noggin2, an antagonist of BMPs, was revealed in the SPBfish cells in vitro.
2. Materials and methods
2.1. Primary culture and isolation of A2B5-expressing cells
Adult snubnose pompano (T. blochii Lacepède, 1801; Perciformes, Carangidae), approximately 35 cm long, were obtained from a local fish farm (Yeongan, Kaohsiung, Taiwan). The fish were anaesthetized with MS-222 (Sigma, St. Louis, MO, USA) and decapitated aseptically. The entire brain was removed and finely chopped with scissors in phosphate-buffered saline (PBS, Ca2+and Mg2+free). The tissue
frag-ments from a brain were then washed several times in an antibiotic solution (PBS containing 500μg/mL streptomycin and 500 IU/mL pen-icillin), separated randomly, and then planted into three 25-cm2
stan-dard cell cultureflasks (Nunc, Roskilde, Denmark). A volume of 2 mL Leibovitz's L-15 medium supplemented with 15% fetal bovine serum (HyClone, South Logan, UT, USA) was added to eachflask, and cells were incubated at 25 °C. Every 4–6 days, half of the medium was removed and replaced with fresh medium until passaging.
Primary cultures were subcultured 6 weeks after the culture was initiated. Cells were then washed twice with PBS and dislodged from theflask surface with trypsin (0.1% w/v, in PBS; Sigma) containing 0.2% EDTA and subcultured at a ratio of 1:2. After thefirst passage, cells were passaged every week. A2B5-expressing cells were isolated at the third passage using monoclonal anti-A2B5 (Sigma) and Dynabeads® rat anti-mouse IgM immunomagnetic beads (Invitrogen, Taipei, Taiwan) according to the manufacturer's protocols. About 1 × 106cells were suspended and labeled with 1μg A2B5 in 1 mL L-15
medium (containing 1% serum) in a sterile 1.5-mL microtube. A vol-ume of 10μL suspended Dynabeads was used to isolate the labeling cells. The isolated cells were cultured and passaged as previously described. The cell line was designated SPB.
2.2. Immunocytochemistry
Fish neural cells are demonstrated expressing antigens that are conserved in mammalian neural cells and can be identified using the antibodies against mammalian antigens. The primary antibodies, targets, and dilutions for immunocytochemistry are listed inTable 1. The specificities of the antibodies on fish cells, except for rabbit anti-DARPP-32 and anti-Olig2, have been described (Wen et al., 2008a, 2009). The anti-DARPP-32 and anti-Olig2 antibodies were predicted cross-reactive withfish according to the amino acid sequences of the antigens. Secondary antibodies were FITC-conjugated anti-rabbit IgG (1:100), anti-mouse IgG (1:100), and anti-mouse IgM (1:50), and Alexa Fluor® 568-conjugated anti-rabbit IgG (1:200).
SPB cells were examined for the expression of certain molecules at selected passages. Cells (2 or 4 × 104) were planted on 12-mm di-ameter uncoated coverslips (Glaswarenfabrik, Sondheim, Germany) in 4-well plates (Nunc). Cells were grown on coverslips at 25 °C for 1– 3 days,fixed in formaldehyde (3.7% in PBS, v/v) for 10 min at room temperature, washed several times in 1% Triton X-100 PBS and then incubated with the primary antibody at 37 °C for 1 h. After several washes, cells were incubated with the appropriate secondary antibody at 37 °C for 30 min. Nuclei were counterstained with 4 ′,6-diamidino-2-phenylindole (DAPI, Sigma) for 1 min. Labeled cells were visualized under an Olympus IX51 invertedfluorescence microscope (Yuan Li instrument Co., Taipei, Taiwan) or an Axiovert 200 fluorescence microscope (Carl Zeiss, Göttingen, Germany). Negative controls (omission of the primary antibody) were included in each experiment. GBC4 and GBC1 cells (Wen et al., 2008b) were additionally used as positive and negative controls in some experiments.
2.3. RT-PCR analyses
Total RNA in SPB cells at passages 40–60 was isolated for RT-PCR using the blood/culture cell total RNA isolation kit (Favorgen, Ping-Tung, Taiwan) according to the manufacturer's protocols. RNA con-centration and purity were determined by measuring the absorbance at 260 nm and 280 nm. RNA (5μg or 5 μL per sample) was used to generate cDNA using the MMLV-RT kit (Promega, Madison, WI, USA). Primer sets used in this study are listed inTable 2. Primers other than those previously reported were desigened using Primer Premier 5 software (Premier Biosoft International, Palo Alto, CA, USA). Primer sets specific for BLBP, Noggin2, and Olig2 were designed, respectively, from spotted green pufferfish (Tetraodon nigroviridis CR684531), three-spined stick-leback (Gasterosteus aculeatus BT028450), and zebrafish (Danio rerio
Table 1
Primary antibody, target, source, and dilution for immunocytochemistry. Antibody Target Species
and clone
Company Dilution Chicken A2B5 Oligodendrocyte
progenitor cells
Mouse 105 Sigma 1:100 Sea urchin
acetylated tubulin
Cilia Mouse 6-11B-1 Invitrogen 1:100
Human BLBP Radial glial cells Rabbit polyclonal Abcam 1:200 Human Cx43 Epithelial gap
junctions
Rabbit polyclonal Sigma 1:200 Human DARPP-32 Tanycytes Rabbit polyclonal Abcam 1:100 Bovine GalC Oligodendrocytes Rabbit polyclonal Chemicon 1:100 Porcine GFAP Astroglia Mouse GA5 NeoMarkers 1:200 Sheep GS Astroglia Mouse GS-6 Chemicon 1:200 Human keratin Epithelia Mouse C11 NeoMarkers 1:200 Mouse Olig2 Oligodendrocytes Rabbit polyclonal Abcam 1:100 Bovine S100 Astroglia Rabbit polyclonal NeoMarkers 1:200 Human Sox2 Neural progenitor
cells
Rabbit polyclonal Abcam 1:200 Porcine VIM Radial glia Mouse V9 NeoMarkers 1:200 C.-M. Wen et al. / Comparative Biochemistry and Physiology, Part A 156 (2010) 224–231
AF442964). PCR amplifications were performed using the Taq DNA Pol Master Mix (Ampliqon, Copenhagen, Denmark). cDNA (1 µL), 1 µM each of forward and reverse primers, and 12.5 µL of Master Mix were added to each 0.2-mL thin-wall PCR tube according to the manufac-turer's protocol. PCR was carried out under the following conditions: 30 cycles of denaturation at 95 °C for 1 min, annealing (as inTable 2) for 30 s, and extension at 72 °C for 1 min, with afinal extension at 72 °C for 7 min. PCR products were electrophoresed on a 1.5% agarose gel. The observed sizes corresponded to the predicted values (Table 2) were cut from the agarose and then purified with Gel purification kit (Favorgen) according to the manufacturer's protocols. Sequencing of the DNA fragments was performed commercially (Genomic BioSci & Tech, Taipei, Taiwan). Sequences were identified using BLASTN (http://www.ncbi. nlm.nih.gov/blast/Blast.cgi).
