Adhesion, Permeability, and Mechanical Properties of Multilayered Blown Films Using Maleated Low-Density
Polyethylene Blends as Adhesion-Promoting Layers
Chi-Hsien HUANG, Jiann-Shing WU, Chun-Chin HUANG,∗†and Li-Shin LIN∗∗ Department of Applied Chemistry, National Chiao Tung University, Hsinchu 300, Taiwan, R. O. C.
∗Department of Mold and Die Engineering, National Kaohsiung University of Applied Science,
415 Chien Kung Road, Kaohsiung 807, Taiwan, R. O. C.
∗∗Chang Chun Petrochemical Co., Ltd., Miaoli 360, Taiwan, R. O. C.
(Received July 22, 2003; Accepted October 7, 2003)
ABSTRACT: We have fabricated three-layer films, comprising a varying content of ethylene–vinyl alcohol copoly-mer (EVOH) as the internal layer and blends of low-density polyethylene (LDPE) and low-density ethylene grafted with maleic anhydride (LDPE-g-MAH) as the external layers, by a coextrusion blown-film process. We chose to use blends to promote the adhesion between EVOH and LDPE and to reduce the number of layers in the coextrusion system. The peel strength increased sharply when the amount of LDPE-g-MAH was greater than 12.5 wt% and we associate it with the promotion of adhesion between layers brought about by specific interactions between EVOH and LDPE-g-MAH. FT-IR spectroscopic analysis showed an increase in the intensity of the absorbance of the ester band upon increasing the content of LDPE-g-MAH, which indicates that a chemical reaction occurs that promotes adhesion at the interface. The tensile strength did not change significantly with increasing LDPE-g-MAH content, which had a small effect on elongation and modulus in both the machine direction (MD) and transverse direction (TD). Tear strength decreased continuously with in-creasing LDPE-g-MAH content, in both the MD and TD, as a result of the greater ease of crack propagation in the EVOH layer. The oxygen permeability of the three-layer films remained almost constant upon varying the amount of LDPE-g-MAH. These three-layer films all followed the theoretical prediction made by the inverse additivity rule. The water vapor permeabilities of the three-layer films, however, were affected by the degree of hydrogen bonding, which was analyzed by FT-IR spectroscopy. The effects of this hydrogen bonding resulted in a discrepancy between the experimental findings and the theoretical predictions, especially when the EVOH content was increased.
KEY WORDS Multilayered Film / Coextrusion / EVOH / Blending / Hydrogen Bonding / Barrier Properties /
Coextrusion is a process in which two or more poly-mers are extruded simultaneously and joined together to form a single structure having different properties in each layer and to achieve a broad range of properties that are not available in any of the individual materi-als alone. In recent years, the packaging and container industries have paid increasing attention to the devel-opment of new or improved products formed by coex-trusion, such as multilayer sheets, multilayer films, and multilayer containers.1–3 The number of layers com-prising these materials depends on the required end-use properties and the availability of polymer combinations suitable for specific applications.
