ARTICLE NO. 0533
The Stability Ratio for a Dispersion of Particles Covered
by an Ion-Penetrable Charged Membrane
JYH-PINGHSU1
ANDYUNG-CHIHKUO
Department of Chemical Engineering, National Taiwan University, Taipei, Taiwan 10617, Republic of China Received January 17, 1996; accepted March 28, 1996
tered in practice ( 2 ) . The solution to a general PBE is
non-The electrostatic interaction between two particles covered by an trivial, except for limited cases. The degree of difficulty ion-penetrable charged membrane suspended in an a :b electrolyte
depends largely on the geometry of a charged surface, the
solution is estimated. A perturbation method is employed to solve
types of electrolyte in the liquid phase, and the boundary
the governing nonlinear Poisson – Boltzmann equation. The
ap-conditions on the surface. In practice, either drastic
assump-proximate analytical expression derived for potential distribution
tions are made and a simplified PBE is solved, or it is solved
yields a sufficiently accurate estimate. Both the stability ratio and
numerically.
the critical coagulation concentration of the counterions of the
Previous efforts on the estimation of the stability ratio of
system under consideration are derived. The results of a numerical
a dispersed system were mainly based on rigid surfaces ( e.g.,
simulation reveal that the stability ratio decreases with an increase
in the size of a particle and increases with the total amount of 3 – 5 ) . Although this model is appropriate for most of the
fixed charges in the membrane phase. For a constant total amount inorganic colloids, it can be unrealistic for some of the dis-of fixed charges and particle size, the stability ratio increases with persed entities in practice. These include biocolloids and a decrease in the thickness of the membrane. We show that mono- particles covered by an artificial membrane ( 6 – 11 ) . A typi-dispersed particles are more stable than polytypi-dispersed particles.
cal example of the former is human erythrocytes, the
periph-q 1996 Academic Press, Inc.
eral zone of which contain a glycoprotein layer about 15 nm
Key Words: stability ratio, spherical particles; critical
coagula-thick. This zone possesses some ionogenic groups and forms
tion concentration, counterions; Poisson – Boltzmann equation,
the outer boundary of the lipid layer ( 12, 13 ) . An example
nonlinear ; membrane, ion-penetrable, charged; electrical potential
of the latter is dispersed entities in polymer-induced
floccu-distribution, approximate, analytical.
lation. Here, an entity comprises a rigid core and a layer of adsorbed polymer molecules, which often bear fixed charges. In an analysis of the interaction between an ion-penetrable
I. INTRODUCTION
particle and a rigid particle, Terui et al. ( 14 ) derived expres-sions for the interaction potential and the electrostatic inter-One of the key characteristics of a dispersed colloidal action force between particles. The analysis was extended suspension is its stability ratio, defined as ( rate of fast coagu- to various combinations of two types of particles, and expres-lation ) / ( rate of slow coaguexpres-lation ) . The numerator denotes sions for critical coagulation concentration of counterions the rate of coagulation in the absence of an interaction energy were derived ( 15 ) . These analyses ( 14, 15 ) were based on barrier, and the denominator is that in the presence of an a low level of surface potential, symmetric electrolyte, and interaction energy barrier. Here, the interaction energy
in-uniformly distributed fixed charges in the surface layer of a cludes the electrostatic repulsive energy and the van der
particle. Waals attractive energy ( 1 ) . The estimation of the former
In a recent study the electrostatic interactions between requires knowledge about the electrostatic potential
distribu-spherical particles coated with an ion-penetrable charged tion between two charged surfaces, which is governed by membrane immersed in an electrolyte solution at a low elec-the Poisson – Boltzmann equation ( PBE ) . This equation in trostatic potential were analyzed ( 16 ) . The result obtained its general form is nonlinear. Although there exists some was adopted to estimate both the stability ratio and the criti-fundamental inconsistency in the PBE which arises from the cal coagulation concentration of counterions. If the potential origin of the corresponding Poisson equation, the deviation is low, the governing PBE can be approximated by a linear from the exact value is tolerable for conditions often encoun- expression, which is readily solvable. In the present study, the linear model of ( 16 ) is extended to a general case in which both the level of potential and the type of electrolyte
1
To whom correspondence should be addressed. E-mail: T8504009@
CCMS.NTU.EDU.TW. are arbitrary.
