Patient subgroups of schizophrenia based on the Positive and Negative
Syndrome Scale: composition and transition between acute and subsided
disease states
Guan-Hua Huang
a, Hsiu-Hui Tsai
a, Hai-Gwo Hwu
b,c,d,e,⁎
, Chen-Hsin Chen
c,f,
Chen-Chung Liu
b, Mau-Sun Hua
d, Wei J. Chen
caInstitute of Statistics, National Chiao Tung University, Hsinchu, Taiwan
bDepartment of Psychiatry, National Taiwan University Hospital and College of Medicine, National Taiwan University, Taipei, Taiwan cInstitute of Epidemiology, College of Public Health, National Taiwan University, Taipei, Taiwan
dDepartment of Psychology, College of Science, National Taiwan University, Taipei, Taiwan eNeurobiology and Cognitive Science Center, National Taiwan University, Taipei, Taiwan
f
Institute of Statistical Science, Academia Sinica, Taipei, Taiwan
Abstract
The present study focuses on schizophrenia patient subgroups with specific symptom pattern using the Positive and Negative Syndrome Scale (PANSS). In this report, we intend to (1) provide a more appropriate analytic method for exploring the subgroups based on PANSS data, (2) validate identified subgroups with external variables, and (3) estimate probabilities of subgroup changes between 2 disease states. The analyzed data include 219 acute-state patients who had completed the PANSS within 1 week of index admission and 225 subsided-state patients who were living in the community and under family care. Regression extension of latent class analysis was performed. We found that acute schizophrenia can be classified into 4 subgroups—whole syndrome, whole syndrome without hostility, partial syndrome with negative symptoms, and partial syndrome with pure reality distortion—and that subsided schizophrenia can be classified into 3 subgroups—florid symptom, marked negative, and remitted. Patients of the whole syndrome, whole syndrome without hostility, partial syndrome with negative symptoms, and partial syndrome with pure reality distortion subgroups at the acute state were most likely to transit to the florid symptom (61%), florid symptom (48%), marked negative (42%), and remitted (56%) subgroups at the subsided state, respectively. Significant relationships of obtained subgroups with sociodemographic variables and neurocognitive variables were identified. These results of different subgroups will provide the background for facilitating current molecular, genetic, and neurobiological studies of schizophrenia.
© 2011 Elsevier Inc. All rights reserved.
1. Introduction
Schizophrenia is a psychotic disorder characterized by several domains of symptoms. Instruments were developed for measuring and quantifying different symptom dimen-sions, such as the Scale for the Assessment of Negative
Symptoms[1]and the Scale for the Assessment of Positive
Symptoms[2]. The Scale for the Assessment of Negative
Symptoms and Scale for the Assessment of Positive
Symptoms may be limited in their potential to identify other than positive and negative symptoms because of the prior selection of symptom dimensions. The Positive
and Negative Syndrome Scale (PANSS) [3] provides
an extensive assessment of the symptom phenomenology of schizophrenia.
Many studies have examined the structure of symptoms in schizophrenia based on the PANSS. A majority of studies have performed exploratory/confirmatory factor analysis [4-11], and there have been some studies using cluster analysis [12-14]. Cluster analysis can classify patients based on their PANSS ratings to identify the subgroups of patients, which could provide the foreground for further genetic and neurobiological studies to take the consideration of heteroge-neity of schizophrenia.
Comprehensive Psychiatry 52 (2011) 469–478
www.elsevier.com/locate/comppsych
⁎ Corresponding author. Department of Psychiatry, National Taiwan University Hospital, Taipei 10002, Taiwan. Tel.: +886 2 23123456x6785; fax: +886 2 23753663.
E-mail address:haigohwu@ntu.edu.tw(H.-G. Hwu).
0010-440X/$– see front matter © 2011 Elsevier Inc. All rights reserved. doi:10.1016/j.comppsych.2010.10.012
Positive and negative subgroups of schizophrenia were well reported in the 1980s [15-17]. In recent years, studies had found evidences in favor of more subgroups, with the
number of subgroups ranging from 3 to 5 [12,13,18].
However, results of previous subgrouping studies have been inconclusive or limited for the following reasons. First, several authors have pointed out that the symptom structure (either symptom factors or patient subgroups) in schizo-phrenia may depend on the phase of chronicity[6,7,19,20]. Most studies did not differentiate between patients in different phases of the disease, which may constitute additional source of bias. Second, few studies have explored the relation of the obtained patient subgroups with
established neuropsychological measures [14,21]. This
analysis can show the external validity of the obtained patient subgroups. Third, most studies have been limited by using symptom components measured cross-sectionally; therefore, how the patients change their subgroups between the acute phase and the subsided phase is still unknown. This is important for evaluating the effectiveness of current treatment and the progressive patterns of the disease. Fourth, studies have submitted PANSS item scales to cluster analysis to estimate patient subgroups a priori; and then estimated subgroups are treated as known and modeled as a function of external variables. However, traditional cluster analysis is suited to continuous manifest variables, whereas PANSS items are of ordinal scales. Furthermore, this 2-step approach ignores the variation of patient subgroup estimation when modeling the association between patient subgroups and external variables; as a result, the significance of the association (ie, P value) can be biased.
In the present study, we had longitudinally collected PANSS measurements and neuropsychological test variables in acute and subsided disease states. The average interval between acute and subsided assessments was about 3 years. The data provided us a unique opportunity to address issues described above. We performed regression extension of
latent class analysis (RLCA) [22], which is useful for
simultaneously classifying patients based on their responses to a set of categorical items and studying the relationship between patient subgroups and external variables. Regres-sion extenRegres-sion of latent class analysis can then examine patient subgroups underlying the PANSS, stability of the composition of patient subgroups across different disease states, transition of subgroups between disease states, and external validity of the obtained subgroups.
2. Methods 2.1. Subjects
The present study is composed of 3 projects: the Multidimensional Psychopathology Group Research Project (MPGRP), the Multidimensional Psychopathological Study on Schizophrenia (MPSS), and the Study on Etiological Factors of Schizophrenia (SEFOS). The initial project MPGRP
investigated the clinical manifestations of schizophrenia in a cohort of schizophrenia patients[14]. The subsequent project MPSS focused on the follow-up neuropsychological evalua-tion of the MPGRP patients[23]. The project SEFOS aimed to search for neurobiological, environmental, and genetic factors underlying schizophrenia.