3. Results
3.1. Establishment of an A2B5-expressing cell line
Four weeks after the start of primary culture, a confluent mono-layer was obtained. Most of the cells that migrated and proliferated in the culture were polygonal epithelial-like cells (Fig. 1). Oligodendro-cyte-like cells, which grew on, or at, the margins of the monolayer, were also frequently observed (Fig. 1, arrows). Epithelial-like cells remained predominant for several passages (data not shown). Im-munocytochemistry at the third passage showed that many of the cells expressed keratin in the cytoplasm and A2B5 on the surface (Fig. 2). The A2B5-expressing cells at the third passage were isolated by resuspending the cells and then purifying them with monoclonal mouse anti-A2B5 and rat anti-mouse IgM immunomagnetic beads. Subsequently, the purified cells attached and grew on the culture flask, and over 95% of the cells had magnetic beads on their surface. The beads were detached from the cells upon treatment with trypsin and subsequent subculturing. Most (75–90%) of the purified cells, dependent on cell density, displayed a polygonal morphology, and a few cells (10–25% cells) had one or more long processes. The purified cells that expressed varying levels of A2B5 were subcultured for more than 50 times and were designated as the SPB continuous cell line. Cells of the established SPB line exhibited morphological and
molecular expression patterns similar to those observed in the early passages of A2B5-expressing cells.
3.2. Immunocytochemical characterization
For immunocytochemistry, SPB cells at every 5–8 passages (at 5– 60 passages) were grown on uncoated coverslips. To characterize expression of oligodendrocyte markers, SPB cells were stained with
Table 2
Primer sets used for RT-PCR. Primer set Sequences (5′–3′)a Product size (bp) Annealing (°C) Reference BLBP F: atggtcgacgccttctgtgc R: ggcccttcatgccttctcgta 405 57 This study BMP4 F: gtttaacctcagcagcatcc R: agccctccactaccatttcc 782 55 Wen et al. (2009) Cx35 F: wgtktgtggtgatcttccg R: tgtcttctccgtgggtct
619 50 Wen et al. (2008a)
Cx43 F: ggctgctcatccccaactg R: gactgctcattctgctgctgg
325 57 Wen et al. (2008a)
GFAP-3 F: aggacctgctcaatgtcaag R: ctccgtagtggactctttaatgat
230 55 Wen et al. (2009)
GS F: aagggctccaacagcgacat R: cagccagcaccgttccagtt
545 55 Wen et al. (2008a)
Noggin2 F: atgggcctctcacaaacgctactc R: tcagcacgaacacttgcactctg 634 57 This study Olig2 F: tgcgsctmaagatcaacaga R: carctggacggrtggaarcc 247 57 This study PDGFRα F: gaggacagcgggaactacaccat R: tttgagcatctttactgccaccttc 735 55 Wen et al. (2009) PLP F: caaatgtttccggtcagg R: ccgaaggtctgcttgaca
344 50 Wen et al. (2008a)
Sox2 F: tcaacgctgttcctgatgta R: ggcacggtgctctggtagtg
941 55 Wen et al. (2009)
aF: forward; R: reverse; degenerate bases, k: g or t; m: a or c; r: a or g; s: c or g; w: a
or t.
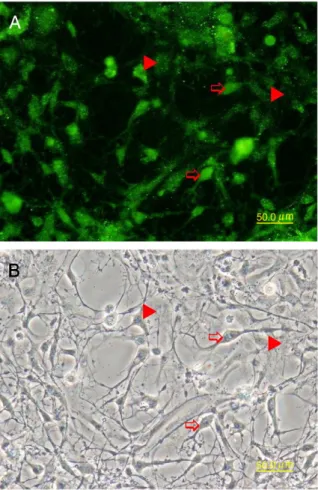
Fig. 1. Monolayer of primary cells isolated from the brain of snubnose pompano. Cells migrated from the adherent explants and proliferated in vitro for 2 weeks. The monolayer consisted mostly of polygonal cells, but oligodendrocyte-like cells (arrows) were also observed. Bar = 50μm.