Recently, it has become common4, 5 in food pack-aging technology to coextrude multilayer films con-sisting of distinct layers that are barriers for oxygen and moisture. Polyethylene is an excellent moisture barrier for packaging, and its low cost, strength and ease of processing make it suitable for many applica-tions. Its inability, however, to act as a barrier for
oxy-gen, aromatics, and oils limits its potential applications. On the other hand, ethylene–vinyl alcohol copolymer (EVOH) possesses excellent barrier properties to oxy-gen, aromatics, and oils.6–9 Unfortunately, EVOH is highly sensitive to moisture, which alters its ability to acts as an oxygen barrier.10, 11 Therefore, using coex-trusion to combine polyethylene and EVOH in a multi-layer structure is very attractive for many demanding packaging applications, such as for food, drugs, and cosmetics. Typical commercial multilayer barrier films for food packaging contain EVOH as an oxygen rier layer and polyethylene resins as the moisture bar-rier layer. This film possesses a multilayer structure in which outer PE layers protect an inner EVOH layer from continuous exposure to moisture. Because of the chemical dissimilarities between PE and EVOH, how-ever, an extrudable adhesive polymer must be incorpo-rated into the film as a tie layer that promotes adhe-sion. Graft copolymers are widely recognized as novel potential adhesive polymers for imparting improved †To whom all correspondence should be addressed (Tel: +886-7-3814526 ext2700, Fax: +886-7-3835015,
adhesion. These copolymers are synthesized mainly by modifying polyolefin resins through the addition of functionality. This process is achieved by adding acid or anhydride units to polyolefins through grafting or by direct synthesis of copolymers. Tanaka et al.12 have successfully developed a new generation of tie layer adhesives, by combining graft and polymer blending, that maintain high adhesive strengths after thermoform-ing and orientation. Botros studied three-layer films, tie/EVOH/tie, using a coextrusion cast-film process13
and found that the tie layers bind to EVOH through covalent and hydrogen bonding. The failure mecha-nisms of the three-layer films were of a mixed cohe-sive/adhesive type. Kim et al.14 have investigated the mechanical and transport properties of various combi-nations of two-layer films, including LDPE/tie, Nylon 6/tie and LDPE/Nylon 6. The tensile strength and mod-ulus of a coextruded film follows the additivity rule and its permeability follows the inverse additivity rule.15 Kamykowski studied the adhesive properties of five-layer coextruded cast films16 and found that the adhe-sion properties generally improved upon increasing the overall film thickness or the relative amount of the ad-hesive (maleic anhydride-grafted polypropylene). The molecular weight of the grafted resin had a small effect on adhesion. Homopolymer diluents outperform ran-dom copolymer diluents in their adhesion properties.
Having additional tie layers in a coextruded film makes the fabrication process more complex and ex-pensive, because of the need in the coextrusion sys-tem for a specially designed die and additional ex-truders for the adhesive polymers. An alternative ap-proach has been reported that overcomes this disadvan-tage by replacing the five-layer film coextrusion system with a three-layer film comprising EVOH as the central layer and LLDPE/LLDPE-g-MAH blends as the exter-nal layers.17, 18
In this paper, we report a method to eliminate the need for tie layers by using blends of low-density polyethylene (LDPE) and linear low-density polyethy-lene grafted with maleic anhydride (LDPE-g-MAH) that promote adhesion between LDPE and EVOH in coextruded three-layer blown films. We investigated the mechanical properties of the films, including their peel strengths, tensile properties, and tear strengths, and compared their oxygen and water vapor permeabil-ity to theoretical predictions.19These blown films could be a viable option for reducing the number of film lay-ers in coextrusion processing.
EXPERIMENTAL
Materials
Commercial-grade, low-density polyethylene
[LDPE, 6030F, M. I. (g/10 min, 190◦C, 2.16 kg) = 0.27, density = 0.922 g cm−3] was supplied in pel-let form by Formosa Plastic Corp. (Taiwan). The ethylene–vinyl alcohol copolymer [EVOH, F101A, ethylene content (mol%) = 32, M. I. (g/10 min, 190◦C, 2.16 kg) = 1.6, density = 1.19 g cm−3] was provided in pellet form by Kuraray Co. (Japan). The adhesive, Modic-AP L502, was obtained in pellet form from Mitsubishi Chemical Corp. (Japan). It is a low-density
polyethylene-grafted maleic anhydride
[LDPE-g-MAH, M. I. (g/10 min, 190◦C, 2.16 kg) = 1.0, density = 0.93 g cm−3].
Preparing Blends
LDPE-g-MAH was dried in a vacuum oven for 24 h period at 50◦C before blending. Blends of LDPE with different amounts of LDPE-g-MAH (0, 5, 7.5, 10, 12.5, 15, 20, and 25 wt%) were prepared in pellet form by melt mixing in a twin-screw extruder operating at 210◦C and 40 rpm.