The recruitment procedures have been detailed in earlier reports[24-26]. Briefly, from August 1, 1993, to June 30, 1998, all patients in the MPGRP were consecutively recruited from the acute inpatient wards of 3 hospitals— National Taiwan University Hospital and the university affiliated Taipei City Psychiatric Center and Taoyuan Psychiatric Center—based on the Diagnostic and Statistical
Manual of Mental Disorders, Fourth Edition,[27] criteria
for schizophrenia. The MPSS project (July 1998-June 2001) then recruited those MPGRP patients who agreed to participate in further follow-ups. The SEFOS project, carried out between January 2002 and December 2005, used the families of schizophrenia as study units. The patients of the SEFOS project were newly recruited, after signing the informed consent, and were different from that of the MPGRP and MPSS projects. There were 3 different types of families, including simplex, multiplex, and normal control families, where simplex families had only one affected offspring and multiplex families had at least 2 affected offsprings. Written informed consent was obtained from all participants after complete description of the study. These studies were approved by the institutional review boards of the participating hospitals.
All subjects at admission of the MPGRP project had received psychiatrists' clinical assessments and the PANSS measurements. After their conditions had stabilized during the index hospitalization, subjects were tested with the Continuous Performance Test (CPT)[28]. At each follow-up project (MPSS and SEFOS), besides PANSS ratings and the CPT, other neuropsychological tests were also completed,
including the Wisconsin Card Sorting Test (WCST) [29],
Wechsler Adult Intelligence Scale–Revised (WAIS-R)[30],
Wechsler Memory Scale–Revised (WMS-R)[31], and Trail
Making Tests A and B (TMT-A and -B)[32,33].
This study included 329 subjects, composed of 226 cases from the MPGRP/MPSS project and 103 cases from the SEFOSS project. There were 219 acute-state patients who had completed the PANSS within 1 week of index admission to the MPGRP project. The patients with PANSS data at the subsided state were from the MPSS and SEFOS projects, where patients were living in the community and under family care. One hundred twenty-two patients at the subsided state were assessed with the PANSS in the first year of the MPSS project. One hundred three patients of the SEFOS project had complete assessments of the PANSS at the subsided state. Therefore, we had 225 patients with PANSS data at the subsided state for analysis. There were 115 subjects who were assessed with the PANSS both in the first week of admission at the MPGRP project and in the
comparison of baseline characteristics assessed at inclusion of the MPGRP project between the follow-ups and the loss to follow-ups into the first-year study of the MPSS project. It seems that the characteristics of the dropout patients were slightly worse in negative and general psychopathological subscales than the completely followed patients.
2.2. Study variables
The PANSS were used to assess patients' symptoms. We examined the external validity of the obtained patient subgroups based on PANSS ratings through their correlations with sociodemographic variables and neuropsychological test variables. Details of these variables are described in the following.
2.2.1. PANSS
The major instrument applied in this study was the PANSS, an assessment of the clinical psychopathological symptoms of schizophrenia. It had 30 items rated on a 7-point scale (1 = absent, 7 = extreme). The PANSS consisted of 3 subscales: positive (7 symptoms: P1-P7), negative (7 symptoms: N1-N7), and general psychopathology (16 symptoms: G1-G16). The Chinese version of the PANSS, the PANSS-CH, was translated from the English version specifically for the MPGRP. The details of development of the PANSS-CH and the reliability test were published in earlier literature[34]. It consisted of the original PANSS plus 3 supplementary excitability items.
The 30 PANSS-CH items of positive, negative, and general psychopathological subscales were used as patients' symptom measurements. The supplementary excitability items were not included in this study because the proportion of subjects who responded to supplementary items was low in the subsided state and the majority of researches about the PANSS structures used the 30 items for analysis. We reduced the 7-point scale on PANSS to the binary scale (no
symptom and having symptom) for RLCA analysis. Note that no symptom was composed of scales 1 (absent) and 2 (minimal) because the patients who were assessed with the minimal scale by psychiatrists had almost no symptom in practice. This reduction in the point scale was done to avoid possible rating errors, reduce the complexity of the fitted model, and ease the interpretation of results. With dichotomized measurements, our obtained subgroups reflect distinct PANSS symptom patterns of patients. 2.2.2. Sociodemographic variables
Sociodemographic variables included sex, age at recruit-ment, onset age of psychotic symptoms, years of education, and occupation (having vs no occupation). The category of no occupation included housewives, students, and unemployed and retired people.
2.2.3. Neuropsychological variables
The neuropsychological batteries assessed reaction time, attention, speed of information processing, and active problem solving. Specifically, the test batteries included several standard neuropsychological instruments with demonstrated reliability and validity, including the CPT, WCST, WAIS-R, WMS-R, and TMT-A and -B.
Individual subtests of the neuropsychological tests were recategorized into constructs of 8 cognitive functional domains that hypothetically reflect basic cognitive processes
following the method of Kremen et al[35]. These domains
comprised (1) verbal ability: subtests of Information, Similarity and Comprehension (WAIS-R); (2) visual/spatial ability: subtests of Block Design and Picture Arrangement (WAIS-R); (3) abstraction/execution: category achieved, perseverative response (WCST) and TMT-B; (4) verbal memory: subtests of Verbal Paired Associates, immediate and delayed recall; (5) visual memory: subtests of Visual Reproduction, immediate and delayed recall (WMS-R); (6) perceptual/motor ability: the TMT-A and the subtest of Digit Symbol Substitution (WAIS-R); (7) mental control: subtests of Arithmetic and Backward Digit Span (WAIS-R); and (8) attention: sensitivity index d' (undegraded CPT) and the subtest of Forward Digit Span (WAIS-R). Performance in the 8 domains was indicative of patient's neuropsychological functioning. Scores in each category were transformed into z scores compared with a control group matched for age, sex, and education years[23]. The z scores were adjusted so that higher scores indicated better performances.
2.3. Regression extension of latent class analysis
Regression extension of latent class analysis [22] is a
statistical method useful in classifying individuals into some J subgroups based on their responses to a set of categorical items and in studying the relationship between individual's subgroup membership and independent variables (predic-tors). In our application, RLCA was applied to 30 dichotomized PANSS items. Sociodemographic variables and neuropsychological test variables were the, say, P
Table 1
Comparison of baseline characteristics between the subjects of follow-ups and loss to follow-ups in the MPGRP and the MPSS
Follow-ups Loss to follow-ups
P valuea (n = 115) (n = 104)
PANSS subscale averaged scores
Positive subscale 3.38 (1.00)b 3.58 (0.99) .14 Negative subscale 2.94 (1.11) 3.31 (1.41) .03 General psychopathology 2.48 (0.72) 2.74 (0.86) .02 Sociodemographic variables Female sex (%) 46.96 52.88 .38 Single (%) 83.48 81.73 .73 Having occupation (%) 21.74 29.13 .21
Age at recruitment (y) 31.53 (7.09) 32.53 (7.42) .31 Education (y) 11.18 (2.86) 10.97 (3.05) .60 Onset age of psychotic symptoms (y) 22.07 (5.64) 23.97 (7.10) .03
a
P values were for the difference between follow-ups and loss to follow-ups.
b
independent variables incorporated into RLCA. The para-meters of RLCA are (1) the (conditional) probability with which members of subgroup j endure symptom on PANSS item m; (2) the prevalence of subgroup j; (3) the odds ratio of belonging to subgroup j vs the (reference) subgroup J comparing across persons who differ on independent
variable p, where 1≤ j ≤ J, 1 ≤ m ≤ 30, and 1 ≤ p ≤ P.
