Correlation between the Urine Profile of
4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone Metabolites
and
N
7
-Methylguanine in Urothelial Carcinoma Patients
Hui-Ling Lee,1Yu-Mei Hsueh,2Chi-Jung Chung,2Yeong-Shiau Pu,3Louis W. Chang,1
Dennis Paul Hsientang Hsieh,1Saou-Hsing Liou,1and Pinpin Lin1
1Division of Environmental Health and Occupational Medicine, National Health Research Institutes, Miaoli County,
Taiwan, People’s Republic of China and2Department of Public Health, School of Medicine, Taipei Medical University; 3Department of Urology, National Taiwan University Hospital, Taipei, Taiwan, People’s Republic of China
Abstract
A major carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), is present in cigarette smoke and its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), is used as an exposure biomarker for environmental tobacco smoke (ETS). This metabolite (NNAL) can be either detoxified into glucuronidated NNAL (NNAL-Gluc) or activated into an unstable reactive metabolite that methylates DNA along with formation of 4-hydroxy-4-(3-pyridyl)-butyr-ic acid [hydroxy acid (HA)]. Therefore, the carcinogen4-hydroxy-4-(3-pyridyl)-butyr-ic risk associated with ETS exposure is greatly modulated by individual variations in metabolic activation and detoxification capabilities. In this study, we defined the urinary HA/total NNAL [HA/total NNAL] ratio as the activation index and NNAL-Gluc/free NNAL [(total NNAL-free NNAL)/free NNAL] ratio as the
detoxifica-tion index of NNK. The major methylated DNA adduct
N7-methylguanine (N7-MeG), considered as the
carci-nogenic biomarker for cigarette smoking, was excreted in urine. The objective of this study was to investigate the effects of these metabolic indexes of NNK on N7-MeG urinary excretion in a population of urothelial carcinoma patients. Urinary levels of total NNAL (free NNAL plus NNAL-Gluc), free NNAL, HA, and
N7-MeG were positively correlated with smoking.
Furthermore, activation index and detoxification index
correlated positively and negatively with N7-MeG
levels, respectively. Our results suggest that these metabolic indices may represent the phenotype of individual metabolism capability and modulate the
carcinogenic risk of ETS exposure. (Cancer Epidemiol
Biomarkers Prev 2008;17(12):3390 – 5)
Introduction
Cigarette smoking is a well-established risk factor for the cancers of the lung, oral cavity, pharynx, and urinary bladder (1). Exposure to environmental tobacco smoke (ETS) is also considered as hazardous for nonsmokers (2). Recently, an epidemiologic study showed that cigarette smoking interacted with other environmental factors and enhanced carcinogenic risks (3). Thus, it is of importance to establish biomarkers to facilitate exposure and cancer risk assessments related to tobacco smoking. Appropriate biomarkers will also allow a better under-standing of the interaction mechanisms between envi-ronmental factors and help in the development of preventive strategies for reducing cancer risks in humans.
Tobacco smoke is a complex mixture of f4,500
chemicals. Nicotine is the most abundant component (4) and its urinary metabolite, cotinine, is readily detectable; thus, it is used as a exposure biomarker related to tobacco smoking (5-8). However, neither nicotine nor cotinine is a
useful cancer-related biomarker for tobacco smoking because their relationship with carcinogenesis is obscure and several foods also contain small amounts of nicotine (9, 10). In this study, we set out to seek more specific cancer-related biomarkers as part of our effort to understand relationship between carcinogenesis and tobacco smoking.