Preparing Multilayer Films
Prior to processing, EVOH was dried in a vacuum oven for 24 h at 60◦C. The extruded blends (the exter-nal layers) were coextruded with EVOH (the interexter-nal layer) through a three-layer coextrusion blown-film die (inner diameter = 97.6 mm; gap thickness = 1.2 mm) at 230◦C. A 42-mm-diameter extruder (L/D = 28) was used for the extruded blends and a 25-mm-diameter ex-truder (L/D = 28) was used for EVOH. All exex-truders were attached with gear pumps to control the thickness of individual component layers precisely. The ratios of take-off and blow-up were 3.5 and 2.5, respectively. Three-layer films were fabricated with EVOH as the in-ternal layer (overall 16.6 wt%) and blends of LDPE and LDPE-g-MAH (0, 5, 7.5, 10, 12.5, 15, 20, or 25 wt% of LDPE) as the external layers (overall 83.4 wt%). The thickness of all the three-layer films produced, irre-spective of the relative amount of LDPE-g-MAH, was 150µm (blend, 130 µm; EVOH, 20 µm). We also pre-pared three-layer films having overall EVOH content of 7.9, 13, 17.1, 21.2 or 24.4 wt% (internal layer) and a blend of LDPE with 15 wt% of LDPE-g-MAH (ex-ternal layers). Table I shows the thicknesses of these three-layer films with respect to the EVOH content. To allow theoretical predictions to be made, we also pre-pared monolayer film samples of LDPE, EVOH, and
LDPE-g-MAH under the same processing conditions.
Preparing Specimens for Measuring Peel Strengths
Film samples were cut into strips (25 mm× 300 mm) and delamination was initiated at one end by soak-ing the tips of each strip in formic acid (HCOOH) for
ca. 4 h. The corner of a strip was pulled repeatedly
with tweezers until we observed a slight indication of delamination. The delamination was then propagated throughout the width of the strip. The peel strengths of the three-layer films were measured at 23◦C by T-type using a tensile tester with a crosshead speed of 25.4 cm min−1, following the procedure described in ASTM D1876. At least ten samples were tested to ob-tain average values.
Analysis by FT-IR Spectroscopy
The interactions at the interface between the layers of the blend and EVOH were analyzed by Fourier trans-form infrared (FT-IR) spectroscopy as a function of the content of LDPE-g-MAH and EVOH using a Bio-Rad FTS-165. At least 32 scans at a resolution of 4 cm−1 were signal-averaged. The hydrogen bonding was also analyzed by FT-IR spectroscopy.
Measuring Tensile Properties
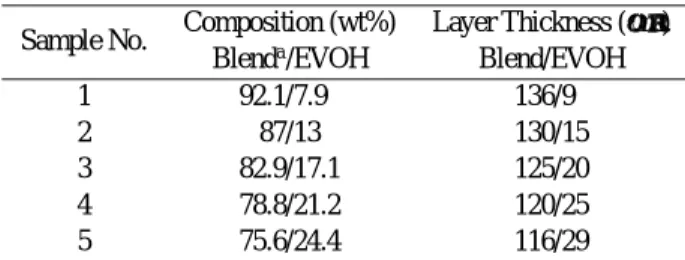
The tensile properties of the three-layer films, includ-ing tensile strength, tensile modulus, and ultimate elon-gation, were measured by a tensile tester at 23◦C and a relative humidity of 50% in both the machine direc-Table I. Thickness of individual layers of three-layer films
with various EVOH contents
Sample No. Composition (wt%) Layer Thickness (µm) Blenda/EVOH Blend/EVOH
1 92.1/7.9 136/9
2 87/13 130/15
3 82.9/17.1 125/20
4 78.8/21.2 120/25
5 75.6/24.4 116/29
aBlend = LDPE (85 wt%)/LDPE-g-MAH (15 wt%).