The conditional probabilities provide information about the degree of association between each of PANSS items and patient subgroups and are analogous to factor loadings in factor analysis [36]. The conditional probabilities give the sensitivity of PANSS items for indicating a particular patient
subgroup. The subgroup prevalence reflects the distribution of patients over all subgroups. An odds ratio significantly greater than 1 indicates that patients with higher values of the pth independent variable are more likely to be in the jth subgroup than in the reference subgroup and are used for external validity.
Before fitting RLCA, the number of subgroups needs to be determined. In this study, the estimated number of subgroups was expected to fix at the number J that
minimizes the Akaike information criterion (AIC)[37]and
the Bayesian information criteria (BIC)[38].
The software for fitting RLCA can be downloaded from
the Internet: http://140.113.114.4/software.htm under the
category“RLCA.” Example programs for implementing the
software to analyze the PANSS are available from the first author.
3. Results
The percentages of having PANSS symptoms are shown inTable 2. The patients at the acute state were more likely to present PANSS symptoms than patients at the subsided state, except for the guilt feelings (G3) item. Table 3shows the sociodemographic and neuropsychological characteristics of patients at acute and subsided states.
Table 2
Proportions of having symptom on PANSS items
Symptom Acute (n = 219) Subsided (n = 225) P valuea % of having symptomb % of having symptomb P1 Delusions 93.6 52.9 b.001 P2 Conceptual disorganization 68.0 40.9 b.001 P3 Hallucinatory behavior 83.6 45.8 b.001 P4 Excitement 53.0 17.8 b.001 P5 Grandiosity 26.5 18.2 .04 P6 Suspiciousness/ persecution 79.0 34.7 b.001 P7 Hostility 51.6 14.2 b.001 N1 Blunted affect 67.1 48.0 b.001 N2 Emotional withdrawal 69.4 44.0 b.001 N3 Poor rapport 51.1 31.6 b.001 N4 Passive/apathetic social withdrawal 66.2 55.1 .02 N5 Difficulty in abstract thinking 75.8 66.7 .03 N6 Lack of spontaneity/flow of conversation 49.8 40.9 .05 N7 Stereotyped thinking 53.9 41.8 .01 G1 Somatic concern 42.5 31.6 .01 G2 Anxiety 55.3 37.8 b.001 G3 Guilt feelings 13.7 17.8 .19 G4 Tension 41.1 22.2 b.001 G5 Mannerisms and posturing 23.7 10.7 b.001 G6 Depression 40.2 23.6 b.001 G7 Motor retardation 36.5 22.7 .001 G8 Uncooperativeness 47.0 15.6 b.001
G9 Unusual thought content 77.2 44.9 b.001
G10 Disorientation 29.2 16.0 b.001 G11 Poor attention 56.2 29.3 b.001 G12 Lack of judgment and insight 96.8 71.1 b.001 G13 Disturbance of volition 50.2 33.8 b.001
G14 Poor impulse control 49.8 20.4 b.001
G15 Preoccupation 63.0 26.2 b.001
G16 Active social avoidance 52.5 30.2 b.001
a P values were for the difference between acute and subsided states.
Because some patients were evaluated in both acute and subsided states, P values were based on the generalized estimating equations approach with the exchangeable correlation structure[52]to adjust for the association between measurements from the same individual.
b
Having symptom = value of 3 to 7 in the 7-point scale.
Table 3
Characteristics of the study subjects at the acute or subsided state of schizophrenia disorder Variable Acute (n = 219) Subsided (n = 225) P valuea Female sex (%) 49.77 47.1 .49 Single (%) 82.60 87.60 .09 Having occupation (%) 25.23 25.30 .99
Age at recruitment (y) 32.00 (7.25)b 34.01 (8.05) .01 Education (y) 11.08 (2.95) 11.80 (2.94) .01 Onset age of
psychotic symptoms (y)
22.97 (6.43) 21.36 (5.90) .01 CPT
Adjusted z
score of undegraded d'
−3.44 (2.46) −1.81 (2.15) b.001 Adjusted z score of degraded d' −3.06 (1.72) −1.90 (1.83) b.001 8 Neuropsychological functional domains
Verbal ability N/Ac −0.73 (1.10)
Visual/spatial ability N/Ac −0.88 (0.91)
Abstraction/execution N/Ac −0.26 (0.62)
Verbal memory N/Ac −1.34 (2.18)
Visual memory N/Ac −1.60 (1.76)
Perceptual/motor ability N/Ac −1.93 (1.39)
Mental control N/Ac −1.24 (1.10)
Attention N/Ac −1.24 (1.42)
aP values were for the difference between acute and subsided states.
Because some patients were evaluated in both acute and subsided states, P values were based on the generalized estimating equations approach with the exchangeable correlation structure[52]to adjust for the association between measurements from the same individual.
b Mean (standard deviation).
cThese neuropsychological variables were not measured in the acute
3.1. Results for patients at the acute state 3.1.1. Composition of patient subgroups
Regression extension of latent class analysis models with the number of subgroups varying from 2 to 8 were fitted for selecting the best number of patient subgroups. When the number of subgroups increased, the RLCA model can become unstable and was difficult to converge because of the model identifiability problem[39]. The AIC and BIC values both decreased from 2 to 5 subgroups, but began to go up and down afterward. Therefore, we chose to fit the RLCA model with 5 or fewer subgroups. We further examined 4- and 5-subgroup models. The composition of the 5-subgroup model had a basic structure similar to the composition of the 4-subgroup model, with a new subgroup that was originally combined with other subgroups under the 4-subgroup model. After reviewing the interpretation and external validity of 2 models, we decided that the 4-subgroup RLCA, with AIC = 6949.4 and BIC = 6991.3, was more appropriate for modeling patients at the acute state.