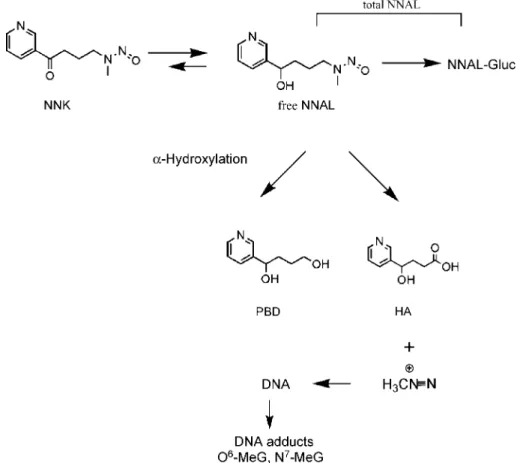
A tobacco-specific nitrosamine, 4-(methylnitrosa-mino)-1-(3-pyridyl)-1-butanone (NNK), is formed by nitrosation of nicotine or of its related minor alkaloid, pseudo-oxynicotine, found only in tobacco products (11). NNK induces lung adenoma in animals (12) and is classified as a human carcinogen (13). Metabolic activa-tion of NNK is required to turn it into carcinogenic metabolites. The general scheme of NNK metabolism is outlined in Fig. 1. NNK is rapidly metabolized to its carbonyl reduction product, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). The ultimate (or reactive) metabolites converted from NNAL methylate DNA and
form DNA adducts, such as N7-methylguanine (N7
-MeG) andO6-methylguanine. Alternatively, NNAL can
be detoxified by forming glucuronidated NNAL (NNAL-Gluc), which is readily excreted in the urine.N7-MeG is by far the most abundant DNA adduct generated from metabolically activated NNK (14).
Urinary N7-MeG has been used as an exposure
biomarker related to tobacco smoking, as it is readily removed from methylated DNA by a DNA repair system Received 8/18/08; revised 9/10/08; accepted 9/29/08.
Grant support:Division of Environmental Health and Occupation Medicine, National Health Research Institutes Taiwan, People’s Republic of China grant EO-097-PP-02. Requests for reprints:Pinpin Lin, Division of Environmental Health and Occupational Medicine, National Health Research Institutes, 35 Keyan Road, Zhunan Town, Miaoli County, Taiwan 350, People’s Republic of China. Phone: 886-37-246166; Fax: 886-37-587406. E-mail: pplin@nhri.org.tw
CopyrightD 2008 American Association for Cancer Research. doi:10.1158/1055-9965.EPI-08-0761
and excreted into the urine (15). Indeed, urinary
excretion of N7-MeG was higher among smokers than
nonsmokers (16). However, N7-MeG is not specific for
tobacco smoking exposure and is more likely to be a biomarker for cancer development after exposure. In comparison, urinary NNAL and NNAL-Gluc are much better biomarkers of NNK uptake in smokers and in people exposed to ETS (17). NNK is found only in tobacco products and seldom in the diet or the general environment unless contaminated by tobacco smoke. Furthermore, because metabolic activation of NNK is critical for its carcinogenic potential, different profiles of NNK metabolites may reflect a variation of cancer risks in different individuals. Therefore, it is of interest to
elucidate the relationship between urinaryN7-MeG and
the profile of NNK metabolites in humans.
Our hypothesis is that the capability of individuals to metabolize NNK will modulate individual cancer risk on exposure to ETS.N7-MeG is considered as a biomarker of tobacco smoking-associated cancer risk. Previously, we reported that cigarette smoking increased the risk of
urothelial carcinoma (18). It has been shown that N7
-MeG levels were high in bladder cancer tissue and modulated by genotypes of a metabolic enzyme, gluta-thioneS-transferase (19). In this study, we examined the association between urinary free NNAL and
NNAL-Gluc, N7-MeG, and the profile of NNK metabolites in
urothelial carcinoma patients. The ultimate goal is to
identify biomarkers that represent the phenotypes of metabolic capacity for NNK and related carcinogens.
Materials and Methods
Study Population.The study population consisted of
126 urothelial carcinoma patients including 15 current smokers, 42 ever smokers, and 69 never smokers from March 2004 to July 2007. All cases were diagnosed as urothelial carcinoma patients with pathologic confirma-tion. All urothelial carcinoma cases had not yet received any treatment before urine collection. The majority of study population lived in Taipei City and were recruited from the National Taiwan University Hospital.
Questionnaire Interview and Participant Specimen
Collection.Standardized personal interviews based on
structured questionnaire were carried out by well-trained personnel. Information collected included de-mographic and socioeconomic characteristics, general potential risk factors for malignancies such as lifestyle, alcohol consumption, cigarette smoking in quantified details, occupational history, and personal and family histories of disease. All patients provided informed consent before questionnaire interview and urine sample collection. Urine samples were stored at 20jC until further use for NNK and DNA adduct level analysis.
