Date 2015/July/12
Type of manuscript: Original article
Manuscript title: Parkinson’s disease and risk of pancreatic cancer: a
population-based case-control study in Taiwan
Running title: Parkinson’s disease and pancreatic cancer
Authors' full names:
Kuan-Fu Liao DM and MS 1,2,3, Cheng-Li Lin MS 4,5, Shih-Wei Lai MD 4,6, Wen-Chi
Chen DM and PhD 3,7
1College of Medicine, Tzu Chi University, Hualien, Taiwan
2Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung,
Taiwan
3Graduate Institute of Integrated Medicine, China Medical University, Taichung,
Taiwan
4College of Medicine, China Medical University, Taichung, Taiwan
5Management Office for Health Data, China Medical University Hospital, Taichung,
Taiwan
6Department of Family Medicine, China Medical University Hospital, Taichung,
Taiwan
7 Department of Urology, China Medical University Hospital, Taichung, Taiwan
Corresponding author: Shih-Wei Lai, Department of Family Medicine, China Medical University Hospital, No 2, Yuh-Der Road, Taichung City, 404, Taiwan
Phone: 886-4-2205-2121; Fax: 886-4-2203-3986 E-mail: wei@mail.cmuh.org.tw
ABSTRACT
Background: The aim of this study was to investigate whether there is a relationship between Parkinson's disease and pancreatic cancer in Taiwan. Methods: This was a case-control study using claim data of the Taiwan National Health Insurance Program. There were 13861 subjects aged 20-84 with newly diagnosed pancreatic cancer as cases and 55444 randomly selected subjects without pancreatic cancer as controls from 1998 to 2011, Cases and controls were matched by sex, age and index year of diagnosing pancreatic cancer. The association of pancreatic cancer with Parkinson's disease was evaluated by the multivariable logistic regression model to estimate the adjusted odds ratio (OR) and 95% confidence interval (95% CI). Results: After adjusting for confounding factors including acute pancreatitis, chronic pancreatitis, diabetes mellitus, biliary stone, alcoholism, hepatitis B and hepatitis C, the
multivariable logistic regression analysis showed the adjusted OR of pancreatic cancer was 0.82 for subjects with Parkinson's disease (95% CI 0.55, 1.21), as compared with subjects without Parkinson's disease. Conclusion: No association is detected between Parkinson's disease and pancreatic cancer.
Key words: biliary stone; diabetes mellitus; pancreatitis; pancreatic cancer; Parkinson's disease
INTRODUCTION
Epidemiological evidence suggests a lower incidence of many common cancers among patients with Parkinson's disease. Although not fully understood, a hypothesis is postulated that a hypothesized process drives cells to opposite directions. That is, in cancer, cells have uncontrolled proliferation and/or survival. In Parkinson's disease, conversely, cells have progressive degeneration and/or death. This opposite direction partially explains why inverse association exists between cancer and Parkinson's disease.
In our published and unpublished studies, after controlling for confounding factors, no significant association is detected between Parkinson’s disease and lung cancer or hepatocellular carcinoma in Taiwan. So far, pancreatic cancer is a major public concern due to its poor prognosis. It is the eighth most common cause of cancer- related deaths in Taiwan in 2011 (1607 deaths, 3.8% of the total). However, there is no conclusive evidence linking Parkinson's disease and pancreatic cancer in Taiwan. Hence, we conducted a population-based case-control study to investigate this issue by analyzing the database from the Taiwan National Health Insurance Program.
MATERIALS AND METHODS Data sources
This population-based case-control study used claim data of the Taiwan National Health Insurance Program. The insurance program also includes a catastrophic illness program to protect vulnerable beneficiaries (including pancreatic cancer patients) by exempting them from co-payments for the corresponding medical services. The insurance program has been well written in previous studies in details.