Fig. 2. Cells at 3rd passage grown on coverslips werefixed with 3.7% formaldehyde and stained with anti-A2B5 and FITC-conjugated secondary antibody. (A) The labeling of A2B5 appeared on the surface of polygonal and oligodendrocyte-like cells. (B) Phase contrast micrograph. Bar = 50μm.
rabbit anti-GalC and anti-Olig2. All cells were labeled by the antib-odies, but the intensity of the labeling varied among cells. GalC staining was especially strong in oligodendrocyte-like cells (Fig. 3). The staining with anti-Olig2 appeared mainly in the nucleus and nucleolus; however, Olig2 in the cytoplasm was also noted (Fig. 4). The Olig2-expression cells also showed GFAP in the cytoplasm (Fig. 4).
Since fish OPCs in vitro have characteristics of tanycytes (Wen et al., 2008a, 2009), the SPB cells were examined for the presence of tanycyte markers, including the presence of a primary cilium and expression of BLBP, Cx43, DARPP-32, GFAP, GS, keratin, S100, Sox2, and VIM. Both polygonal and process-bearing SPB cells expressed these proteins (Fig. 5A–H). SPB cells have a single primary cilium (Fig. 5A, F) as determined by anti-acetylated α-tubulin staining (Alieva et al., 1999). DARPP-32 labeling in SPB cells was high in the nucleus and scarce in the cytoplasm (Fig. 5A, D). Similar to the DARPP-32 results, anti-BLBP labeling was concentrated in the nucleus of SPB cells and was scarce in the cytoplasm (Fig. 5B). Bundles of GFAP were observed in the cytoplasm in radial glia-like SPB cells, but GFAP labeling was faint in polygonal SPB cells (Fig. 5B). The staining with anti-VIM (Fig. 5C, D, G) was similar to that of GFAP, but was more intense, while the staining with anti-Sox2 was approximately homog-enous in the nucleus and cytoplasm (Fig. 5C, E). However, the Sox2-and BLBP-positive polygonal cells present in SPB cells Sox2-and in previous studies (Wen et al., 2008a, 2009), showed sparse GFAP (Fig. 5B) and VIM (Fig. 5D) labeling. Keratin was observed in the cytoplasm of the polygonal cells (Fig. 5E) and in those cells with long processes, but the polygonal cells had higher levels (data not shown). The staining for the gap junction protein Cx43 was punctate, and either
adjoined to, or disconnected from, the polygonal cells (Fig. 5F, G), but Cx43 staining was scarce in the SPB cells with long processes (Fig. 5G). Except for a few oligodendrocyte-like cells that exhibited high levels of GS and S100, staining for these proteins was sparse in most SPB cells (Fig. 5H).
3.3. Specific gene expression
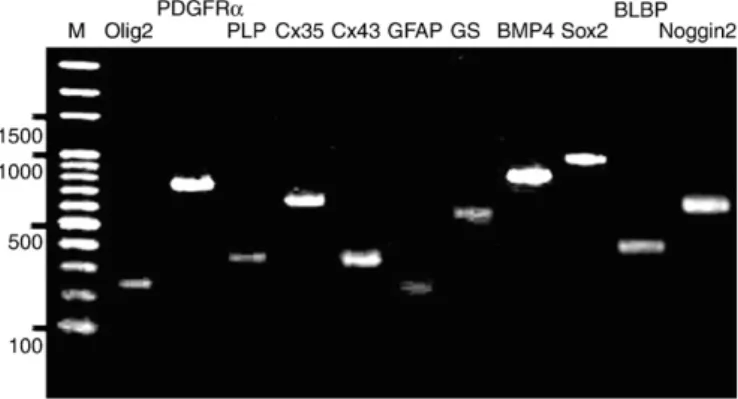
Purified DNA fragments from the SPB cell line used for sequenc-ing were confirmed by ensuring that their sizes corresponded to the predicted values (Fig. 6). All of the SPB nucleotide sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index. html; BLBP GU001777, BMP4 GU001778, Cx35 GU001779, Cx43 GU001780, GFAP GU001781, GS GU001782, Noggin2 GU001783, Olig2 GU001784, PDGFRα GU001785, PLP GU001786, and Sox2 GU001787). The BLAST program revealed that the nucleotide sequences encoding the BMP4, Cx35, Cx43, GFAP, GS, PDGFRα, PLP and Sox2 proteins of SPB respectively had 93, 96, 100, 100, 93, 89, 94, and 96% identities with the respective BMP4 (FJ436409), Cx35 (EU798295), Cx43 (EU798290), GFAP (EU798291), GS (EU798292), PDGFRα (EU798293), PLP (EU798294), and Sox2 (FJ432695) proteins of GBC4 (orange-spotted grouper E. coioides, data not shown). Respectively, the SPB BLBP, Noggin2, and Olig2 nucleotide sequences had 91, 93, and 85% identities with pufferfish (T. nigroviridis) BLBP (CR684531) and Noggin2 (AY779056), and with zebrafish (D. rerio) Olig2 (AF442964) (data not shown). These data confirm that SPB cells express protein molecules characteristic of both OPCs and tanycytes.