184
T are, respectively, the Boltzmann constant and the absolute
temperature; i represents a region index ( iÅ0 denotes the double-layer region, i Å1 the membrane phase ) ; and r is the distance measured from the center of a particle. The boundary conditions associated with [1] are assumed to be
cr 0 and ( d c / d X )r0 as Xr ` [1a ] cÉxr(x 0/d )0ÅcÉxr(x0/d )/ Åcd [1b ] ( d c / d X )Éxr(x 0/d )0 Å( d c / d X )Éxr(x0/d )/ [1c ] cr ccand ( d c / d X )r 0 as Xr X0. [1d ] In these expressions, ccand cdare, respectively, the dimen-sionless electrical potential at the outer boundary of the rigid core of a particle and that at the membrane-liquid interface. Suppose that c can be expanded in the perturbation series ( 17 ) cÅ
∑
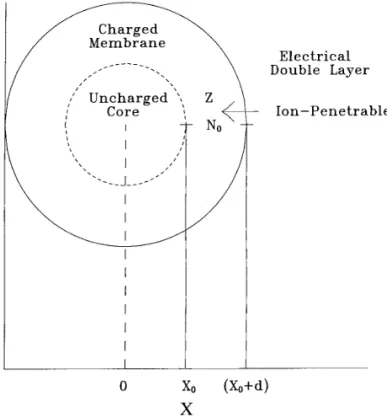
` £Å0 h£ X£FIG. 1. A schematic representation of the structure of a particle under consideration. Z and N0are, respectively, the valence and the density of
the charged groups. The region Xú( X0/d ) represents the liquid phase,
d being the dimensionless thickness of the membrane. The region XõX0 à h0/
S
h1
X
D
/S
h2
X2
D
. [ 2 ] denotes the rigid core of a particle; X0õXõ( X0/d ) is the ion-penetrablemembrane phase.
Substituting [ 2 ] into [1] with iÅ0 and solving the resultant
II. MODELING
expression give the electrical potential distribution for the double-layer region ( Appendix A ) ,
By referring to Fig. 1, we consider a spherical particle composed of a rigid core and an ion-penetrable, charged membrane immersed in an a :b electrolyte solution. Denote the dimensionless sizes of the rigid core and the membrane
h0Å
S
2 aD
ln 1/[ tanh ( acd/ 4 ) ] 1 exp [0k3( X 0X00 d ) ] 10[ tanh ( acd/ 4 ) ] 1exp [0k3( X0X00d ) ] [ 3 ] as X0 and d , respectively. Here, X0Å kr0, where r0 and kare the size of the rigid core and the reciprocal Debye length, respectively. Without loss of generality, we assume that the fixed charges in the membrane are negative and distributed
h1 Å
S
1 ak3D
sinhS
ah0 2DF
tanh 2S
acd 4D
uniformly.The electrical potential distribution in spherical geometry is described by the PBE
0tanh2
S
ah0 4D
02 lnF
tanh ( acd/ 4 ) tanh ( ah0/ 4 )GG
[ 4 ] d2c d X2/ 2 X d c d X Å g/iN a/b , iÅ 0, 1, [1] h2 ÅX ( h0) a sinh ( ah0/ 2 ) 8k3F
u*
h0 0 h2 1sinh ( ah0/ 2 ) X ( h0) dh0 where cÅef / kBT , gÅ[ exp ( bc )0exp (0ac ) ] , XÅkr ,k2
Åe2
a ( a/b ) n0
a/ e0erkBT , and NÅZNAN0/ an0a. In these
expressions, f denotes the electrical potential; e is the ele- /
*
c0h0
uh2
1sinh ( ah0/ 2 )
X ( h0) dh0
G
, [ 5 ] mentary charge; N0and Z are, respectively, the density andthe valence of the charged groups in the membrane (0ZeNAN0 is the density of fixed charges ) ; NA represents
the Avogadro number ; erand e0are the relative permittivities where k3, u , and X ( h0) are defined in Appendix A. If the membrane is sufficiently thick, the dimensionless of the solution and the vacuum, respectively; n0
ais the
S
2 a/bD
1 / 2H
1 b[ exp ( bcd)01]/ 1 a[ exp (0acd)01]J
1 / 2 0 1 X0/dF
4 atanhS
acd 4DG
/ 4 ( X0/d )2 1F
tanh 2( acd/ 4 )02 ln [ cosh ( acd/ 4 ) ]
k3sinh ( acd/ 2 )
G
Å
S
2a/b
D
1 / 2
H
1b[ exp ( bcd)0exp ( bcDon) ]
/1
a[ exp (0acd)0exp (0acDon) ]
/N ( cd0cDon)
J
1 / 2 0 4 X0/d 1H
1 atanhF
a ( cd0cDon) 4GJ
/ 4( X0/d )2 FIG. 2. Schematic representation of a system containing two particles.