Figure 1. Overview of NNK metabolism showing structures of most urinary metabolites (for more details, see ref. 24). PBD, 1-(3-pyridyl)-1,4-butanediol;O6-MeG, O6-methylguanine.
Analysis of NNK Metabolites.Urinary NNK metab-olite concentrations were determined using the liquid chromatography-tandem mass spectrometry method (20, 21).
Analysis of UrinaryN7-MeG Level.A 50AL aliquot of
urine was diluted with 85% acetonitrile (450 AL)
containing 0.1% formic acid followed by the addition of
3 ng/mL [2H3]-N7-MeG as an internal standard for
quantitation of N7-MeG by liquid
chromatography-tandem mass spectrometry method (21, 22). The [2H3
]-N7-MeG was synthesized based on published references
with minor modifications at purity of >98% (22).
Statistical Analysis. Concentration of urinary
meta-bolites was expressed as milligram creatinine to correct for variation in urine flow. Total NNAL concentration (ng/mg creatinine) was the sum of urinary free NNAL and NNAL-Gluc. The detoxification capability index was defined as the ratio between NNAL-Gluc and free NNAL levels. Activation index was defined as the ratio between 4-hydroxy-4-(3-pyridyl)-butyric acid [hydroxy acid (HA)] and free NNAL. All significant analyses of difference between NNK metabolites and DNA adducts were based on logarithmic transformed values. Pearson’s correlation was used to assess the relationship between urinary NNK metabolites and DNA adducts levels. Simultaneously, we developed a simple linear regression to estimate the joint effects of various indices and DNA adducts on urothelial carcinoma risk adjusted for total NNAL. All data were analyzed using the SAS statistical package.P < 0.05 was considered significant.
Results
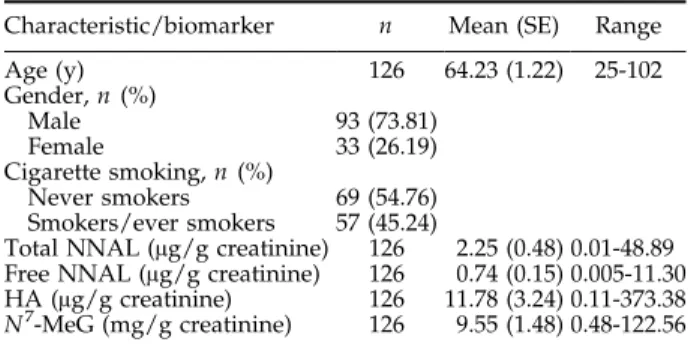
A total of 126 subjects [93 (73.81%) males and 33 (26.19%)
females] with a mean age of 64.23 F 1.22 years were
recruited for the study. Their demographic character-istics, smoking status, and biomarkers data are summa-rized in Table 1. NNK metabolites, including free NNAL,
total NNAL, HA, andN7-MeG, were detectable in urine
(Table 1). Among NNK metabolites, the HA levels were much higher than free NNAL and total NNAL. The relationship between biomarkers (NNK metabolites and
N7-MeG) and subject characteristics are presented in
Table 2. Neither gender or age affected the levels of these biomarkers. We combined current smokers and ever smokers into one group. These biomarkers were not significantly different between smokers/ever smokers and never smokers.
Total NNAL, sum of free NNAL and NNAL-Gluc, is an accepted biomarker for NNK uptake (23). When free NNAL is not conjugated, it is metabolically activated
to HA accompanied with N7-MeG formation.
NNAL-Gluc and HA represented the ultimate metabolites after detoxification and activation of NNK, respectively.
N7-MeG is an effective product of NNK activation.
The urinary concentration of total NNAL were well correlated with those of free NNAL (P < 0.0001), HA (P < 0.0001), and N7-MeG (P < 0.0039; Table 3). These results suggest that free NNAL, total NNAL, HA, and
N7-MeG are also good urinary biomarkers for ETS
exposure in humans.
