VOL. 2, NO. 1 APRIL, 1964
CHINESE JOURNAL OF PHYSICS
Angular Momentum Distributions in the Thomas-Fermi and Thomas-Fermi-Dirac Models of the Atom
JE N N- LI N HW A N G ($$ $6 #) Dtpartnmt of Physics, National Taiwan Chiversity,
(Received March 8, 1964)
Taipei, Taiwajz
The angular momentum distributions in the Thomas-Fermi and Thomas-Fermi-Dirac models of the atom are calculated by an’improved method proposed previously. The maximum energy (El),== for a given orbital angular momentum state is not neglected and is treated as an eigenvalue by the WKB method.
The effective central field potentials in terms of the universal approximate Thomas-Fermi function proposed by Latter are utilized. The determination of the maximum energy (El),n,, and, therefore, the number of electrons rzl for a given angular momentum state 2 is greatly facilitated by the term values computed by Latter; The calculated angular momentum distributions are checked with the dia-grams of term values against Z given in the Latter’s paper to assure that our.pro-posed method prouduces no appreciable error. .
Comparing with the empirical data, we then tind that the accuracy of the Thomas-Fermi-Dirac model is far better than that of the Thomas-Fermi model. The prediction of the first appearance of the s, p, d and f electrons by the Thomas-Fermi-Dirac model is almost perfectly exact.
I. INTRODUCTION
It has been believed that the problem of how many electrons of a given angular momentum Zh are to be found in an atom of a given atomic number 2 is not subject to treatment by the statistical model, because in this model the electrons have a con-tinuous distribution in angular momentum. In the previous .paperl), however, we have shown that the problem can be almost exactly solved by the WKB method, once the exact form of potential V(r) for the atom is given. The number of electrons nl with a given angular momentum Zfi may be determined from the following equation:
0
n/ = 2(2;;t1) 2 2nz((EJ,,,-- v(‘.)3-@p%++J,
[
JJ
(1)
where the maximum energy (E,),,, for a given angular momentum state involved in the WKB integral
-_-. _ _ ___ -.
1) J. L. Hwang, Chinese J. Phys. 1, 74 (1963) 28
ANGULAR MOMENTUM DISTRIBUTIONS IN ATOMS 29
(2) N - l , 2 , 3,-e....,
should be determined as an eigenvalue. At a glance, it seems that the Eqn (1) spoils the statistical ‘nature of the model. However, as mentioned in the footnote of the previous paper, the additional labor required in evaluating the Eqn (2) is practically._ . -limited within a small amount.
In the present paper an application of Eqn (1) will be made to the Fermi-Thomas
P
model and also to the Fermi-Thomas-Dirac model of the atom.
. __- . _
II. ATOMIC POTENTIALS
The effective central held potentials used in the present calculation are cited below from the paper of Latter21 .,...in which he has made accurate fits to these potentials.
(a) The Thomas-Fermi .Potential
V ( r ) =ZrcP(r/ft)/r, i f V(r)>e/r.
V(r) = 0, otherwise. (3)
(b) The Thomas-Fermi-Dirac Potential
(4)
Ve,(rj =e/r, otherwise.
Here the universal Thomas-Fermi function @(r/p) is approximated to @(r/p) =il +0.02747(r/p)‘/2+ 1.243(r/p) -0.1486(r/p)s~2+0.2302(r~,u)”
_. . . . - -t 0.007298(rt;u~5h + 0.006944(r/p)3]-1, (5) p = 0.8853a,/Z”3, and a,, is the Bohr radius. The maximum error in Eqn (3) is less than 0.3 percent and that in Eqn (4) is within 5 percent.