A2Å 0
F
4 27k3 / 2 9a4( a 2 2 02a 2 2a3)G
tanh 2S
acd,n 4D
1H
tanh2 [ a ( cd0cDon) / 4 ] 02 ln [ cosh [ a ( cd0cDon) / 4 ] ] k3sinh [ a ( cd0cDon) / 2 ] . [ 6 ] 1{ 3k3( X0,n/dn) cosh [ 3k3( X0,n/dn) ] 03k3X0,ncosh ( 3k3X0,n)The electrostatic interaction energy between two particles
0sinh [ 3k3( X0,n/dn) ]/sinh ( 3k3X0,n) } [ 7b ]
Iel( R ) can be estimated by substituting [ 2 ] – [ 5 ] into [ B16 ] ( see Appendix B ) to yield
A3Å
F
3 32a4( 2a 2 2a30a 2 20a2)G
tanh 4S
acd,n 4D
Iel( R )Å 16p ak3 3k3R∑
2 n Å1 { Pn ,n =∑
3 t Å1 { Atexp [ k3( 2t01 ) 1{ 5k3( X0,n/dn) cosh [ 5k3( X0,n/dn) ] 05k3X0,ncosh ( 5k3X0,n) 1( X0,n/dn0R ) ] } } , nxn*, [ 7 ] 0sinh [ 5k3( X0,n/dn) ]/sinh ( 5k3X0,n) } [ 7c ] where the subscripts n and n* denote particles n and n*,respectively, dnis the dimensionless thickness of the mem- a1Å
S
12
D
tanh 2S
acd,n4
D
/k3( X0,n/dn)03/a5
a4
brane phase of particle n , and R is the dimensionless center- [ 7d ]
to-center distance between two particles ( Fig. 2 ) . The values of A1, A2, A3, and Pn ,n =are defined by
a2Åtanh 2
S
acd,n 4D
/lnH
1 2F
1/coshS
acd,n 2DGJ
[ 7e ] A1Å ( 3 /a1) ( X0,n/dn) cosh [ k3( X0,n/dn) ] a3Å coth ( acd,n/ 2 ) sinh ( acd,n/ 2 ) 0 1 2 0( 3 /a1) X0,ncosh ( k3X0,n)0S
3 k3 /a1D
1lnH
1 2F
1/coshS
acd,n 2DGJ
[ 7f ] 1( X0,n/dn) sinh [ k3( X0,n/dn) ] /S
3 k3 /a1D
1X0,nsinh ( k3X0,n) a4Å600k43( X0,n /dn) [ 7g ] [ 7a ]shown that the value of R at which Ithas its primary maxi-a5Åa 2 2a3sinh 2
S
acd,n 4D
mum, Rmax, is Rmaxà[ b6/( b37/b26)1 / 2]1 / 3 /F
2a2 2a3/ 1 2a 2 2/ a2G
1sinh 4S
acd,n 4D
[ 7h ] /[ b60( b3 7/b 2 6)1 / 2 ]1 / 3 0b3 3 , [10 ] Pn ,n =ÅLnF
tanhS
acd,n = 4DG
where 1exp [ k3( X0,n = /dn =0X0,n0dn) ] , [ 7i ] b3Åb202 D20 A132 12 D3b1 [10a ] where the energy density Lnis defined in Appendix B.b6Å
( 9b3b4027b502b3)
54 [10b ]
The electrostatic interaction force between two particles,
fel( R ) , can be calculated by b7Å ( 3b30b2 3) 9 [10c ] fel( R )Å 0k
S
dIel d RD
b5ÅD 2 2b2 [10d ] b4ÅD2( D202b2) [10e ] ÅkIel R / 16p ak2 3k2R∑
2 n Å1 { Pn ,n =∑
3 t Å1 { At( 2t0 1 ) b2Å 16p ak3 3k3b1∑
2 n Å1 { Pn ,n =∑
3 t Å1 { At1exp [ k3( 2t01 ) 1exp [ k3( 2t01 ) ( X0,n/dn0R ) ] } } 1( X0,n =/dn =) ] } } , nxn* [10f ] Åk( k3R /1 ) R Iel/ 32p ak2 3k2R b1Å 16p ak2 3k3∑
2 n Å1 { Pn ,n =∑
3 t Å1 { At( 2t01 ) 1∑
2 n Å1 { Pn ,n =∑
3 t Å2 { At( t01 ) 1exp [ k3( 2t01 ) ( X0,n =/d n =) ] } } , nxn*. [10g ] 1exp [ k3( 2t01 ) ( X0,n/dn0R ) ] } } ,nxn*. [ 8 ] In these expressions, A132denotes the Hamaker constant, and
D2and D3are defined in [ C2c ] and [ C2b ] , respectively ( see Appendix C ) . Substituting [ 7 ] , [ C1] , [ C2e ] , [ C5a ] , and Note that if Rr `, both Ieland fel vanish, as expected.