4. Discussion
Here, an A2B5-expressing SPB cell line from the brain of snubnose pompano was established and characterized. SPB exhibited markers for oligodendrocytes and astroglia similar to those reported in GBC4, a tanycyte-like cell line (Wen et al., 2009). In addition, SPB cells ex-pressed Olig2 and Noggin2. Moreover, SPB cells have a single primary cilium that has not been observed in GBC4 cells. These differences indicate the presence of heterogeneity infish OPCs and tanycytes. Our results suggest that SPB cells are tanycyte-like neural stem cells with more OPC characteristics than those in GBC4 cells. Several studies have reported the cultivation of neural progenitor and neural stem cells from adultfish brain (Hinsch and Zupanc, 2006; Wen et al., 2008a,b; Servili et al., 2009). Manyfish neural progenitor and neural stem cells show neuroepithelial characteristics and exhibit mature neural
Fig. 3. A2B5 immunomagnetic beads purified SPB cells after 10 passages were planted on uncoated coverslips and incubated for 4 days. The cells werefixed with 3.7% formaldehyde and stained with anti-GalC and FITC-conjugated secondary antibody. (A) The labeling of GalC on the surface of oligodendrocyte-like cells (arrows) was higher than on polygonal cells (arrowheads). (B) Phase contrast micrograph. Bar = 50μm.
Fig. 4. SPB cells from passage 48 were grown on uncoated coverslips andfixed and stained with rabbit anti-Olig2 and mouse anti-GFAP, and secondary antibodies Alexa Fluor® 568-conjugated anti-rabbit IgG and FITC-conjugated anti-mouse IgG. The nuclei (blue) were stained with DAPI. Note the localizations of Olig2 (red) mainly in the nucleus (arrows) and sparse in the cytoplasm (arrowhead). GFAP (green) are shown filamentous in the cytoplasm. Bar=20 μm.
markers in vitro. A review of the results of those studies suggests the presence of heterogeneous neural progenitor and neural stem cells among these teleost cultures. However, due to a lack of type-specific markers, the relationship between neural progenitor and neural stem cells is unclear.
4.1. Molecular expression offish OPCs in vitro
Fish OPCs in vitro are characterized by the expression of A2B5. They are eitherfibroblast-like or epithelial-like, and may or may not have GFAP. In this study, SBP cells have been shown to not only exhibit OPC markers A2B5, PLP, PDGFRα, and Sox2, a stem cell marker, but also exhibit markers for astroglia and neurons as has been previously reported in TB2, a tilapia (Oreochromis mossambicus and O. niloticus hybrid) cell line, and in GBC4 cells from grouper (Wen et al., 2008a, 2009). Fish OPCs may generate from radial glial cells or be a subtype of radial glial cells; therefore, the detection of astroglia markers in the cells was not surprising. SPB and GBC4 cells show a greater degree of epithelial characteristics than that in TB2 cells (Wen et al., 2008a). Moreover, SPB cells express Olig2 and Noggin2, whereas GBC4 cells do not (Wen et al., 2009). GBC4 and TB2 cells have exhibited expression of neuron marker Cx35 and tyrosine hydroxylase (TH) in previous studies (Wen et al., 2008a, 2009); however, SPB cells did not express TH (data not shown), whereas they did express Cx35.
Expression of Sox10 has been observed in OPCs in zebrafish CNS (Cunliffe and Casaccia-Bonnefil, 2006; Li et al., 2007). Here, RT-PCR examination of Sox10 expression in SPB was negative (data not shown), similar to the results reported for GBC4 cells (Wen et al., 2009). Because the Sox10 protein is needed for oligodendrocyte terminal differentiation and myelination (Stolt et al., 2002; Li et al., 2007), the results indicate the SPB cells are in an early OPC stage. Although GalC expression is restricted in mature oligodendrocytes in mammals (Liu and Rao, 2004), it does appear in SPB and otherfish neural progenitor cell lines, including GBC4 and TB2 (Wen et al., 2008a, 2009). SPB also shows radial glia markers, including BLBP, GFAP, GS, S100 and VIM, although the expression of GS and S100 is sparse. Fish neural stem and neural progenitor cells
show characteristics different from their counterparts in mammals. They retain neuroepithelial properties, including keratin, Cx43 type gap junctions, desmosome junctions, and PDGFRα expression (Wen et al., 2008a, 2009), as well as exhibiting apical–basal polarization and ventricle contact (Kaslin et al., 2009).
4.2. SPB cells exhibit specific molecules of tanycyte
Tanycytes comprise a heterogeneous population of specialized cells lining the ependymal layer of CNS and were classified broadly into ependymal and extraependymal cells (Horstmann, 1954). In adult mammals, tanycytes are abundant in the third ventricle (hypothalamus) wall and have been studied extensively. On the basis of morphology, topography, and metabolism, four types of hypothalamic tanycytes (α1, α2, β1, and β2) are distinguishable (Akmayev and Fidelina, 1976; Rodriguez et al., 2005). Numerous proteins, including GFAP, DARPP-32, Nestin, O4, S100, and VIM have been detected in hypothalamic tanycytes; however, none of the proteins are uniquely present in those cells (Rodriguez et al., 2005). Tanycytes share some features with radial glia but display several properties, including DARPP-32, keratin 8 and 18, glutamate transporters, and glucose transporters, that clearly distin-guish them from subependymal radial glia.