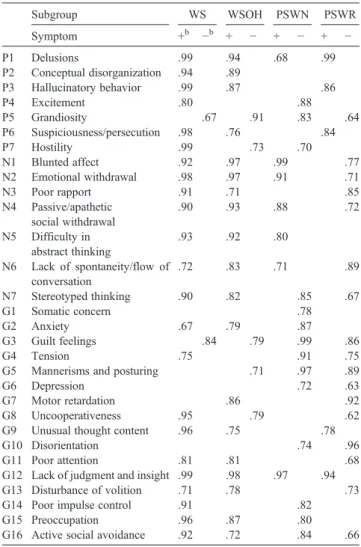
Table 4 shows conditional probabilities of having the presence of PANSS symptom items for certain subgroup in acute schizophrenia. The first subgroup had widespread whole syndrome of hostility/excitement, disorganization, and negative symptoms in addition to reality distortion (delusion and hallucination), covering most of positive, negative, and general psychopathological items of the PANSS. It was named the whole syndrome (WS) subgroup. The second subgroup was composed of widespread negative and disorganization symptoms in addition to reality distortion but without hostility, and was named the whole syndrome without hostility (WSOH). In the third subgroup, other than the delusional symptom, the main symptoms were 5 negative items (N1, N2, N4-N6) and had no hostility. It was named the subgroup of partial syndrome with negative symptoms (PSWN). The fourth subgroup could be labeled the subgroup of partial syndrome with pure reality distortion (PSWR) because it was composed of delusion and hallucination only and did not have most of the negative symptoms. The prevalence of these 4 subgroups varied, with the lowest prevalence of 16% for the PSWN subgroup, the highest prevalence of 39% for the PSWR subgroup, and the WS and WSOH having prevalences of 23% and 21%, respectively.
3.1.2. External validity
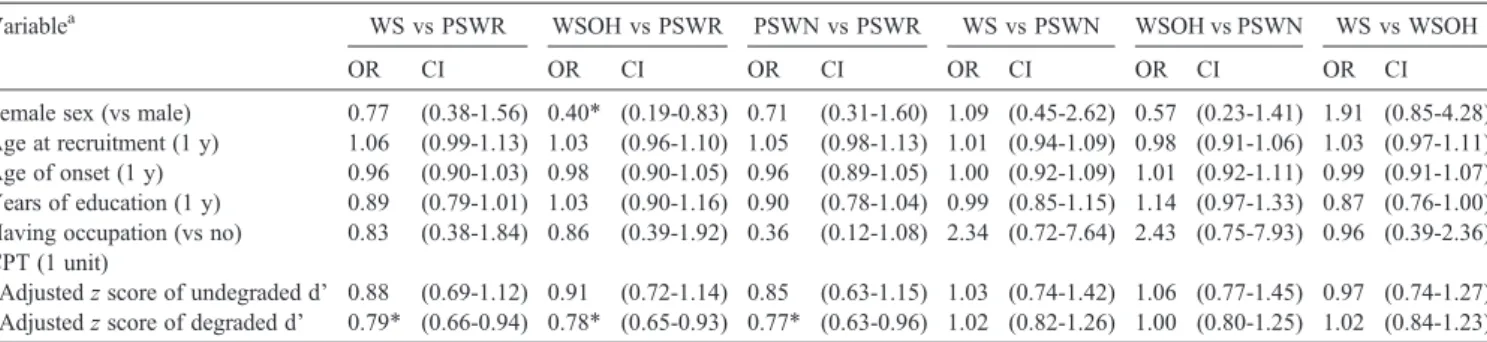
Table 5 shows the odds ratios for the relationship between subgroups of schizophrenia at the acute state and sociodemographic/neuropsychological variables. Odds ratios comparing any 2 subgroups were presented, and the CPT was the only neuropsychological test performed in the acute state.
By comparing with the patients of the PSWR subgroup
(ie, based on the first 3 odds ratios of Table 5), we can
characterize other 3 subgroups as follows. Patients of the WS subgroup were more likely to have lower z-standardized
degraded d' on the CPT (impaired sustained attention). Patients of the WSOH subgroup were more likely to be male and have lower z-standardized degraded d' on the CPT. Patients of the PSWN subgroup tended to have lower z-standardized degraded d' on the CPT. There was no difference among 3 groups of WS, WSOH, and PSWN in all external validating variables.
3.2. Results for patients at the subsided state 3.2.1. Composition of patient subgroups
In the subsided phase, the AIC and BIC values both decreased from 2 to 4 subgroups, but began to go up and down afterward. After considering the AIC and BIC criteria and the interpretation and validity of obtained subgroups, in the subsided phase, we adopted a 3-subgroup RLCA model with AIC = 6899.5 and BIC = 6932.1.
Table 4
Composition of symptom items, shown by conditional probabilities,a assessed by the PANSS in the 4-subgroup model defined by the RLCA for psychopathology at the acute state of schizophrenia disorder
Subgroup WS WSOH PSWN PSWR Symptom +b −b + − + − + − P1 Delusions .99 .94 .68 .99 P2 Conceptual disorganization .94 .89 P3 Hallucinatory behavior .99 .87 .86 P4 Excitement .80 .88 P5 Grandiosity .67 .91 .83 .64 P6 Suspiciousness/persecution .98 .76 .84 P7 Hostility .99 .73 .70 N1 Blunted affect .92 .97 .99 .77 N2 Emotional withdrawal .98 .97 .91 .71 N3 Poor rapport .91 .71 .85 N4 Passive/apathetic social withdrawal .90 .93 .88 .72 N5 Difficulty in abstract thinking .93 .92 .80 N6 Lack of spontaneity/flow of conversation .72 .83 .71 .89 N7 Stereotyped thinking .90 .82 .85 .67 G1 Somatic concern .78 G2 Anxiety .67 .79 .87 G3 Guilt feelings .84 .79 .99 .86 G4 Tension .75 .91 .75
G5 Mannerisms and posturing .71 .97 .89
G6 Depression .72 .63
G7 Motor retardation .86 .92
G8 Uncooperativeness .95 .79 .62
G9 Unusual thought content .96 .75 .78
G10 Disorientation .74 .96
G11 Poor attention .81 .81 .68
G12 Lack of judgment and insight .99 .98 .97 .94
G13 Disturbance of volition .71 .78 .73
G14 Poor impulse control .91 .82
G15 Preoccupation .96 .87 .80
G16 Active social avoidance .92 .72 .84 .66
a
The conditional probabilities were shown while they were significantly different from .5 at the .05 level.
b
“+” and “−” represent “having symptom” and “no symptom” levels, respectively.