Table II. Properties of films for individual component materials Property
Material
LDPE LDPE-g-MAH EVOH (130µm) (130µm) (20µm) Tensile Strength MD/TD (MPa) 24.2/21.4 23.6/20.6 63/45 Tensile Elongation MD/TD (%) 670/720 690/750 280/210 Tensile Modulus MD/TD (MPa) 205/220 220/235 1590/1610
Tear Strength MD/TD (N) 9.9/12 10.3/12.4 <0.1/<0.1 Oxygen Permeabilitya
1300 1250 11
(mL-mm/m2-24 h-atm)× 102
Water Vapor Permeabilityb
9 8.7 700
(g-mm//m2-24 h)× 102
a23◦C, 50% relative humidity.b38◦C, 90% relative humidity.
tion (MD) and the transverse direction (TD), following the procedure described in ASTM D882. At least ten samples were tested to obtain average values.
Measuring Tear Strength
The tear strengths of the three-layer films were mea-sured by a tensile tester at 23◦C and a relative humid-ity of 50% in both the machine direction (MD) and the transverse direction (TD), following the procedure de-scribed in ASTM D1938. At least ten samples were tested to obtain average values.
Measuring Oxygen Permeability
The oxygen permeability properties of the three-layer films were measured using a Lyssy L-100-5000 Gas Permeability Tester,20 following the ASTM D1434. The oxygen permeability of the films was mea-sured at 23◦C and a relative humidity of 0%. The tem-perature was controlled by a water bath.
Measuring Water Vapor Permeability
Water vapor permeability properties of the three-layer films were measured using Lyssy L-80-5000 Wa-ter Vapor Permeability TesWa-ter,21 following the ASTM
E96. The water vapor permeability of the films was measured at 38◦C and a relative humidity of 90%. The temperature was controlled by a water bath.
RESULTS AND DISCUSSION
Table II lists the tensile, tear and barrier proper-ties of individual component materials. It is seen that the LDPE-g-MAH exhibit almost the same values in all mechanical and barrier properties as LDPE. The EVOH has much better tensile strength, modulus, tear strength, and oxygen barrier property than both LDPE and LDPE-g-MAHH. On the other hand, LDPE and LDPE-g-MAH exhibit higher tensile elongation and water vapor barrier property. We assume that these properties of the blends are not affected significantly
Figure 1. Peel strength between LDPE/LDPE-g-MAH blend and EVOH of three-layer films as a function of LDPE-g-MAH con-tent.
by the relative amount of LDPE-g-MAH in LDPE due to the same level of properties as shown in Table II.
Peel Properties
Figure 1 shows the peel strength as a function of the relative amount of LDPE-g-MAH. The peel strength in-creased slightly upon increasing the amount of
LDPE-g-MAH and then increased sharply when the amount
of LDPE-g-MAH was greater than 12.5 wt%. When the LDPE-g-MAH content was above 15 wt%, we ob-served in the three-layer films a concomitant elongation and break in the blend layer of the film in addition to peeling at the interface between layers of EVOH and blend. As mentioned above, this observation indicates that good adhesion exists between the blend and EVOH layers and, as a result, it changes the failure mechanism from adhesive to cohesive.
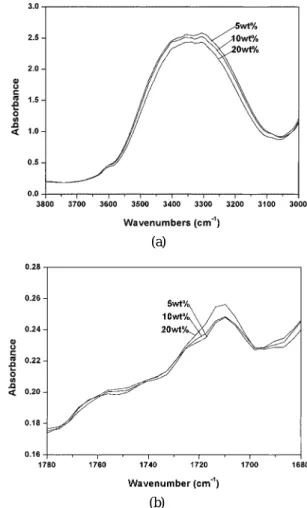
The interactions at the interface between the blend and EVOH layers were analyzed by FT-IR spec-troscopy as shown in Figure 2. Table III lists the ab-sorbances of the ester bands near 1710 cm−1 and the hydroxyl bands near 3350 cm−1. We observe a contin-uous increase in the intensity of the ester absorbance and a decrease in that of the hydroxyl absorbance upon increasing the amount of LDPE-g-MAH. The increase in the ester band absorbance is attributed to covalent bonding between the carbonyl groups in LDPE-g-MAH and the hydroxyl groups in EVOH. These stronger in-teractions lead to an increase in the adhesion between the blend and EVOH layers, which is a property sup-ported by the increasing peel strengths shown in Fig-ure 1.