[10 ] into [ C4 ] yields The stability ratio can be regarded as the reciprocal
colli-sion efficiency which leads to coagulation. It can be
mea-sured by the ratio of particle fluxes without and with an W àexp
HF
Iel( Rmax)0
A132D3
12 ( Rmax0D2)
GY
kBTJ
. [11] energy barrier ( 1 ) . Therefore the kinetic property can bedescribed by the interaction energy between two particles. According to Fuchs ( 18 ) and Overbeek ( 19 ) , the stability
The critical coagulation concentration ( CCC ) can be de-ratio of the system under considede-ration, W , can be estimated
termined by by Iel( Rc) 0 A132D3 12 ( Rc0D2) Å0 [12 ] WÅ
*
` 1S
1 R2 rD
expS
It kBTD
d Rr, [ 9 ] fel( Rc) / kA132D3 12 ( Rc0D2)2Å0, [13 ] where RrÅR / D2, and Itis the total interaction energybe-tween two particles. The latter includes the electrostatic re- where Rc is the dimensionless center-to-center distance be-tween two particles at the CCC. Solving these equations pulsion energy and the van der Waals attraction energy. On
TABLE 1
Average Deviation Q (%) in the Potential Distribution of the Present Perturbation Method, c, from the Exact Numerical Calcu-lation, c*a N0(M) X0 1003 511003 1002 1 3.77 4.84 5.61 2 3.13 4.27 4.85 4 2.32 3.50 4.39 6 2.17 3.28 3.96
aFor the case ZÅ1, dÅX
0/4, ionic strengthÅ1003M, erÅ78, and
TÅ298.15 K. t equally spaced points in the range [X0/d, X0/d/3]
are chosen, and the potentials at these points are evaluated. Q is defined by 100% [(3
aÅ1(
3
bÅ1( t
jÅ1É(c*0c)/c*]/9t, where a and b are the valences
of the cation and anion, respectively.
Rc àg/[ g( g/D2) ] 1 / 2
, [14 ]
FIG. 3. Variation of log W as a function of the radius of the rigid core of where
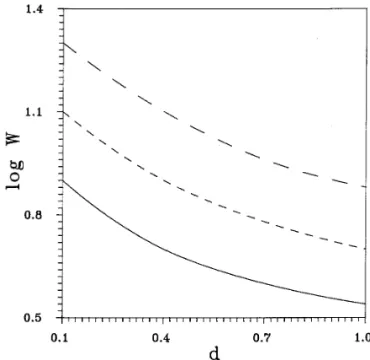
a particle for the case X0,1ÅX0,2ÅX0, d1Åd2ÅX0/4. — , N0Å1003M;
---, N0Å511003M; – – – , N0Å1002M. Key: erÅ78, TÅ298.15 K,
Z1ÅZ2Å1, aÅ2, bÅ1, A132Å10020J, and ionic strengthÅ1003M.
gÅD20
A132D3
12b1 . [14a ]
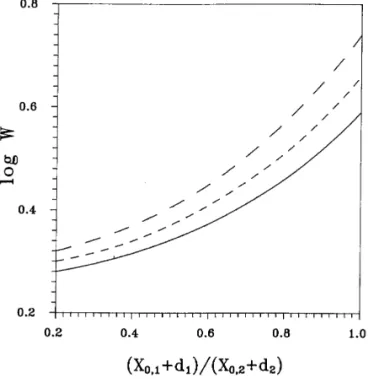
Figure 5 illustrates the variation in the stability ratio as a The CCC for counterions can be estimated by substituting
function of the size ratio of two interacting particles, ( X0,1 [14 ] into either [12 ] or [13 ] .
/d1) / ( X0,2/d2) , at a fixed mean size of two particles, [ ( X0,1
/d1) ( X0,2/d2) ]1 / 2
. This figure shows that the smaller the
III. DISCUSSION
size ratio ( the greater the difference in the sizes of two Table 1 shows the average percentage deviation in the
potential distribution estimated by [ 2 ] from the exact values, estimated by solving [1] numerically. As can be seen from this table, the performance of the present perturbation method is satisfactory.