Table 6shows the conditional probabilities of having the presence of specific PANSS symptom items for certain subgroup in subsided schizophrenia. The first subgroup was characterized by the existence of prominent reality distortion, disorganization, and negative symptoms at the subsided state. This subgroup was named the florid symptom subgroup. The second subgroup can be labeled the marked negative one because there were only significant negative symptoms. In the third subgroup, the patients had no significant symptoms identified; and it can be labeled the remitted subgroup. In addition, the remitted subgroup had the highest prevalence of 46%; and the prevalence of florid symptom and marked negative subgroups was 30% and 24%, respectively.
3.2.2. External validity
Table 7 contains the odds ratios for the relationship between subgroups of subsided schizophrenia and socio-demographic/neuropsychological variables. By comparing with the patients of the remitted subgroup (ie, based on the first 2 odds ratios ofTable 7), we can characterize the other 2 subgroups as the following. Patients of the florid symptom subgroup tended to be older, be younger at onset age of psychotic symptoms, be less educated, be prone to have no occupation, and show significantly worse performance in all neuropsychological functioning. Patients of the marked negative subgroup were more likely to be male, be less educated, have no occupation, and tend to perform significantly worse in all neuropsychological functioning except for the abstraction/execution and verbal memory ability where the difference was not significant. When comparing with the marked negative subgroup, the florid symptom subgroup tended to be older, younger at onset age of psychotic symptoms, and more educated. However, there was no difference in all neuropsychological function domains between these 2 subgroups.
3.3. Transition of subgroups between acute and subsided states There were 115 subjects who were assessed with the PANSS in both the acute and subsided disease states (the
averaged follow-up period was 1143 days with a standard
deviation of 417 days). Table 8 shows the tabular
cross-classification of these 115 subjects by their acute- and subsided-state subgroups. The P value of theχ2test for the contingency table is .01, which reveals the strong association between acute and subsided subgroups. A majority of patients (61%) belonging to the WS subgroup at the acute state maintained to be in the florid symptom subgroup at the subsided state. The WSOH patients of the acute state were more likely to be in the florid symptom subgroup at the subsided state. Fifty-six percent of the patients attributed to the PSWR subgroup at the acute state would become remitted at the subsided state. Patients of the PSWN subgroup at the acute state showed higher probability to be retained in the marked negative subgroup than to change to other subgroups at the subsided state.
4. Discussion
From a descriptive psychopathological viewpoint, this study reveals the underlying patient subgroups of schizo-phrenia based on the PANSS ratings. Using RLCA, this article presents that (1) there exist different structures of patient subgroups in the acute and subsided disease phases, (2) there are different external validity indicators related to the identified subgroups, and (3) the transition probabilities between subgroups of acute and subsided states can be fairly well predicted. These efforts will surely contribute to the progress in the delineation of complex genetic and neurobiological pathogenesis of schizophrenia.
We have demonstrated the usefulness of RLCA in studying patient subgroups. Regression extension of latent class analysis is a more appropriate approach for analyzing ordinal-scale's PANSS than traditional cluster methods (eg, Ward method), which are good only for continuous variables. However, we pay the price of fitting a much more complicated statistical model and risking the numerical instability of RLCA due to estimating a large number of parameters with 30 PANSS items simultaneously. To
Table 5
External validity of the 4-subgroup model of acute state schizophrenia defined by the RLCA
Variablea WS vs PSWR WSOH vs PSWR PSWN vs PSWR WS vs PSWN WSOH vs PSWN WS vs WSOH
OR CI OR CI OR CI OR CI OR CI OR CI
Female sex (vs male) 0.77 (0.38-1.56) 0.40⁎ (0.19-0.83) 0.71 (0.31-1.60) 1.09 (0.45-2.62) 0.57 (0.23-1.41) 1.91 (0.85-4.28) Age at recruitment (1 y) 1.06 (0.99-1.13) 1.03 (0.96-1.10) 1.05 (0.98-1.13) 1.01 (0.94-1.09) 0.98 (0.91-1.06) 1.03 (0.97-1.11) Age of onset (1 y) 0.96 (0.90-1.03) 0.98 (0.90-1.05) 0.96 (0.89-1.05) 1.00 (0.92-1.09) 1.01 (0.92-1.11) 0.99 (0.91-1.07) Years of education (1 y) 0.89 (0.79-1.01) 1.03 (0.90-1.16) 0.90 (0.78-1.04) 0.99 (0.85-1.15) 1.14 (0.97-1.33) 0.87 (0.76-1.00) Having occupation (vs no) 0.83 (0.38-1.84) 0.86 (0.39-1.92) 0.36 (0.12-1.08) 2.34 (0.72-7.64) 2.43 (0.75-7.93) 0.96 (0.39-2.36) CPT (1 unit)
Adjusted z score of undegraded d' 0.88 (0.69-1.12) 0.91 (0.72-1.14) 0.85 (0.63-1.15) 1.03 (0.74-1.42) 1.06 (0.77-1.45) 0.97 (0.74-1.27) Adjusted z score of degraded d' 0.79⁎ (0.66-0.94) 0.78⁎ (0.65-0.93) 0.77⁎ (0.63-0.96) 1.02 (0.82-1.26) 1.00 (0.80-1.25) 1.02 (0.84-1.23) OR indicates odds ratio; CI, 95% confidence interval of OR.
a Parentheses identify the unit of increase or the reference group for which the odds ratio was calculated.
overcome the numerical instability, we followed the standard practice of using multiple sets of initial values for parameter estimation. All different sets of initial values converged to similar results. We also used the statistical modeling software Mplus[40], which can fit a mixture model similar to RLCA but with different parameterization (results are not shown). Patient subgroups from Mplus were similar to the ones we report here. Mplus' conclusion about relationships between subgroups and external variables was consistent with the conclusion from RLCA but with weaker signifi-cance in estimated relations.
The PANSS item ratings used a 7-point scale. It is a good rating scale for severity measurement in assessment of clinical treatment responses. In this study, the rating was lumped into a dichotomized assignment of yes or no. Other than the strength of statistical purpose as stated above, this way of coding is of practical convenience in assessing the subgroups of schizophrenia. To define the subgroups of schizophrenia, the clinical practitioners and researchers can easily code yes or no for specific PANSS items.