Tensile Properties
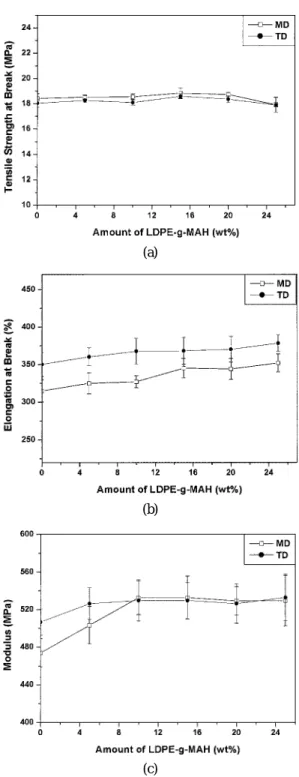
Figure 3 shows the tensile properties in both the ma-chine direction (MD) and the transverse direction (TD) as a function of the amount of LDPE-g-MAH (0 to 25 wt%). In Figure 3 (a), we see that the tensile strength
(a)
(b)
Figure 2. IR absorption spectra of three-layer films at the (a) hydroxyl band; (b) ester band with various LDPE-g-MAH contents.
Table III. IR absorbance of hydroxyl and ester bands of three-layer films with different LDPE-g-MAH contents LDPE-g-MAH content Hydroxyl Band Ester Band (wt%) 3350 cm−1 1710 cm−1 0 623.9 0.461 5 610.2 0.547 7.5 600.7 0.583 10 592.8 0.616 12.5 587 0.671 15 575.7 0.714 20 560.1 0.789
does not change significantly in either the MD or the TD upon changing the relative amount of LDPE-g-MAH. The tensile strengths of three-layer films, how-ever, are all lower than those of the films of the indi-vidual materials (see table II). These results are much different from those reported for three-layer films of LDPE/tie/PS15 and two-layer films of LDPE/tie, Ny-lon 6/tie and LDPE/NyNy-lon 6.14 In these previous stud-ies, the tensile strengths of the two- or three-layer films were found to follow the additivity rule.15 Figure 3 (b) shows that the elongation at break increases slightly as the relative amount of LDPE-g-MAH increases. It is believed that high-elongation materials, such as LDPE
(a)
(b)
(c)
Figure 3. Tensile Properties of three-layer films as a function of LDPE-g-MAH content for both MD and TD: (a) strength at break; (b) elongation at break; (c) modulus.
or LDPE-g-MAH, as shown in Table II, block trans-verse crack propagation in the EVOH layer, which causes the elongation to be about twice that of the sep-arate single EVOH film in both the MD and TD.15 Figure 3 (c) shows that the tensile modulus increases initially——up to 10 wt% for the MD and 5 wt% for the TD——with increasing the relative amount of LDPE-g-MAH, and then it stays constant This property is due to the much higher tensile modulus of EVOH, as shown in Table III, which leads to improved tensile moduli for the three-layer films as a result of strong adhesion
be-Figure 4. Tear strength of three-layer films as a function LDPE-g-MAH content for both MD and TD.
Figure 5. Oxygen permeabilities of three-layer films as a func-tion of LDPE-g-MAH content.
tween LDPE and EVOH.
Tear Properties
Figure 4 presents the tear strengths of the three-layer films investigated in both the MD and TD. A continu-ous decrease in tear strength is observed upon increas-ing the relative amount of LDPE-g-MAH. This prop-erty is due to the poor tear resistance of EVOH (see Table II) and the increase in adhesion, i.e., the increase of the degree of chemical bonding. The crack propaga-tion in EVOH continues relatively easily and this fail-ure mechanism transfers to the blend layers because of the chemical bonding in the three-layer film structures. Thus, the better the adhesion, the easier it becomes for crack growth to occur through the entire three-layer film, which results in lower tear strengths.