The International Classification of Disease, 9th Revision of Clinical Modification (ICD-9 code) is available in the claims data to define disease status. The index date was defined as the date of diagnosing pancreatic cancer. Subjects aged 20 or older with new diagnosis of pancreatic cancer between 1998 and 2011 (ICD-9 code 157) were selected as the case group. For each case identified, four subjects without pancreatic cancer were randomly selected as the control group. Both groups were matched by sex, age (every 5 year) and index year of diagnosing pancreatic cancer. Cases with pancreatic cancer were identified from the Registry of Catastrophic Illnesses Patient Database (RCIPD), a dataset containing health claims data for treatment of catastrophic illness, which consists of thirty categories of diseases that require long-term care. Claims data were used to identify comorbidities including Parkinson's disease (ICD-9 code 332.0), acute pancreatitis (ICD-9 code 577.0), chronic pancreatitis (ICD-9 code 577.1), diabetes mellitus (ICD-9 code 250), biliary stone (ICD-9 code 574), alcohol-related disease (ICD-9 codes 291, 303, 305.00, 305.01, 305.02, 305.03, 790.3 and V11.3), hepatitis B infection (ICD-9 codes V02.61, 070.20, 070.22, 070.30 and 070.32) and hepatitis C infection (ICD-9 codes V02.62, 070.41, 070.44, 070.51 and 070.54). The diagnosis accuracy of comorbidities based on ICD-9 codes, such as Parkinson's disease, acute pancreatitis, biliary stone, diabetes mellitus, hepatitis B infection and hepatitis C infection, has been well documented in previous studies. In order to avoid being mistakenly diagnosed or being mistakenly coded by accident, we defined that subjects should have at least 3 consensus same diagnoses during outpatient visits and/or hospitalization to ensure the validity of diagnosis. Therefore, Parkinson's disease and other comorbidities were recorded for 3 or more outpatient visits and/or hospitalization. To reduce biased results, subjects who were diagnosed with Parkinson's disease or other comorbidities only within 5 years of
diagnosing pancreatic cancer were excluded from this study. That is, only those whose pancreatic cancer was diagnosed > 5 years after Parkinson's disease diagnosis were included in the study. Subjects who had any cancer (ICD-9 codes 140-208) or secondary Parkinsonism (ICD-9 code 332.1) before the index date were excluded from the study.
Statistical analysis
Data analysis first compared cases with controls on the proportional distributions of demographic status and comorbidities using the Chi-square test and Fisher-exact test for categorical variables. Initially, all covariables were examined by the univariable unconditional logistic regression model. Only those observed to be significant in the univariable unconditional logistic regression model were further examined by the multivariable unconditional logistic regression model to estimate the adjusted odds ratio (OR) and 95% confidence interval (95% CI) for pancreatic cancer. Analyses were performed using the SAS 9.2 (SAS Institute Inc., Carey, North Carolina, USA), with P <0.05 considered as statistically significant.
RESULTS
Characteristics of the study population
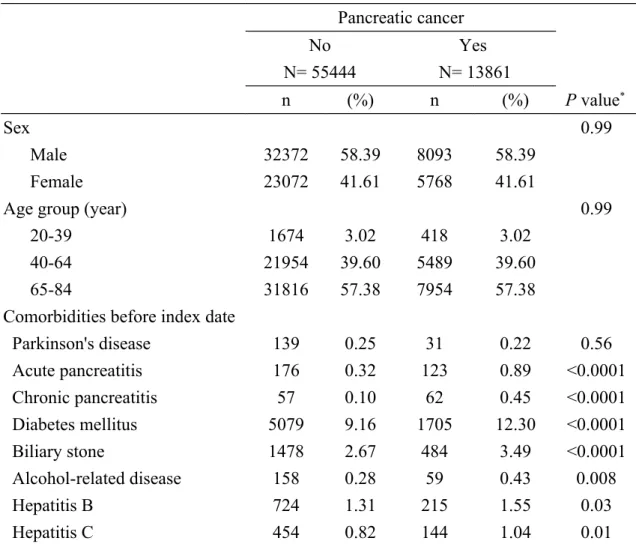
Table 1 displays the characteristics between two groups. The case group had 13861 subjects with new diagnosis of pancreatic cancer and the control group had 55444 subjects without pancreatic cancer. The mean ages (standard deviation) were 65.7(12.1) years in the pancreatic cancer group and 64.9 (12.3) years in the control group.