In the adult teleost CNS, most radial glial cells have cell bodies located at the ependyma and are recognized as ependymal radial glial cells or tanycytes. In contrast to mammals, adultfish CNS tanycytes are a subtype of radial glial cells (Kálmán, 1998). Molecular expression of tanycytes infish CNS is unclear, yet may be similar to that in fish radial glial cells, which exhibit AroB, BLBP, Nestin, Olig2, GS, S100, and Sox2, in addition to GFAP and VIM (Forlano et al., 2001; Menuet et al., 2003; Pellegrini et al., 2005, 2007; Ari and Kálmán, 2008; Kim et al., 2008a; Lam et al., 2009; Tong et al., 2009). Fish tanycytes, and other ependymocytes, have keratins, desmosomes, and gap junctions (Bodega et al., 1993, 1995) and may or may not exhibit cilia on the ventricular surface (Ma, 1993; Shioda et al., 1997). In this study, SPB cells are recognized as tanycytes as they have DARPP-32, primary cilia, radial glia markers, and a long GFAP- and VIM-positive process. DARPP-32 is a specific marker for dopaminoceptive cells including choroidal epithelial cells, neurons, pituicytes, and tanycytes (Meister et al., 1988), but was not detected in carp (Cyprinus carpio) (Hemmings and Greengard, 1986) and goldfish (Carassius auratus) brain ( Hem-mings et al., 1992). The DARPP-32 negative results in those previous studies and the positive result in this study might be due to the use of different antibodies.
4.3. Identity of SPB
Olig2-expressing radial glial cells infish CNS have been observed to generate neurons and oligodendrocytes, but astrocyte generation has not been reported (Park et al., 2002, 2007; Reimer et al., 2008; Zannino and Appel, 2009). In mammals, the fate and morphogenesis of neural progenitor cells are controlled by BMPs and Noggin (Bani-Yaghoub et al., 2000; Gulacsi and Lillien, 2003; See et al., 2004; Weible and Chan-Ling, 2007). BMPs promote neuronal and astroglial differen-tiation, but suppress oligodendrocytic differentiation (Gomes et al., 2003); whereas Noggin inhibits astroglial differentiation but promotes
Fig. 6. RT-PCR analyses of SPB cells to detect transcripts for BLBP (405 bp), BMP4 (782 bp), Cx35 (619 bp), Cx43 (325 bp), GFAP (230 bp), GS (545 bp), Noggin2 (634 bp), Olig2 (247 b), PDGFRα (735 bp), PLP (344 bp), and Sox2 (941 bp). The amplicons having the predicted size were purified, cloned, and sequenced to verify the specificity of the PCR reaction. M: 100-bp ladder.
Fig. 5. SPB cells from passages 12 to 30 were double stained with antibodies that recognize tanycytes. The mouse antibodies were labeled with FITC-conjugated anti-mouse IgG, and the rabbit antibodies were labeled with Alexa Fluor® 568-conjugated anti-rabbit IgG. Nuclei were stained with DAPI (blue). (A) The labeling of rabbit anti-DARPP-32 (red) was primarily in the nucleus and sparse in the cytoplasm, whereas mouse anti-acetylated tubulin labeled the cilia (arrows). (B) SPB cells seeded at high density (4 × 104cells). The
staining for rabbit anti-BLBP (red) was concentrated in the nucleus, whereas faint labeling was seen in the cytoplasm. GFAP (green) was highly expressed in the long processes and not observed in the cytoplasm of polygonal cells. (C) Bipolar and multipolar radial glial cells showed intense labeling for VIM (green). Labeling with rabbit anti-Sox2 antibodies (red) was observed in the nuclei and cytoplasm. (D) Polygonal SPB cells showed DARPP-32 (red) in the nuclei and cytoplasm. Labeling of VIM (green) in the cells was weaker than in the process-bearing cells. (E) Polygonal SPB cells showed Sox2 (red) in the nuclei and keratin throughout the cytoplasm (green). (F) Cx43 (red) was seen at the junctions between the polygonal cells and a cilium (green, arrows) located at the surface near the nucleus. (G) Radial glial cells with high levels of VIM (green) within their processes grew on the monolayer of polygonal cells. Note that the polygonal cells showed sparse VIM staining but were strongly labeled for Cx43 at junctions (red). Cx43 was only faintly expressed in radial glial cells. (H) GS (green) and S100 (red) labeling was sparse in the polygonal cells. Intense labeling of GS and S100 was observed in oligodendrocyte-like cells. Bar = 20μm.
oligodendrocytic differentiation (Kasai et al., 2005). Additionally, Olig2 represses the generation of astrocytes from progenitor cells, and an export of Olig2 to the cytoplasm is essential for astrocyte dif-ferentiation (Setoguchi and Kondo, 2004). The role of BMPs and Noggins in the development offish neural cells is uncertain and ex-pression of BMP4 and Noggin2 in the Olig2-expressing cells infish CNS needs to discover.
SPB cells exhibit markers for OPCs as well as for tanycytes and share some properties with type B astroglia in the subventricuar zone (SVZ) of the adult mammalian brain. Type B astroglia have a single primary cilium, PDGFRα, and GFAP, are the neural stem cells in adult mammalian brain, and are heterogeneous (García-Verdugo et al., 1998; Danilov et al., 2009). SPB cells are suggested OPCs, but the ability to generate neurons is questionable.
4.4. Functions offish tanycytes
The function of tanycytes remains largely speculative. Tanycytes act as barrier cells, as do other ependymocytes, and are commonly suggested to be a conduit between the ventricle and the neural and portal capillaries; thereby allowing uptake and transport of substances from the central spinalfluid, guiding migration and regeneration of neurons and axons, and regulating endocrine, immune, and repro-ductive functions (Rodriguez et al., 2005; Lechan and Fekete, 2007).