To our knowledge, this is the first study demonstrating how schizophrenia patients change their subgroups between different disease states empirically. A majority of studies had proven that the factor structure of symptoms was stable over time [5,8,10,41]. These findings suggest that, although symptomatological presentation during episodes of illness
may largely overlap [10], individual patients can endure
different symptoms over time. Theoretically, this clinical phenomenon suggests the possibility of the existence of different subgroups of schizophrenia across the illness course. This study presents a 4-subgroup model at the acute state and a 3-subgroup model at the subsided state of schizophrenia disorder. At the acute state, the schizophrenia syndromes defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria allow the possibility of the
existence of the“whole syndrome” encompassing the whole
spectrum of symptomatology assessed by the operational
scale of PANSS and the“partial syndrome” with part of the
spectrum of schizophrenia psychopathology. This study performed by RLCA did find these 2 (whole and partial)
Table 6
Composition of symptom items, shown by conditional probabilities,a assessed by the PANSS in the 3-subgroup model defined by the RLCA for psychopathology at the subsided state of schizophrenia disorder
Subgroup Florid symptom Marked negative Remitted
Symptom +b −b + − + − P1 Delusions .93 .69 .62 P2 Conceptual disorganization .86 .61 .88 P3 Hallucinatory behavior .84 .67 .73 P4 Excitement .88 .96 P5 Grandiosity .62 .96 .87 P6 Suspiciousness/persecution .72 .83 .80 P7 Hostility .67 .91 .95 N1 Blunted affect .66 .92 .87 N2 Emotional withdrawal .72 .80 .93 N3 Poor rapport .65 .97 N4 Passive/apathetic social withdrawal .77 .94 .79
N5 Difficulty in abstract thinking .94 .89 .63
N6 Lack of spontaneity/ flow of conversation .76 .88 N7 Stereotyped thinking .72 .85 G1 Somatic concern .78 .78 G2 Anxiety .67 .84 .70 G3 Guilt feelings .76 .90 .82 G4 Tension .62 .81 .86
G5 Mannerisms and posturing .75 .92 .97
G6 Depression .64 .86 .80
G7 Motor retardation .67 .96
G8 Uncooperativeness .66 .82 .98
G9 Unusual thought content .88 .78 .71
G10 Disorientation .75 .74 .96
G11 Poor attention .64 .64 .96
G12 Lack of judgment and insight .97 .86
G13 Disturbance of volition .88
G14 Poor impulse control .84 .94
G15 Preoccupation .66 .83 .95
G16 Active social avoidance .69 .87
a
The conditional probabilities were shown while they were significantly different from .5 at the .05 level.
b
subsyndromes at the acute state (Table 4). The whole syndrome was divided into the whole syndrome with (WS)
and without (WSOH) the symptoms of hostility (Table 4).
The partial syndrome was further divided into the partial syndrome with prominent negative syndrome (PSWN) and the partial syndrome with prominent reality distortion (delusion and hallucination) and without negative symptoms (PSWR) (Table 4).
Using the generalized association plot analysis[42], we also found a 3-subgroup model of the remitted (RM), marked blunt (MB), and persistent delusion/hallucination (PDH) subgroups using the PANSS data assessed at 6 months after
inclusion of the study of the MPGRP project (Liu et al, unpublished data). Theoretically, the RM, MB, and PDH subgroups were corresponding to the remitted, negative symptom, and florid symptom subgroups, respectively. The generalized association plot analysis performed based on correlation structure of PANSS data emphasized the quantitative nature of the data. This RLCA approach emphasized the qualitative nature of PANSS data. The concordant finding of a 3-subgroup model with similar symptom profile did provide the validity of this 3-subgroup model at the subsided state of schizophrenia. Taking the neurobiological hypothesis of schizophrenia into conside-ration, the remitted (or RM) subgroup could be of pure
dopamine psychosis[43,44], the negative symptom (or MB)
subgroup could be of neurodevelopment psychosis[45-47],
and the florid symptom (or PDH) subgroup could be of continual neurodegeneration in the sustaining psychotic course of the illness[48]. In this study, the prevalence of the remitted, negative symptom, and florid symptom subgroups based on PANSS data at the subsided state was 46%, 24%, and 30%, respectively, for those subjects who only received PANSS assessment at the subsided state and 40%, 25%, and 35%, respectively, for those patients who were assessed at both acute and subsided states. For our other study (Liu et al, unpublished data), the prevalence of the RM, MB, and PDH subgroups was 38%, 31%, and 32%, respectively. These prevalence data were very much similar even using different statistical approaches in different sampling structures; and this provided the clinical validity of this 3-subgroup model, too. This 3-subgroup model may provide the new orientation of genetic and neurobiological studies on the pathogenesis of schizophrenia, taking the clinical and genetic heterogeneity into consideration.
In the present study, the pure positive subgroup was only identified in the acute phase. Dollfus et al [12] in their
Table 7
External validity of the 3-subgroup model of subsided state schizophrenia defined by the RLCA
Variablea Florid symptom vs Remitted Marked negative vs Remitted Florid symptom vs Marked negative
OR CI OR CI OR CI
Female sex (vs male) 0.68 (0.35-1.30) 0.44⁎ (0.22-0.90) 1.52 (0.73-3.18)
Age at recruitment (1 y) 1.06⁎ (1.01-1.12) 0.95 (0.90-1.00) 1.12⁎ (1.05-1.19)
Age of onset (1 y) 0.93⁎ (0.87-0.99) 1.04 (0.96-1.13) 0.89⁎ (0.82-0.97)
Years of education (1 y) 0.84⁎ (0.75-0.95) 0.73⁎ (0.64-0.84) 1.15⁎ (1.00-1.32)
Having occupation (vs no) 0.24⁎ (0.10-0.55) 0.41⁎ (0.17-0.95) 0.59 (0.22-1.58)
8 Neuropsychological functional domains (1 unit)
Verbal ability 0.58⁎ (0.40-0.85) 0.66⁎ (0.44-0.99) 0.88 (0.58-1.34) Visual/spatial ability 0.48⁎ (0.24-0.97) 0.36⁎ (0.18-0.71) 1.33 (0.65-2.77) Abstraction/execution 0.42⁎ (0.20-0.93) 0.46 (0.22-1.01) 0.91 (0.39-2.14) Verbal memory 0.70⁎ (0.54-0.92) 0.86 (0.68-1.09) 0.82 (0.63-1.06) Visual memory 0.62⁎ (0.48-0.79) 0.72⁎ (0.56-0.93) 0.85 (0.68-1.08) Perceptual/motor ability 0.64⁎ (0.42-0.97) 0.66⁎ (0.46-0.96) 0.96 (0.66-1.41) Mental control 0.36⁎ (0.23-0.56) 0.49⁎ (0.31-0.79) 0.73 (0.46-1.14) Attention 0.45⁎ (0.31-0.67) 0.55⁎ (0.37-0.83) 0.81 (0.58-1.38) a
Parentheses identify the unit of increase or the reference group for which the odds ratio was calculated. ⁎ P value b .05.