Figure 3 illustrates the simulated variation in the stability ratio W as a function of the linear size of the rigid core of a particle at various densities of fixed charges in the membrane phase. This figure suggests that W decreases with increases of X0. This is consistent with the classic DLVO theory to-gether with the Fuchs model ( 1 ) , and the result for the case of low potential ( 16 ) . For a fixed X0, the greater the density of fixed charges N0, the greater the W . This is expected, since the greater the N0, the greater the electrostatic repulsive force between two particles.
Figure 4 shows the effect of membrane thickness on the stability ratio at a constant total amount of fixed charges and particle size. As can be seen from Fig. 4, the stability ratio increases with decreases in the membrane thickness. This is because at a constant total amount of fixed charges, the
FIG. 4. Variation of log W as a function of the membrane thickness at smaller the thickness of membrane, the more concentrated
a constant total amount of fixed charges, TC, and particle size for the case the fixed charges, and the greater the electrostatic repulsion d
1Åd2Åd , X0,1/ d1ÅX0,2/ d2Å5. — , TCÅ 2.261 10022mol;
energy. Figure 4 also suggests that the stability ratio in- - - -, TCÅ 1.13110021mol; – – – , TCÅ2.26110021mol. Key: see
the legend to Fig. 3. creases with the total amount of fixed charges, as expected.
where H is the dimensionless surface-to-surface distance be-tween the particle and the surface.
3.3. Two Parallel Flat Surfaces
In the case of two parallel flat surfaces, it can be shown that Iel( H )Å 8p ak4 3k 3
∑
2 n Å1H
LnF
tanhS
acd,n 4DG
1H
3 [10exp (0k3dn) ] exp (0k3D3H ) 0 4 27( 3k301 ) [10exp (03k3dn) ] 1F
tanhS
acd,n 4DG
exp (03k3D3H )JJ
. [17 ]FIG. 5. Variation of log W as a function of the relative size of two interacting particles at a fixed mean size of two particles aV for the case d1
3.4. Rigid Particles and 1:1 Electrolytes
Åd2Å 1 and N0Å 811003M. — , aVÅ11; - - -, aVÅ13; – – – , aVÅ
15. Key: see the legend to Fig. 3.
For 1:1 electrolytes and rigid particles, [ 3 ] – [ 5 ] reduce to those derived by Dukhin et al. ( 20 ) .
particles ) , the smaller the stability ratio. In other words,
monodispersed particles are more stable than polydispersed 3.5. Single Particle
particles. This can be deduced from Eqs. [ C2 ] – [ C2d ] . The
Suppose that the radius of one of the two interacting parti-smaller the size ratio, the parti-smaller the value of D4, the greater
cles approaches zero, i.e., ( X0,n/dn)r0, nÅ1 or 2. Then
the absolute van der Waals attraction energy, and the smaller
dn r0 and cndoes not exist. In this case, [ 7 ] implies that
the stability ratio.
Iel( R )r 0 and Ivdwr0 by [ B2 ] . Under certain conditions, the results of the present study
can be either simplified or reduced to those reported in the
APPENDIX A
literature. We consider five special cases.
3.1. Two Identical Particles Substituting [ 2 ] into [1] and collecting terms of the same