By contrast, tanycytes infish brain, when functioning as radial glial cells, are active in neurogenesis and neuron guiding. However, addi-tional functions offish tanycytes, other than neurogenesis, are unclear. Nevertheless, tanycytes in the adult teleost CNS contain NADPH-diaphorase suggesting that they can generate nitric oxide to modulate a variety of neural functions, including synaptic transmission, cerebral blood flow, and excitotoxicity (Ma, 1993). Expression of AroB as-sociates with estrogen receptors (Menuet et al., 2003; Pellegrini et al., 2005) indicates thatfish tanycytes are regulated by endocrine (Mouriec et al., 2008).
4.5. Conclusion
Previously, GBC4 neural progenitor cells from adultfish brain have been reported to expressfish radial glia specific markers including AroB, BLBP, GFAP, VIM, and Sox2 in vitro (Wen et al., 2009). In this study, the tanycyte marker DARPP-32 and the OPC marker Olig2 were expressed in SPB in vitro. Also, primary cilia were noted on the apical surface of SPB cells but were not present on GBC4 cells. Though both GBC4 and SPB express BMP4, Noggin2 expression was only observed in SPB. Both Olig2 and Noggin2 promote oligodendrocyte generation; therefore, oligodendrocyte-like cells appear to be more common in SPB than in GBC4. Fish neural progenitor and neural stem cells and some of the genes responsible for the expression of these proteins have been identified; however, the microenvironmental factors that may induce neuron and oligodendrocyte generation are unknown. Revealing the conditions that generate large numbers of mature neurons and/or oligodendrocytes in vitro is a topic for future work. Acknowledgment
This work was supported partially by the Council of Agriculture of Republic of China under grant 98AS-5.4.2-ST-a2.
References
Adolf, B., Chapouton, P., Lam, C.S., Topp, S., Tannhauser, B., Strahle, U., Gotz, M., Bally-Cuif, L., 2006. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293.
Akmayev, I.G., Fidelina, O.V., 1976. Morphological aspects of the hypothalamic–hypophyseal system. Cell Tissue Res. 173, 407–416.
Alieva, I.B., Gorgidze, L.A., Komarova, Y.A., Chernobelskaya, O.A., Vorobjev, I.A., 1999. Experimental model for studying the primary cilia in tissue culture cells. Membr. Cell Biol. 12, 895–905.
Ari, C., Kálmán, M., 2008. Glial architecture of the ghost shark (Callorhinchus milii, Holocephali, Chondrichthyes) as revealed by different immunohistochemical mar-kers. J. Exp. Zool. B: Mol. Dev. Evol. 310, 504–519.
Arochena, M., Anadón, R., Díaz-Regueira, S.M., 2004. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 469, 413–436.
Bani-Yaghoub, M., Felker, J.M., Sans, C., Naus, C.C.G., 2000. The effects of bone morphogenetic protein 2 and 4 (BMP2 and BMP4) on gap junctions during neurodevelopment. Exp. Neurol. 162, 13–26.
Baracskay, K.L., Kidd, G.J., Miller, R.H., Trapp, B.D., 2007. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 55, 1001–1010.
Bodega, G., Suárez, I., Rubio, M., Villalba, R.M., Fernández, B., 1993. Astroglial pattern in the spinal cord of the adult barbel (Barbus comiza). Anat. Embryol. (Berl) 187, 385–398. Bodega, G., Suárez, I., Rubio, M., Fernández, B., 1994. Ependyma: phylogenetic evolution
of glialfibrillary acidic protein (GFAP) and vimentin expression in vertebrate spinal cord. Histochemistry 102, 113–122.
Bodega, G., Suárez, I., Rubio, M., Fernández, B., 1995. Type II cytokeratin expression in adult vertebrate spinal cord. Tissue Cell 27, 555–559.
Bruni, J.E., 1998. Ependymal development, proliferation, and functions: a review. Micro. Res. Tech. 41, 2–13.
Chapouton, P., Adolf, B., Leucht, C., Tannhauser, B., Ryu, S., Driever, W., Bally-Cuif, L., 2006. her5 expression reveals a pool of neural stem cells in the adult zebrafish midbrain. Development 133, 4293–4303.
Cunliffe, V.T., Casaccia-Bonnefil, P., 2006. Histone deacetylase 1 is essential for oligoden-drocyte specification in the zebrafish CNS. Mech. Dev. 123, 24–30.
Danilov, A.I., Gomes-Leal, W., Ahlenius, H., Kokaia, Z., Carlemalm, E., Lindvall, O., 2009. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia 57, 136–152.
Ekström, P., Johnsson, C.M., Ohlin, L.M., 2001. Ventricular proliferation zones in the brain of an adult teleostfish and their relation to neuromeres and migration (secondary matrix) zones. J. Comp. Neurol. 436, 92–110.
Forlano, P.M., Deitcher, D.L., Myers, D.A., Bass, A.H., 2001. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleostfish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 21, 8943–8955. García-Verdugo, J.M., Doetsch, F., Wichterle, H., Lim, D.A., Alvarez-Buylla, A., 1998.
Architecture and cell types of the adult subventricular zone: in search of the stem cells. J. Neurobiol. 36, 234–248.
Germanà, A., Marino, F., Guerrera, M.C., Campo, S., de Girolamo, P., Montalbano, G., German, G.P., Ochoa-Erena, F.J., Ciriaco, E., Vega, J.A., 2008. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 71, 248–255.
Gomes, W.A., Mehler, M.F., Kessler, J.A., 2003. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 255, 164–177.
Grandel, H., Kaslin, J., Ganz, J., Wenzel, I., Brand, M., 2006. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 295, 263–277.