Table 8
Contingency table of the acute-state subgroup vs the subsided-state subgroup for 115 repeatedly measured subjects
Subgroups of the acute state
Subgroups of the subsided state Total Florid symptom Marked negative Remitted
WS 11a (61)b (27)c 4 (22) (14) 3 (17) (7) 18 (100) (15) WSOH 12 (48) (30) 5 (20) (17) 8 (32) (17) 25 (100) (22) PSWN 6 (25) (15) 10 (42) (34) 8 (33) (17) 24 (100) (21) PSWR 11 (23) (27) 10 (21) (34) 27 (56) (59) 48 (100) (42) Total 40 (35) (100) 29 (25) (100) 46 (40) (100) 115 (100) (100)
a The number of subjects in the cell. b The row percentage.
c
combined population of acute and subsided patients reported that the pure positive subgroup contained patients essentially (90.5%) in the acute phase, which is consistent with our
findings. However, Lykouras et al [13] showed that there
was no difference in the proportions of acute patients across 5 identified subgroups, which revealed that there was no association between patient subgroups and disease phases and is different from our findings of only the acute phase containing the pure positive subgroup.
The validity of patient subgroups based on PANSS data can also be supported by the correlation of the subgroups with external validating data of demographic and neuropsy-chological variables (Tables 5 and 7). Taking the subgroups assessed at the subsided state as the core subgroups of schizophrenia hypothetically, there must be more correlated external validating variables at the subsided state than that assessed at the acute state. However, the core pathology of the specific subgroup assessed at the subsided state could also be observed at the acute state by combining appropriate subgroups for comparison. This study did show that this hypothesis was true. In contrast to the remitted subgroup, the florid symptom subgroup and the negative symptom subgroup at the subsided state had prominent negative symptoms. The presence of negative symptoms might contribute to the worse neuropsychological functioning profile of these 2 subgroups (Table 7). Analogous results had been reported in some previous studies. Mahurin et al
[49] found that greater verbal memory impairment was
associated with the negative subgroup. In the literature, the negative subgroup was thought to have perceptual motor skill problems[49,50]. This was also true for the 3 subgroups of WS, WSOH, and PSWN that presented negative symptoms at the acute state of schizophrenia. The sustained attention was also worse in these 3 subgroups, comparing with the PSWR that had no negative symptoms. These findings confirm the suggestions of Liu et al[24], Hwu et al
[14], and Brazo et al [21]. Comparing with the remitted
subgroup, the presence of negative symptoms of the florid symptom and marked negative subgroups at the subsided state might also be significantly related to demographical variables of being male, being less educated, and tending to have no occupation. The florid symptom subgroup tended to be older than the remitted subgroup. At the acute state, those subgroups having negative symptoms including WS, WSOH, and PSWN also tended to have a higher chance of being male and being older at the time of the study. Based on these findings, we emphasized the core psychopathological meaning of negative symptoms assessed by PANSS at both acute and subsided states.
The subgroup of schizophrenia without negative symptoms was the PSWR at the acute state and the remitted at the subsided state. This subgroup was similar to the traditional
nomenclatural paranoid subtype [51]. This PSWR and
remitted subgroup could be due to the neurobiological pathogenesis of pure dopamine hyperactivity and no cognitive impairment. We hypothesize that this acute-PSWR and
remitted subgroup at the subsided state may have dopamine transmission problems and have no structural-cognitive pathology in the illness course of schizophrenia. This hypothesis deserves to be proven using molecular, genetic, and brain imaging approaches.
On the other hand, those cases with negative symptoms could be divided into 2 subgroups of florid symptom and marked negative symptom. Both subgroups had worse cognitive impairments in similar cognitive domain and similar degree of impairment; however, there might exist some different characteristics. The florid symptom subgroup tended to be at older age to reach clinical severity and have younger onset age of psychotic symptoms than the marked negative symptom subgroup. This marked negative symp-tom subgroup might be thought to have early neurodevelop-mental problems with widespread cognitive impairment because of its relatively delayed neurologic treatment response. In contrast, the florid symptom subgroup with widespread cognitive impairment tended to have a longer duration between disease onset and clinical severity. This clinical course fits the degenerative etiological process. Thus, both subgroups are hypothesized to have brain structural pathology but may have differential genetic etiology. This is a testable hypothesis for current nosology study of schizophrenia using genetic and brain imaging approaches; and we suggest that these subgroups of schizophrenia, for clinical and research purpose, could be considered in the future design of subclassification system of schizophrenia such as in International Classification of Diseases, 11th Revision, and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Acknowledgment
Funding for this study was provided by the National Health Research Institutes, Taiwan (DOH 83, 84, 85, 86, and 87-HR-306; DOH 88-HR-825; NHRI-GT-EX89-P825P; NHRI-EX90-8825PP); National Science Council, Taiwan (NSC 95-3112-B-001-018-Y, NSC 97-3112-B-001-020, NSC 98-3112-B-001-027, NSC 94-2118-M-009-008, NSC 95-2118-M-009-002, NSC 96-2118-M-009-001-MY2, NSC 98-2118-M-009-002-MY2); and National Taiwan University Hospital (NTUH-90S1562).
References
[1] Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983.
[2] Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984.
[3] Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. [4] Kay SR, Sevy S. Pyramidal model of schizophrenia. Schizophr Bull
1990;16:537-45.
[5] Dollfus S, Petit M. Principal-component analyses of PANSS and SANS-SNPS in schizophrenia: their stability in an acute phase. Eur Psychiatry 1995;10:97-106.
[6] White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology 1997;30:263-74.
[7] Nakaya M, Suwa H, Komahashi T, Ohmori K. Is schizophrenic symptomatology independent of the phase of the illness? Psychopa-thology 1999;32:23-9.
[8] Lancon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr Res 2000;42:231-9.
[9] Emsley R, Rabinowitz J, Torreman M. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res 2003;61:47-57.
[10] Reicherberg A, Riechmann N, Harvey PD. Stability in schizophrenia symptoms over time: finding from the Mount Sinai Pilgrim Psychiatric Center longitudinal study. J Abnorm Psychol 2005;114:363-72. [11] Van den Oord EJ, Rujescu D, Robles JR, Giegling I, Birrell C,
Bukszár J, et al. Factor structure and external validity of the PANSS revisited. Schizophr Res 2006;82:213-23.
[12] Dollfus S, Everitt B, Ribeyre JM, Assouly-Besse F, Sharp C, Petit M. Identifying subtypes of schizophrenia by cluster analyses. Schizophr Bull 1996;22:545-55.
[13] Lykouras L, Oulis P, Daskalopoulou E, Psarros K, Christodoulou GN. Clinical subtypes of schizophrenic disorders: a cluster analytic study. Psychopathology 2001;34:23-8.