The pancreatic cancer group was more likely to have acute pancreatitis, chronic pancreatitis, diabetes mellitus, biliary stone, alcohol-related disease, hepatitis B and hepatitis C than the control group, with statistical significance (P < 0.05 for all). In further analysis, among 31 subjects with Parkinson's disease in the pancreatic cancer
group, 30 subjects were aged 65-84 (96.77%) and 1 subject was aged 40-64 (3.23%). Among 139 subjects with Parkinson's disease in the control group, 129 subjects were aged 65-84 (92.81%) and 10 subjects were aged 40-64 (7.19%). There was no
significant difference in the proportion of age group between the pancreatic cancer group and control group among subjects with Parkinson's disease (Fisher-exact test for P = 0.69).
Parkinson's disease and comorbidities associated with pancreatic cancer
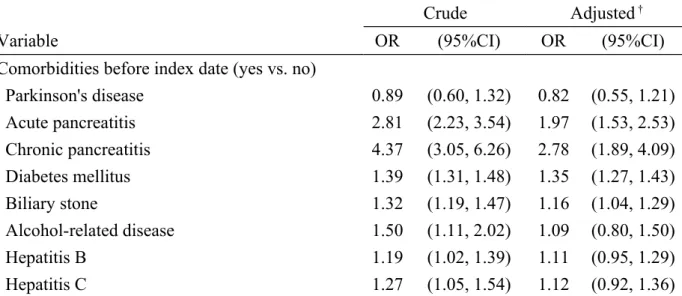
Table 2 displays the odds ratio of pancreatic cancer associated with Parkinson's disease and other comorbidities. After adjusting for potential confounding factors, the multivariable unconditional logistic regression analysis displayed that the adjusted OR of pancreatic cancer was 0.82 for subjects with Parkinson's disease (95% CI 0.55, 1.21), when compared with subjects without Parkinson's disease. Acute pancreatitis (adjusted OR1.97, 95% CI 1.53, 2.53), chronic pancreatitis (adjusted OR 2.78, 95% CI 1.89, 4.09), diabetes mellitus (adjusted OR 1.35, 95% CI 1.27, 1.43) and biliary stone (adjusted OR 1.16, 95% CI 1.04, 1.29) were other comorbidities significantly associated with pancreatic cancer.
DISCUSSION
In this population-based case-control study, we found that no association could be detected between Parkinson's disease and pancreatic cancer, which is compatible with Moller et al’s study in Denmark showing no association between Parkinson's disease and pancreatic cancer (relative risk = 0.86, 95% CI = 0.5-1.3). However, a study by Kareus et al in USA showed that an association could be detected between
Parkinson's disease and pancreatic cancer (relative risk = 0.26, 95% CI = 0.05-0.76). These conflicting findings indicate that the relationship between Parkinson's disease and pancreatic cancer remains inconclusive. It also indicates a future research direction that only after controlling for comorbidities, the relationship between
Parkinson's disease and any other cancer can be totally elucidated.
The latency from initiating mutation of the pancreatic cell to pancreatic cancer death
is relatively long. In the few years before diagnosing pancreatic cancer, the cancer
typically becomes low-level symptomatic, creating an important issue of potential reverse causality. This is an important problem for examining that histories of
Parkinson's disease and other comorbidities should exist before diagnosing pancreatic cancer. In fact, nobody knows what cut-point is suitable, but any shown relationship would be more convincing if comorbidities were present for a much longer period before diagnosing pancreatic cancer. Therefore, in order to reduce the above-mentioned biased results, patients who were diagnosed with Parkinson's disease or other comorbidities only within 5 years of diagnosing pancreatic cancer were
excluded from this study. That is, only those whose pancreatic cancer was diagnosed > 5 years after Parkinson's disease diagnosis were included in the study.