order in X , we obtain the potential distribution in the double-If the interacting particles are identical, then X0,1 Å X0,2
layer region, ÅX0, d1 Åd2Å d , cd,1Å cd,2Å cd, P1,2 ÅP2,1 ÅP ( X0, d , cd) , AtÅAt( X0, d , cd) , tÅ1, 2, 3, and [ 7 ] gives d2 h0 dX2 Å g0 /iN a/b [ A1] Iel( H )Å 32p ak3 3k3R P ( X0, d , cd) d2h 1 d X2 0 1 a/b[ b exp ( bh0) 1
∑
3 t Å1 { At( X0, d , cd) 1exp [ k3( 2t01 ) ( X0/d0R ) ] } . [15 ] /a exp (0ah0) ] h1Å02 dh0 dX [ A2 ] 3.2. A Spherical Particle and a Flat Surfaced2 h2 dX2 0 2 X dh2 dX / 2 X2h2 If particle 1 is spherical and particle 2 becomes a flat
surface, [ 7 ] reduces to 0 1 a/b[ b exp ( bh0) /a exp (0ah0) ] h2 Iel( H )Å 24p ak4 3k 3L1
F
tanhS
acd,2 4DG
[10 exp (0k3d2) ] Å 1 2 ( a/b ) [ b 2 exp ( bh0)0a2 exp (0ah0) ] h21, [ A3 ] 1exp [ k3( X0,1 /d10 D3H ) ] , [16 ]where g0Åg ( h0) . The boundary conditions associated with
S
dh1 dXD
(X0/d )/ Å 04 atanhS
acd 4D
[ A11]these equations are
h£and ( dh£/ d X )r 0 as Xr `, £Å 0, 1, 2, . . . [ A4 ] h0 rcd, h£ r0 as Xr ( X0/d ) , £Å 1, 2, . . . . [ A5 ]
S
dh2 dXD
(X 0/d )/ Å 4 tanh2 ( acd/ 4 ) 0 8 ln [ cosh ( acd/ 4 ) ]k3sinh ( acd / 2) . [ A12 ] Solving [ A1] with i Å 0 subject to [ A4 ] and [ A5 ] gives
[ 3 ] with
Equation [1d ] can be rewritten in terms of h£as
k3Å
H
[ ( k 02 ) k1 /2k2] / k , if k£4 [ 2k1/( k02 ) k2] / k , if kú4, [ A6 ]S
dh0 dXD
r 0,S
dh£ dXD
r0, h0r cc, and h£r 0 where k1Å2 / { k1 / 2 [ ( k / 2 )2 / ( k 02 ) 01] } , k2Å2 / k1 / 2 , and k as Xr X0,£Å1, 2, . . . . [ A13 ]Å2/2b / a ( 17 ) . Similarly, solving [ A2 ] and [ A3 ] subject to [ A4 ] and [ A5 ] gives [ 4 ] and [ 5 ] with
Integrating [ A1] – [ A3 ] with i Å 1 subject to [ A13 ] and applying [ A5 ] , we obtain uÅ cosh ( acd/ 2 ) sinh2 ( acd/ 2 ) 0 cosh ( ah0/ 2 ) sinh2 ( ah0/ 2 )
S
dh0 dXD
(X0/d )0 ÅF
2 a/bG
1 / 2H
1 b[ exp ( bcd) 0exp ( bcc) ] 0lnF
tanh ( ah0/ 4 ) tanh ( acd/ 4 )G
[ A7 ] /1 a[ exp (0acd)0exp (0acc) ] X ( h0) Å 1 2k3lnFS
cosh ( ah0/ 2 ) /1 cosh ( ah0/ 2 ) 01DS
cosh ( acd/ 2 )01 cosh ( acd/ 2 )/1DG
/N ( cd0 cc)J
1 / 2 [ A14 ] /X0 /d . [ A8 ]S
dh1 dXD
(X0/d )0 Å 0 4 atanhS
a ( cd 0cc) 4D
[ A15 ]Substituting [ 3 ] – [ 5 ] into [ 2 ] gives the potential distribu-tion in the double-layer region. For 1:1 electrolytes, [ 3 ] – [ 5 ] reduce to the results proposed by Dukhin et al. ( 20 ) .
Differentiating [ 2 ] with respect to X and applying [ A5 ] ,
S
dh2dX
D
(X0/d )0 Å 4 tanh2 ( a ( cd0cc) / 4 ) 08 ln [ cosh ( a ( cd0cc) / 4 ) ] k3sinh ( a ( cd0cc) / 2 ) . [ A16 ] we obtainS
d c d XD
X 0/d Å∑
` £Å0FS
dh£ dXDY
X £G
X0/d If the membrane phase is sufficiently thick, there is no net
charge as XrX0, and c approaches cDon, the dimensionless Donnan potential. In this case, letting iÅ 1 in [1] yields
à
S
dh0 dXD
X0/d / 1 X0/ dS
dh1 dXD
X0/d g ( c Don)/N Å0. [ A17 ]cDoncan be estimated by solving this equation. On the basis
/ 1
( X0 /d )2
S
dh2
dX
D
X0/d. [ A9 ]
of [1b ] , [ A9 ] , [ A10 ] – [ A12 ] , and [ A14 ] – [ A16 ] , [ 6 ] can be recovered.