111 ANGULAR MOMENTUM DISTRIBUTIONS
The main reason for adopting the potentials Eqs (3) and (4) is that the eigenvalues of the Schrodinger equation with these potentials have been calculated by Latter with use of an electronic computer. These eigenvalues serve us the first guess for determining (EJ,,,,.L. of Eqn (2), and facilitate LLS greatly when evaluating the integral. Furthermore,
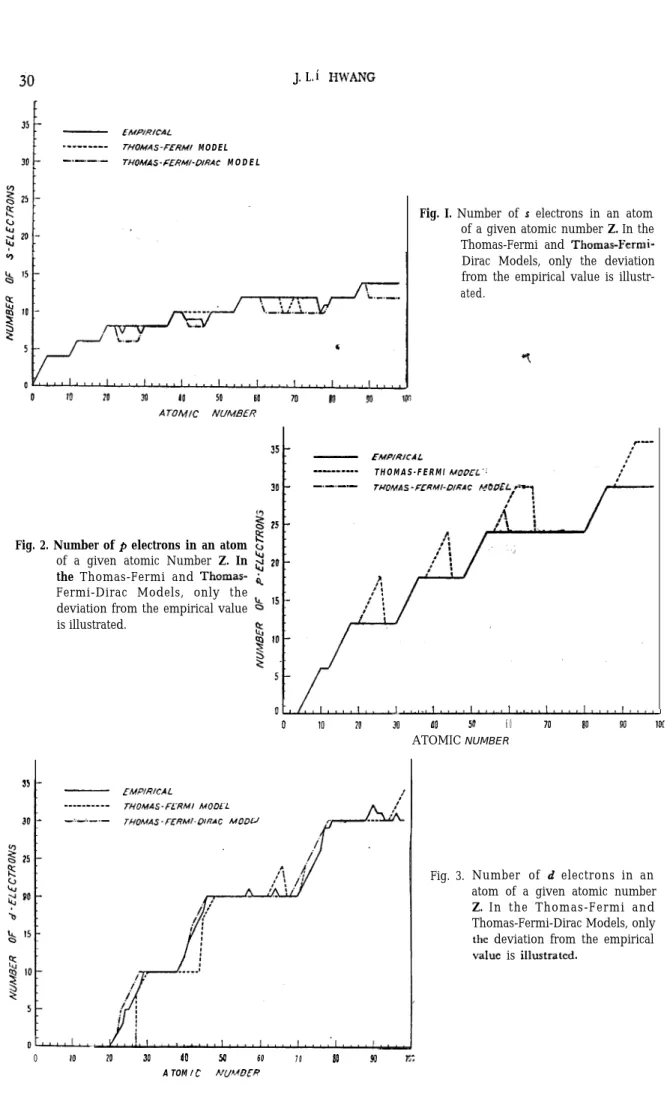
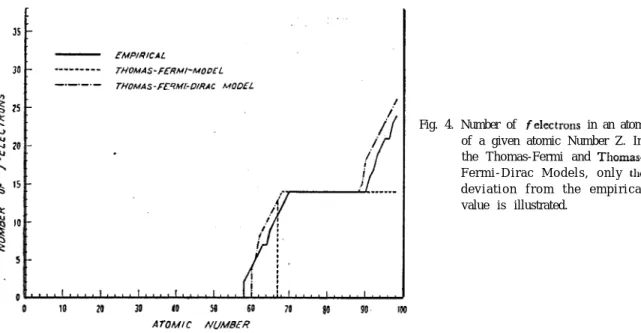
the diagrams of the term value VS. the atomic number 2 furnish us with a good standard, for checking the calculated n,‘s. The results are illustrated in Figs l-4, where the angular momentum distributions for both models of the atom are compared with the exact one. The latter is determined from the empirical data of the ground state electron
- Ehff%QICAL
.___m____ THOMAS-FfRhfl M O D E L -.-.-._ THOMAS-b-ERM/-DIRAC M O D E L
J. L.’ HWANG
ATDMK NUMBER
Fig. 2. Number of p electrons in an atom
of a given atomic Number Z. In
the Fermi and Thomas-Fermi-Dirac Models, only the deviation from the empirical value is illustrated.
- EMPIR/CAL
-____-__._ TUOMAS-FL-RMI MO0L.L
_:_‘.-.- THOMAS-F.9?Mt~DlRAC MODU
Fig. I. Number of s electrons in an atom of a given atomic number Z. In the Thomas-Fermi and Thomas-Fermi-Dirac Models, only the deviation from the empirical value is illustr-ated. - FMPIUJCAL _I... T H O M A S - F E R M I MOOfL’. -._.s- TUOMAS-FERMI-DffiAC J 0 IO 20 30 00 50 60 70 80 90 lO[ ATOMIC NUMBER
Fig. 3. Number of d electrons in an atom of a given atomic number
Z. In the Thomas-Fermi and Thomas-Fermi-Dirac Models, only the deviation from the empirical value is illustrated.
0 JO 70 30 40 so 60 70 a0 90 lc:
A TOM I C NUUDER
ANGULAR MOMENTUM DISTKIBUTIONS IN ATOMS 31
Fig. 4. Number of ~felectrons in an atom of a given atomic Number Z. In the Fermi and Thomas-F e r m i - D i r a c M o d e l s , o n l y the d e v i a t i o n f r o m t h e e m p i r i c a l value is illustrated.
configuration of the elements given in the text of Condon and Shortley3). Since the calculated distributions agree exactly with those predicted from the Latter’s diagrams over almost the whole range of 2, we can assure that the use of our proposed Eqn (1) does not yield any appreciable error. Therefore, the discrepancy existing between the calculated and empirical ones merely means the inherent defects in each model of the atom.
On viewing the Figs. l-4 we readily notice that the accuracy of the Thomas-Fermi-Dirac model is far better than that of the Thomas-Fermi model. For the case of Thomas-Fermi model there are four spurious peaks in the distribution of p electrons. It is these peaks that’ make the appearence of 34 4d, 4f and SJ-‘ electrons lag behind the empirical values. ‘On the other hand, for the case of Thomas-Fermi-Dirac model the distributions are all in good conformity with the empirical data, apart from a slight lead of appearence of 5d and
Sf
electrons and a slight lag of that of4f
and 7s electrons. This is the important result of the exchange effect among the electrons which Oliphant has attempted to show.‘)ACKNOWLEDGMENT
A grant from the National Council on Science Development is gratefully acknow-ledged.
-3) E. U. Condon and G. H. Shortley, The Theo& of Atomic Spectra iuniversity Press, Cambridge, 1935) 4) T. A. Oliphant, Jr. Phys. Rev. 104, 954 (1956)