This present study has some limitations inherent to the database. First, body mass index and smoking status were not recorded in this database. Some risk factors of pancreatic cancer, such as obesity and cigarette smoking, cannot be included for analysis. Second, before analysis, nobody knows whether patients with pancreatic cancer have higher prevalence rate of Parkinson's disease. In this present study, there were 31 patients with Parkinson's disease in the pancreatic cancer group (0.22%) and 139 patients with Parkinson's disease in the control group (0.25%), without a
statistical significance. The prevalence of Parkinson's disease is lower in this study than that in our previous study (2.8%). The low prevalence could be due to the rigorous inclusion criteria studied. That is, only those whose pancreatic cancer was diagnosed > 5 years after Parkinson's disease diagnosis were included in the study. The statistical power in the multivariable analysis may be questionable. Therefore,
further studies with more Parkinson's disease patients are needed to clarify this issue. Third, the diagnosis of comorbidities might be under- or over-estimated according to outpatient visits due to the limitation of this database. In further analysis, we included all patients with Parkinson's disease to compare comorbidities studied between the pancreatic cancer group and non-pancreatic cancer group. There was no significant difference in comorbidities between the pancreatic cancer group and non-pancreatic cancer group (Table not shown). This means that the result is not confounded by comorbidities studied. Fourth, although this is a negative study for investigating the association between Parkinson's disease and pancreatic cancer, it is a clinically relevant topic with some scientific novelty. It provides the updated evidence for this country.
We conclude that no association is detected between Parkinson's disease and pancreatic cancer. Acute pancreatitis, chronic pancreatitis, diabetes mellitus and biliary stone are significantly associated with pancreatic cancer.
Acknowledgement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical
Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation in Taichung in Taiwan, Taiwan Brain Disease Foundation in Taipei in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Specific author contributions
Kuan-Fu Liao and Shih-Wei Lai substantially contributed to the conception of this article. They planned and conducted this study. They initiated the draft of the article and critically revised the article.
Cheng-Li Lin and Wen-Chi Chen conducted the data analysis and critically revised the article.
Conflict of Interest Statement
Table 1. Characteristics of pancreatic cancer cases and control subjects Pancreatic cancer No N= 55444 Yes N= 13861 n (%) n (%) P value* Sex 0.99 Male 32372 58.39 8093 58.39 Female 23072 41.61 5768 41.61
Age group (year) 0.99
20-39 1674 3.02 418 3.02
40-64 21954 39.60 5489 39.60
65-84 31816 57.38 7954 57.38
Comorbidities before index date
Parkinson's disease 139 0.25 31 0.22 0.56 Acute pancreatitis 176 0.32 123 0.89 <0.0001 Chronic pancreatitis 57 0.10 62 0.45 <0.0001 Diabetes mellitus 5079 9.16 1705 12.30 <0.0001 Biliary stone 1478 2.67 484 3.49 <0.0001 Alcohol-related disease 158 0.28 59 0.43 0.008 Hepatitis B 724 1.31 215 1.55 0.03 Hepatitis C 454 0.82 144 1.04 0.01
Data are presented as the number of subjects in each group, with percentages given in parentheses.
Table 2. Odds ratio and 95% confidence interval of pancreatic cancer associated with Parkinson's disease and other comorbidities
Crude Adjusted †
Variable OR (95%CI) OR (95%CI)
Comorbidities before index date (yes vs. no)
Parkinson's disease 0.89 (0.60, 1.32) 0.82 (0.55, 1.21) Acute pancreatitis 2.81 (2.23, 3.54) 1.97 (1.53, 2.53) Chronic pancreatitis 4.37 (3.05, 6.26) 2.78 (1.89, 4.09) Diabetes mellitus 1.39 (1.31, 1.48) 1.35 (1.27, 1.43) Biliary stone 1.32 (1.19, 1.47) 1.16 (1.04, 1.29) Alcohol-related disease 1.50 (1.11, 2.02) 1.09 (0.80, 1.50) Hepatitis B 1.19 (1.02, 1.39) 1.11 (0.95, 1.29) Hepatitis C 1.27 (1.05, 1.54) 1.12 (0.92, 1.36)
†Covariables which were significantly associated with pancreatic cancer in the univariable
unconditional logistic regression model were further examined by the multivariable unconditional logistic regression model.
Additionally adjusted for acute pancreatitis, chronic pancreatitis, diabetes mellitus, biliary stone, alcohol-related disease, hepatitis B and hepatitis C