Oxygen Permeability
Figure 5 presents the oxygen permeabilities of the three-layer films as a function of the relative amount of LDPE-g-MAH, and shows that it is almost constant. For the sake of comparison, this figure also shows the theoretical predictions of oxygen permeabilities using
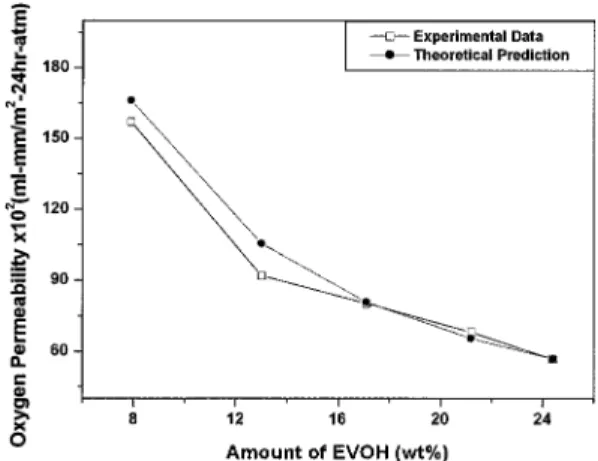
Figure 6. Oxygen permeability of three-layer films as a func-tion of EVOH content.
Figure 7. Water vapor permeability of three-layer films as a function of LDPE-g-MAH content.
the inverse additivity rule.19 1 Pthree-layer = ϕblend Pblend + ϕEVOH PEVOH (1) whereϕ is the volume fraction and P is the oxygen per-meability. Table II lists the oxygen permeabilities of the individual component materials. A good agreement exists between the experimental and theoretical data. Figure 6 displays the experimental and theoretical oxy-gen permeabilities of the three-layer films as a function of EVOH content. As expected, the permeability de-creases with increasing the relative amount of EVOH, and it agrees reasonably well with the theoretical pre-diction.
Water Vapor Permeability
Figures 7 and 8 present the water vapor permeabil-ities of the three-layer films as a function of the rel-ative amounts of LDPE-g-MAH and EVOH, respec-tively. For the sake of comparison, the two figures also show the theoretical predictions made by eq 1 and data of individual component materials (see Table II). In Figure 7, we see that the water vapor permeability of the three-layer films is almost constant with respect
Figure 8. Water vapor permeability of three-layer films as a function of EVOH content.
Figure 9. IR absorption spectra of three-layer films at the hy-droxyl band with various EVOH contents.
to the LDPE-g-MAH content, but a significant discrep-ancy exists between the experimental data and the theo-retical prediction. Figure 8 shows that upon increasing the EVOH content the water vapor permeability of the three-layer films decreases slightly and the discrepancy between the experimental data and theoretical predic-tion becomes larger. From these observapredic-tions, we con-clude that a constant relative amount of EVOH leads to an almost constant discrepancy between the exper-imental and theoretical data, but that increasing levels of EVOH result in larger discrepancies. This feature may be due to the increased amount of intramolecu-lar hydrogen bonding in the EVOH layer, which allows barely any water vapor to pass through the three-layer films. Figure 9 shows the IR absorption spectra, viewed in the region of the hydroxyl band, of the three-layer films as a function of EVOH content. With increasing EVOH content, the absorption peaks of the hydroxyl bands near 3350 cm−1 shift towards lower wavenum-bers as a result of the increasing degree of intramolecu-lar hydrogen bonding.22, 23 The magnitude of the band shift is proportional to the strength of hydrogen bond-ing. The increased strength of hydrogen bonding makes
it more difficult for water vapor to pass through the films, and results in a larger discrepancy observed be-tween the experimental data and the theoretical predic-tion.