Gulacsi, A., Lillien, L., 2003. Sonic hedgehog and bone morphogenetic protein regulate interneuron development from dorsal telencephalic progenitors in vitro. J. Neurosci. 23, 9862–9872.
Hemmings, H.C., Greengard, P., 1986. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distri-bution. J. Neurosci. 6, 1469–1481.
Hemmings, H.C., Girault, J.A., Nairn, A.C., Bertuzzi, G., Greengard, P., 1992. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J. Neurochem. 59, 1053–1061.
Hernández, C., Martín, M., Bodega, G., Suáez, I., Pérez, J., Fernández, B., 1999. Response of carp central nervous system to hyperammonemic conditions: an immunocyto-chemical study of glutamine synthetase (GS), glialfibrillary acidic protein (GFAP) and 70 kDa heat-shock protein (HSP70). Aquat. Toxicol. 45, 195–207.
Hinsch, K., Zupanc, G.K.H., 2006. Isolation, cultivation, and differentiation of neural stem cells from adultfish brain. J. Neurosci. Methods 158, 75–88.
Hökfelt, T., Foster, G., Schultzberg, M., Meister, B., Schalling, M., Goldstein, M., Hemmings, H.C.J., Ouimet, C., Greengard, P., 1988. DARPP-32 as a marker for D-1 dopaminoceptive cells in the rat brain: prenatal development and presence in glial elements (tanycytes) in the basal hypothalamus. Adv. Exp. Med. Biol. 235, 65–82.
Horstmann, E., 1954. Die Faserglia des Selachegehirns. Z. Zellforsch 39, 588–617. Jakovcevski, I., Filipovic, R., Mo, Z., Rakic, S., Zecevic, N., 2009. Oligodendrocyte
development and the onset of myelination in the human fetal brain. Front. Neuroanat. 3, 5.
Jeserich, G., Stratmann, A., 1992. In vitro differentiation of trout oligodendrocytes: evidence for an A2B5-positive origin. Brain Res. Dev. Brain Res. 67, 27–35. Kálmán, M., 1998. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed
by immunohistochemical staining against glialfibrillary acidic protein (GFAP). Anat. Embryol. (Berl) 198, 409–433.
Kasai, M., Satoh, K., Akiyama, T., 2005. Wnt signaling regulates the sequential onset of neurogenesis and gliogenesis via induction of BMPs. Genes Cells 10, 777–783. Kaslin, J., Ganz, J., Geffarth, M., Grandel, H., Hans, S., Brand, M., 2009. Stem cells in the
adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J. Neurosci. 29, 6142–6153.
Kim, H., Shin, J., Kim, S., Poling, J., Park, H.-C., Appel, B., 2008a. Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev. Dyn. 237, 2081–2089.
Kim, S., Kim, S.-H., Kim, Ho., Chung, A.-Y., Cha, Y.I., Kim, C.-H., Huh, T.-L., Park, H.-C., 2008b. Frizzled 8a function is required for oligodendrocyte development in the zebrafish spinal cord. Dev. Dyn. 237, 3324–3331.
Kucenas, S., Snell, H., Appel, B., 2008. nkx2.2a promotes specification and differentiation of a myelinating subset of oligodendrocyte lineage cells in zebrafish. Neuron. Glia Biol. 4, 71–81.
Lam, C.S., März, M., Strähle, U., 2009. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev. Dyn. 238, 475–486. Lazzari, M., Franceschini, V., 2004. Glialfibrillary acidic protein and vimentin
immuno-reactivity of astroglial cells in the central nervous system of the African lungfish, Protopterus annectens (Dipnoi: Lepidosirenidae). J. Morphol. 262, 741–749. Lechan, R.M., Fekete, C., 2007. Infundibular tanycytes as modulators of neuroendocrine
function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed. 78 (Suppl. 1), 84–98.
Li, H., Grumet, M., 2007. BMP and LIF signaling coordinately regulate lineage restriction of radial glia in the developing forebrain. Glia 55, 24–35.
Li, H., Lu, Y., Smith, H.K., Richardson, W.D., 2007. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J. Neurosci. 27, 14375–14382.
Liu, Y., Rao, M.S., 2004. Glial progenitors in the cns and possible lineage relationships among them. Biol. Cell 96, 279–290.
Liu, Y., Wu, Y., Lee, J.C., Xue, H., Pevny, L.H., Kaprielian, Z., Rao, M.S., 2002. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia 40, 25–43.
Ma, P.M., 1993. Tanycytes in the sunfish brain: NADPH-diaphorase histochemistry and regional distribution. J. Comp. Neurol. 336, 77–95.
Meister, B., Hökfelt, T., Tsuruo, Y., Hemmings, H., Ouimet, C., Greengard, P., Goldstein, M., 1988. DARPP-32, a dopamine- and cyclic AMP-regulated phosphoprotein in tanycytes of the mediobasal hypothalamus: distribution and relation to dopamine and luteinizing hormone-releasing hormone neurons and other glial elements. Neuroscience 27, 607–622.
Menn, B., Garcia-Verdugo, J.M., Yaschine, C., Gonzalez-Perez, O., Rowitch, D., Alvarez-Buylla, A., 2006. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907–7918.
Menuet, A., Anglade, I., Guevel, R.L., Pellegrini, E., Pakdel, F., Kah, O., 2003. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J. Comp. Neurol. 462, 180–193. Merkle, F.T., Tramontin, A.D., Garcia-Verdugo, J.M., Alvarez-Buylla, A., 2004. Radial glia
give rise to adult neural stem cells in the subventricular zone. Proc. Natl Acad. Sci. USA 101, 17528–17532.