Integrating [ A1] – [ A3 ] with i Å 0, subject to [ A4 ] and
APPENDIX B
employing [ A5 ] , we have
Let E be the strength of electric field. We have
S
dh0 dXD
(X0/d )/ ÅF
2 a/bG
1 / 2H
1 b[ exp ( bcd)01] Ç r( fE )ÅfÇrE/ErÇf, [ B1]whereÇ is the differential operator. Since EÅ 0Çf, the /1
a[ exp (0acd)01]
J
1 / 2
[ A10 ]
Ç rEÅ 0 ÇrÇf Ft elÅ 0
S
e0er 2D
*
A fErn d A01 2*
V1 rfixfd V1, [ B9 ] Å 0[( M £Å1z£en 0 £exp (0z£c )0irfix] e0er , [ B2 ]where n denotes the unit outer normal vector of surface where n0
£ and z£denote, respectively, the number concentra- element d A . Since both E and f are continuous, and fE is
tion of the£th ion species in the bulk liquid phase and its bounded, the surface integral in [ B9 ] vanishes, and we have
valence, M is the number of ion species, and rfixrepresents the density of fixed charges evaluated by
Ft elÅ 0
1 2
*
V1rfixfd V1. [ B10 ]
rfixÅZeNAN0. [ B2a ]
For a rigid surface E is not continuous at the solid – liquid For the present system, the density of internal energy, uel, interface. Thus, if n is parallel to E , letting V1Å0 in [ B9 ] is identical to the electric field energy, i.e., leads to
uelÅ
S
e0er
2
D
ErE . [ B3 ] FtelÅsf0A
2 , [ B11]
where f0denotes the surface potential, and A is the area of For dilute solutions the density of entropy, sel, is
the surface.
The electrostatic energy of a system containing K
parti-selÅ 0
S
kB 2D
∑
M £Å1 n£lnS
n£ n0 £D
, [ B4 ] cles, Iel( R ) , can be calculated by
Iel( R )ÅFtel( R )0Ftel(`) , [ B12 ] where n£is the number concentration of species£. According
to the Gibbs – Helmholtz equation, we have where R is the set of distances Rn ,n =, n , n* Å1, 2, . . . , K , n
xn*, Rn ,n =being the dimensionless center-to-center distance
felÅuel0Tsel, [ B5 ] between particle n and n*, with Rn ,n = ÅRn =,n. Suppose that
the double layer around a particle is thin. Employing [ B10 ] where felis the density of electrical free energy. Substituting and [ B2a ] , we obtain
[ B3 ] and [ B4 ] into this expression, employing the Boltz-mann distribution law for ions, and using [ B1] and [ B2 ] ,
Ft el( R )Å
∑
K n Å1*
V1,n Lncd V1,n [ B13a ] we have Ft el(`) Å∑
K n Å1*
V1,n Lncn ,i Å1d V1,n [ B13b ] felÅ 0S
e0er 2D
Çr( fE ) 0 irfixf 2 . [ B6 ]Denote the volume of the membrane phase and that of the liquid phase as V1 and V2, respectively, and let V Å V1 /
V2. The total free energy of the system, F t el, is cÅ cn ,i Å1/
∑
n = cn =,i Å0, X0,nõXnõ( X0,n/dn) , n , n* Å1, 2, . . . , K , n* xn cn ,i Å0/∑
n = cn =,i Å0, ( X0,n/dn)õXn, ( X0,n =/dn =)õXn =, n , n* Å1, 2, . . . , K , n* xn , [B14] Ft el Å*
feld V . [ B7 ]Substituting [ B6 ] into [ B7 ] gives
where LnÅ 0znNAN0, n kBT / 2, d V1,nÅ2pX 2 nsin und Xndun/ Ft elÅ 0
S
e0er 2D
*
V Çr( fE ) d V 01 2*
V1 rfixfd V1, [ B8 ] k3, and un is the angle between line segments defined by
Rn ,n = and Xn. Ftel(`) is the electrostatic free energy for the case any pair of two particles are infinitly apart. Since cn ,i Å1
is a function of Xn, Ftel(`) is independent of R. For a system where d V and d V1represent volume elements. Applying the
ftÅ 0
S
k D3DS
dIt dHD
cÅ c1,i Å1/c2,i Å0, X0,1õX1õ( X0,1/d1) c1,i Å0/c2,i Å0, ( X0,1/d1)õX1, ( X0,2/d2)õX2 c1,i Å0/c2,i Å1, X0,2õX2õ( X0,2/d2) . [ B14a ] Å fel0S
k D3DS
dIvdw dHD
. [ C3 ]The stability ratio of the system, W , can be estimated by By referring to Fig. 2, we have [ 9 ] . As an approximation, the total interaction energy is replaced with its primary maximum, It,max, and the integra-tion procedure suggested by Overbeek ( 19 ) leads to
X1Å ( R 2 /X2 20 2 RX2cos u2) 1 / 2 [ B15a ] X2Å ( R 2 /X2 102 RX1cos u1) 1 / 2 , [ B15b ] W àexp
S
It,max kBTD
. [ C4 ]where R denotes the dimensionless center-to-center distance between two particles, and the angles u1and u2are defined
in Fig. 2. For a two-particle system, [ B12 ] becomes As Hr0, [ C2 ] reduces to
Iel( R )Å
∑
2 n Å1*
V1,n = Lncn =,i Å0d V1,n =, n* x n . [ B16 ] IvdwÅ 0A132 12H [1/H ( 1 0D4) /2H ln H/ rrr] APPENDIX C à0A132 12H . [ C5a ]The total interaction energy Itincludes the van der Waals
energy Ivdwand the electrostatic energy Iel, i.e., If the size of one of the two interacting particles approaches infinity, i.e., X0,n r `, nÅ1 or 2, [ C2 ] reduces to
ItÅIvdw/Iel. [ C1] IvdwÅ 0A132 6
F
1/2H 2H ( 1/H ) /lnS
H 1/ HDG
. [ C5b ] We assume that ( 1 ) IvdwÅ 0A132 12HF
1 1/D4H / H1/H/D4H2 If the sizes of two interacting particles approach infinity, the problem reduces to the interaction of two parallel planar surfaces. In this case, [ C2 ] gives
/2H ln
S
H/D4H 2 1/H/D4H2
DG
, [ C2 ]IvdwÅ 0A132Ap[12p( D3H )2] , [ C5c ] where Ap is the average surface area of two particles, and where
D3H represents the dimensionless surface-to-surface distance between two particles.