CONCLUSIONS
We have fabricated three-layer films, consisting of EVOH as the internal layer and blends of low-density polyethylene and low-density ethylene grafted with maleic anhydride (LDPE-g-MAH) as external layers, by a coextruded blown-film process. By investigating the adhesion, permeability and mechanical properties, we make the following conclusions: (1) The adhesion of three-layer films, consisting of incompatible layers, can be improved by using blends of maleated low-density polyethylene and low-low-density polyethylene as a result of hydrogen bond formation, which can be an-alyzed by FT-IR spectroscopy. (2) The tensile strength does not change with increasing LDPE-g-MAH con-tent, but it results in a slight increase in elongation of both the MD and TD. The modulus is only slightly in-creased at low levels of the LDPE-g-MAH content, and is almost constant in the MD and TD when the amount of LDPE-g-MAH is greater than 10 and 5 wt%, respec-tively. (3) The tear strengths in both the MD and TD decrease significantly with increasing LDPE-g-MAH content as a result of the greater ease of crack propaga-tion in EVOH that is transferred to the entire three-layer film by the improved adhesion. (4) The oxygen per-meabilities of the three-layer films follow the inverse additivity rule upon varying the EVOH and LDPE-g-MAH contents. The water vapor permeabilities of the three-layer films, however, did not follow this rule be-cause increasing degrees of hydrogen bonding result in reduced permeability.
REFERENCES
1. D. K. Lee, U. S. Patent 6 544 661 (Apr. 8, 2003).
2. T. J. Kraft, E. P. Socci, and M. K. Akkapeddi, U. S. Patent 200 3045 640 (Mar. 6, 2003).
3. H. Siewert and M. Thielen, Kunst. Plast. Euro., 88, 48 (1998). 4. F. Hensen, Plastics Extrusion Technology, Hanser, New York
N.Y., 1997.
5. A. P. Sullivan, E. Baer, A. Hiltner, G. J. Castle, C. E. Bibbons, and I. D. Sand, U. S. Patent 2 002 150 704 (Oct. 17, 2002). 6. R. H. Foster, Packaging, 32, 70 (1987).
7. T. Iwanami and Y. Hirai, Tappi J., 66, 1404 (1983).
8. J. M. Lagaron, A. K. Powell, and G. Bonner, Polym. Test., 20, 569 (2001).
9. H. U. Beckmann and Ch. Herschbach, Kunst. Plast. Euro., 86, 5 (1996).
10. Z. Zhang, I. J. Britt, and M. A. Tung, J. Appl. Polym. Sci., 82, 1866 (2001).
11. R. Gopalakrishnan, J. M. Schultz, and R. Gohil, J. Appl. Polym. Sci., 56, 1749 (1995).
12. H. Tanaka, H. Shigemoto, and H. Kawachi, J. Plast. Film Sheet., 12, 279 (1996).
13. M. G. Botros, J. Plast. Film Sheet., 12, 195 (1996).
14. Y. J. Kim, C. D. Han, B. K. Song, and E. Kouassi, J. Appl. Polym. Sci., 29, 2359 (1984).
15. W. J. Schrenk and T. Alfrey, Polym. Eng. Sci., 9, 393 (1969). 16. G. W. Kamykowski, J. Plast. Film Sheet., 16, 237 (2000). 17. S. S. Valdes, F. O. Villarreal, M. L. Quintanilla, I. Y. Flores,
and L. F. Ramos de Valle, Polym. Eng. Sci., 38, 127 (1998). 18. J. V. Olmos, S. S. Valdes, and I. G. Y´anez Flores, Polym. Eng.
Sci., 39, 1597 (1999).
19. J. B. Faisant, A. A¨ıt-Kadi, M. Bousmina, and L. Deschˆenes, Polymer, 39, 533 (1998).
20. Automatic Manometric Gas Permeability Tester Operator Manual, Model L100-5000, Lyssy AG (2001).
21. Automatic Manometric Water Vapor Permeability Tester Op-erator Manual, Model L800-5000, Lyssy AG (2001). 22. L. Dai and L. Ying, Macromol. Mater. Eng., 287, 509 (2002). 23. J. C. Speakman, “The Hydrogen Bond and Other