Mouriec, K., Pellegrini, E., Anglade, I., Menuet, A., Adrio, F., Thieulant, M.L., Pakdel, F., Kah, O., 2008. Synthesis of estrogens in progenitor cells of adultfish brain: evolutive novelty or exaggeration of a more general mechanism implicating estrogens in neurogenesis? Brain Res. Bull. 75, 274–280.
Park, H.-C., Appel, B., 2003. Delta–Notch signaling regulates oligodendrocyte specifi-cation. Development 130, 3747–3755.
Park, H.-C., Mehta, A., Richardson, J.S., Appel, B., 2002. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 248, 356–368. Park, H.-C., Shin, J., Roberts, R.K., Appel, B., 2007. An olig2 reporter gene marks oligodendrocyte precursors in the postembryonic spinal cord of zebrafish. Dev. Dyn. 236, 3402–3407. Pellegrini, E., Menuet, A., Lethimonier, C., Adrio, F., Gueguen, M.-M., Tascon, C., Anglade,
I., Pakdel, F., Kah, O., 2005. Relationships between aromatase and estrogen receptors in the brain of teleostfish. Gen. Comp. Endocrinol. 142, 60–66.
Pellegrini, E., Mouriec, K., Anglade, I., Menuet, A., Page, Y.L., Gueguen, M.-M., Marmignon, M.-H., Brion, F., Pakdel, F., Kah, O., 2007. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501, 150–167.
Rao, M.S., Mayer-Proschel, M., 1997. Glial-restricted precursors are derived from multi-potent neuroepithelial stem cells. Dev. Biol. 188, 48–63.
Reimer, M.M., Sorensen, I., Kuscha, V., Frank, R.E., Liu, C., Becker, C.G., Becker, T., 2008. Motor neuron regeneration in adult zebrafish. J. Neurosci. 28, 8510–8516. Rodriguez, E.M., Blazquez, J.L., Pastor, F.E., Pelaez, B., Pena, P., Peruzzo, B., Amat, P.,
Kwang, W.J., 2005. Hypothalamic tanycytes: a key component of brain–endocrine interaction. Int. Rev. Cytol. 247, 89–164.
Schebesta, M., Serluca, F.C., 2009. olig1 expression identifies developing oligodendrocytes in zebrafish and requires hedgehog and notch signaling. Dev. Dyn. 238, 887–898. See, J., Zhang, X., Eraydin, N., Mun, S.-B., Mamontov, P., Golden, J.A., Grinspan, J.B., 2004.
Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell. Neurosci. 26, 481–492.
Servili, A., Bufalino, M.R., Nishikawa, R., de Melo, I.S., Muñoz-Cueto, J.A., Lee, L.E.J., 2009. Establishment of long term cultures of neural stem cells from adult sea bass, Dicentrarchus labrax. Comp. Biochem. Physiol. 152, 245–254.
Setoguchi, T., Kondo, T., 2004. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J. Cell Biol. 166, 963–968.
Shioda, S., Honma, Y., Yoshie, S., Hosoya, Y., 1997. Scanning electron microscopy of the third ventricular wall in the lamprey, Lampetra japonica. Arch. Histol. Jpn. 40, 41–49. Sivron, T., Jeserich, G., Nona, S., Schwartz, M., 1992. Characteristics offish glial cells in
culture: possible implications as to their lineage. Glia 6, 52–66.
Sivron, T., Cohen, I., Schwartz, M., 1994. Intermediatefilaments reminiscent of immature cells expressed by goldfish (Carassius auratus) astrocytes and oligodendrocytes in vitro. Cell Tissue Res. 275, 327–337.
Stolt, C.C., Rehberg, S., Ader, M., Lommes, P., Riethmacher, D., Schachner, M., Bartsch, U., Wegner, M., 2002. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16, 165–170.
Tong, S.-K., Mouriec, K., Kuo, M.-W., Pellegrini, E., Gueguen, M.-M., Brion, F., Kah, O., Chung,, B.-c., 2009. A cyp19a1b-GFP (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis 47, 67–73.
Weible, M.W., Chan-Ling, T., 2007. Phenotypic characterization of neural stem cells from human fetal spinal cord: synergistic effect of LIF and BMP4 to generate astrocytes. Glia 55, 1156–1168.
Wen, C.-M., Cheng, Y.-H., Huang, Y.-F., Wang, C.-S., 2008a. Isolation and characteriza-tion of a neural progenitor cell line from tilapia brain. Comp. Biochem. Physiol. 149, 167–180.
Wen, C.M., Lee, C.W., Wang, C.S., Cheng, Y.H., Huang, H.Y., 2008b. Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanoda-virus and megalocytibetanoda-virus infectivity and propagation. Aquaculture 278, 14–21. Wen, C.-M., Huang, J.-Y., Ciou, J.-H., Kao, Y.-L., Cheng, Y.-H., 2009. Immunochemical and
molecular characterization of GBC4 as a tanycyte-like cell line derived from grouper brain. Comp. Biochem. Physiol. 153, 191–201.
Zannino, D.A., Appel, B., 2009. Olig2+precursors produce abducens motor neurons and
oligodendrocytes in the zebrafish hindbrain. J. Neurosci. 29, 2322–2333. Zupanc, G.K., Horschke, I., 1995. Proliferation zones in the brain of adult gymnotiform
fish: a quantitative mapping study. J. Comp. Neurol. 353, 213–233. C.-M. Wen et al. / Comparative Biochemistry and Physiology, Part A 156 (2010) 224–231