[14] Hwu HG, Chen CH, Hwang TJ, Liu CM, Cheng JJ, Lin SK, et al. Symptom patterns and subgrouping of schizophrenic patients: significance of negative symptoms assessed on admission. Schizophr Res 2002;56:105-19.
[15] Potkin SG, Cannon HE, Murphy DL, Wyatt RJ. Are paranoid schizophrenics biologically different from other schizophrenics? N Engl J Med 1978;298:61-6.
[16] McGuffin P, Farmer AE, Yonace AH. HLA antigens and subtypes of schizophrenia. Psychiatry Res 1981;5:115-22.
[17] Farmer AE, McGuffin P, Spitznagel EL. Heterogeneity in schizophrenia: a cluster-analytic approach. Psychiatry Res 1983;8:1-12.
[18] Morrison RL, Bellack A, Wixted JT, Mueser KT. Positive and negative symptoms in schizophrenia: a cluster-analytic approach. J Nerv Ment Dis 1990;178:377-84.
[19] Kendler KS, Gruenberg AM, Tsuang MT. Subtype stability in schizophrenia. Am J Psychiatry 1985;142:827-32.
[20] Mohr PE, Cheng CM, Claxton K, Conley RR, Feldman JJ, Hargreaves WA, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res 2004;71:83-95.
[21] Brazo P, Marié RM, Halbecq I, Benali K, Segard L, Delamillieure P. Cognitive patterns in subtypes of schizophrenia. Eur Psychiatry 2002; 17:155-62.
[22] Huang GH, Bandeen-Roche K. Building an identifiable latent variable model with covariate effects on underlying and measured variables. Psychometrika 2004;69:5-32.
[23] Liu SK, Hsieh MH, Huang TJ, Liu CM, Liu CC, Hua MS. Patterns and clinical correlates of neuropsychologic deficits in patients with schizophrenia. J Formos Med Assoc 2006;105:978-91.
[24] Liu SK, Hwu HG, Chen WJ. Clinical symptom dimensions and deficits on the Continuous Performance Test in schizophrenia. Schizophr Res 1997;25:211-9.
[25] Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry 1998;155:1214-20.
[26] Chang CJ, Chen WJ, Liu SK, Cheng JJ, Yang WC, Chang HJ, et al. Morbidity risk of psychiatric disorders among the first degree relatives of schizophrenia patients in Taiwan. Schizophr Bull 2002;28:379-92. [27] American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington, DC: American Psychiatric Press; 1994.
[28] Rosvold H, Mirsk AF, Sarason I, Bransome ED, Bech LH. A continuous performance test of brain damage. J Consult Psychol 1956; 20:343-50.
[29] Heaton R, Chelune G, Talley J, Kay G, Curtis G. Wisconsin Card Sorting Test Manual. Odessa (Fla): Psychological Resources; 1993. [30] Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale–
Revised. New York (NY): Psychological Corporation; 1982. [31] Wechsler D. The Wechsler Memory Scale–Revised. New York (NY):
Psychological Corporation; 1987.
[32] Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General's Office; 1994. [33] Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test
Battery: therapy and clinical interpretation. Tucson (Ariz): Neuropsy-chological Press; 1985.
[34] Cheng JJ, Ho H, Chang CJ, Lane SY, Hwu HG. Positive and Negative Syndrome Scale (PANSS): establishment and reliability study of a mandarin Chinese language version. Chin Psychiatry 1996;10:251-8. [35] Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT.
Heterogeneity of schizophrenia: a study of individual neuropsycho-logical profiles. Schizophr Res 2004;71:307-21.
[36] McCutcheon AL. Latent class analysis. Sage University Papers, Series on Quantitative Applications in the social Sciences, No. 07-064. Beverly Hills (Calif): Sage Publications; 1987.
[37] Akaike H. Factor analysis and AIC. Psychometrika 1987;52:317-32. [38] Schwarz G. Estimating the dimension of a model. Ann Stat 1978;6:
461-4.
[39] Huang GH. Model identifiability. In: Everitt BS, Howell DC, editors. Encyclopedia of statistics in behavioral science. New York (NY): Wiley; 2005. p. 1249-51.
[40] Muthén LK, Muthén BO. Mplus user's guide. 4th ed. Los Angeles (Calif): Muthén & Muthén; 2006.
[41] Rey ER, Bailer J, Brauer W, Handel M, Laubenstein D, Stein A. Stability trends and longitudinal correlations of negative and positive syndromes within a three-year follow-up of initially hospitalized schizophrenics. Acta Psychiatr Scand 1994;90:405-12.
[42] Chen CH. Generalized association plots: information visualization via iteratively generated correlation matrices. Stat Sin 2002;12:7-29. [43] Garver DL, Zemlan F, Hirschowitz J, Hitzemann R, Mavroidis ML.
Dopamine and non-dopamine psychoses. Psychopharmacology (Berl) 1984;84:138-40.
[44] Garver DL, Steinberg JL, McDermott BE, Yao JK, Ramberg JE, Lewis S, et al. Etiologic heterogeneity of the psychoses: is there a dopamine psychosis? Neuropsychopharmacology 1997;16:191-201.
[45] Murray RM, O'Callaghan E, Castle DJ, Lewis SW. A neurodevelop-mental approach to the classification of schizophrenia. Schizophr Bull 1992;18:319-32.
[46] Garver DL, Nair TR, Christensen JD, Holcomb J, Ramberg JE, Kingsbury SJ. Atrophic and static (neurodevelopmental) schizophrenic psychoses: premorbid functioning, symptoms and neuroleptic response. Neuropsychopharmacology 1999;21:82-92.
[47] Garver DL, Holcomb JA, Christensen JD. Heterogeneity of response to antipsychotics from multiple disorders in the schizophrenia spectrum. J Clin Psychiatry 2000;61:964-72 quiz 973.
[48] Knoll JL, Garver DL, Ramberg JE, Kingsbury SJ, Croissant D, McDermott B. Heterogeneity of the psychoses: is there a neurode-generative psychosis? Schizophr Bull 1998;24:365-79.
[49] Mahurin RK, Velligan DI, Alexander L, Miller AL. Executive-frontal lobe cognitive dysfunction in schizophrenia: a symptom subtype analysis. Psychiatry Res 1998;79:139-49.
[50] Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT. Neuropsychological impairments in deficit vs non-deficit forms of schizophrenia. Arch Gen Psychiatry 1994;51:804-11. [51] Tsuang MT, Winokor G. Criteria for subtyping schizophrenia: clinical differentiation of hebephrenic and paranoid schizophrenia. Arch Gen Psychiatry 1974;31:43-7.
[52] Liang KY, Zeger SL. Longitudinal data and analysis using generalized linear models. Biometrika 1986;73:13-22.