D4Å
D3
2 D2 [ C2a ] At the critical cogulation contration,
ItÅ0 and ftÅ 0. D3Å D1 D2 [ C2b ] ACKNOWLEDGMENT D2Å X0,1 /d1/X0,2 /d2 [ C2c ]
This work was supported by the National Science Council of the Republic
D1Å 2 ( X0,1/d1) ( X0,2 /d2) [ C2d ]
of China under Grant NSC84-2214-E002-030.
HÅ ( R0D2)
D3
, [ C2e ]
REFERENCES
1. Hunter, R. J., ‘‘Foundations of Colloid Science,’’ Vol. I. Oxford Univ. where A132denotes the Hamaker constant and D3represents Press, London, 1989.
the dimensionless reduced radius. The interaction force be- 2. Hiemenz, P. C., ‘‘Principles of Colloid and Surface Chemistry,’’ 2nd ed. Dekker, New York, 1986.
3. Wiese, G. R., and Healy, T. W., Trans. Faraday Soc. 66, 490 ( 1970 ) . 13. Kawahata, S., Ohshima, H., Muramatsu, N., and Kondo, T., J. Colloid Interface Sci. 138, 182 ( 1990 ) .
4. Metcalfe, I. M., and Healy, T. W., Faraday Discus. Chem. Soc. 90,
335 ( 1990 ) . 14. Terui, H., Taguchi, T., Ohshima, H., and Kondo, T., Colloid Polym. Sci. 268, 76 ( 1990 ) .
5. Overbeek, J. Th. G., Pure Appl. Chem. 52, 1151 ( 1980 ) .
6. Donath, E., and Pastushenko, V., Bioelectrochem. Bioenerg. 6, 543 15. Taguchi, T., Terui, H., Ohshima, H., and Kondo, T., Colloid Polym. Sci. 268, 83 ( 1990 ) .
( 1979 ) .
7. Wunderlich, R. W., J. Colloid Interface Sci. 88, 385 ( 1982 ) . 16. Hsu, J. P., and Kuo, Y. C., J. Chem. Phys. 103, 465 ( 1995 ) . 17. Hsu, J. P., and Kuo, Y. C., J. Chem. Soc. Faraday Trans. 91, 1223 8. Levine, S., Levine, M., Sharp, K. A., and Brooks, D. E., Biophys. J.
42, 127 ( 1983 ) . ( 1995 ) .
18. Fuchs, N., Z. Phys. 89, 736 ( 1934 ) . 9. Sharp, K. A., and Brooks, D. E., Biophys. J. 47, 563 ( 1985 ) .
10. Reiss, H., and Bassignana, I. C., J. Membrane Sci. 11, 219 ( 1982 ) . 19. Overbeek, J. Th. G., in ‘‘Colloid Science’’ ( H. R. Kruyt, Ed.) , Vol. 1. Elsevier, Amsterdam, 1952.
11. Selvey, C., and Reiss, H., J. Membrane Sci. 23, 11 ( 1985 ) .
12. Seaman, G. V., in ‘‘The Red Blood Cells’’ ( D. M. Sergenor, Ed.) , 20. Dukhin, S. S., Semenikhin, N. M., and Shapinskaya, L. M., Dokl. Phys. Chem. 193, 540 ( 1970 ) .