N7-MeG is both an exposure biomarker and an
effective biomarker. The formation of N7-MeG shall be
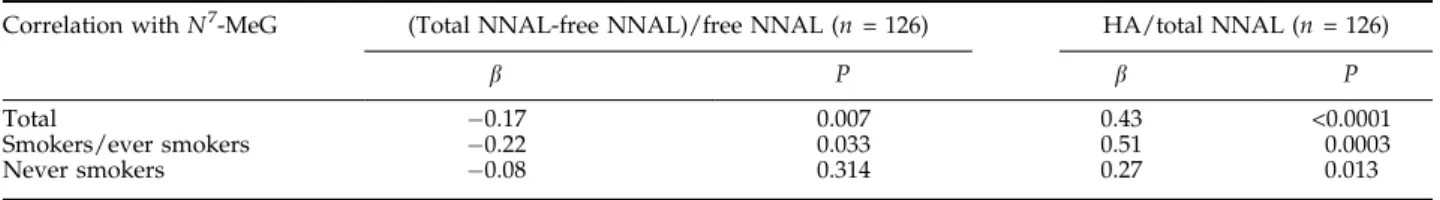
modulated by metabolic activation and detoxification capabilities. The NNAL-Gluc/free NNAL ratio [(total NNAL-free NNAL)/free NNAL] and the HA/total NNAL ratios were defined as the detoxification index and activation index, respectively, and their relationship with urinaryN7-MeG levels was investigated (Table 4). N7-MeG was negatively (b = 0.17; P = 0.007) correlated with the detoxification index [(total NNAL-free NNAL)/ free NNAL] but positively (b = 0.43; P < 0.0001) correlated with the activation index (HA/total NNAL). After stratification with smoking status, the correlation
betweenN7-MeG and the detoxification index remained
significant in smokers but not in never smokers. On the
other hand, N7
-MeG correlated with activation index among both smokers and never smokers. These results Table 1. Characteristics of the studied participants
and biomarker levels in urine
Characteristic/biomarker n Mean (SE) Range
Age (y) 126 64.23 (1.22) 25-102 Gender,n (%) Male 93 (73.81) Female 33 (26.19) Cigarette smoking,n (%) Never smokers 69 (54.76) Smokers/ever smokers 57 (45.24)
Total NNAL (Ag/g creatinine) 126 2.25 (0.48) 0.01-48.89 Free NNAL (Ag/g creatinine) 126 0.74 (0.15) 0.005-11.30
HA (Ag/g creatinine) 126 11.78 (3.24) 0.11-373.38
N7-MeG (mg/g creatinine) 126 9.55 (1.48) 0.48-122.56
Table 2. Relationship between NNK metabolites,N7-MeG, and characteristics
Total NNAL
(Ag/g creatinine) (Ag/g creatinine)Free NNAL (Ag/g creatinine)HA N
7-MeG (mg/g creatinine) Stratification by gender Male 2.13F 0.59 0.84F 0.20 13.24F 4.37 10.13F 1.96 Female 2.58F 0.77 0.48F 0.10 7.66F 1.22 7.94F 1.26 P 0.68 0.12 0.22 0.35
Stratification by age (y)
<58 2.04F 0.59 0.78F 0.27 7.16F 1.54 10.00F 2.69 58-75 2.97F 1.22 0.81F 0.30 12.85F 3.74 10.25F 3.00 z75 1.65F 0.33 0.62F 0.19 16.52F 10.28 8.15F 1.42 P 0.53 0.88 0.50 0.83 Smoking status Never smokers 1.49F 0.28 0.48F 0.10 7.37F 0.99 7.57F 0.76 Smokers/ever smokers 3.16F 0.99 1.06F 0.31 17.10F 7.03 11.96F 3.13 P 0.11 0.08 0.18 0.18
suggest that individual variation in detoxification and activation capabilities might influence their carcinogenic effect (DNA methylation) of NNK in humans.
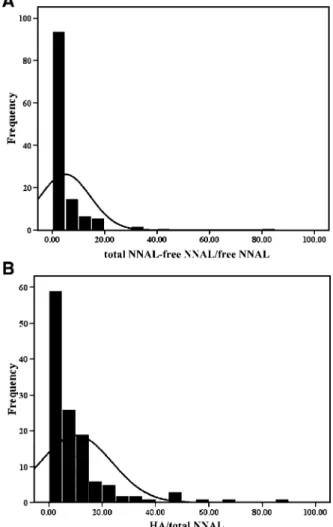
The frequency distribution of the detoxification index
[(total NNAL-free NNAL)/free NNAL] was 5.0 F 9.55
(n = 126; Fig. 2A). The detoxification index was >0.36 in f10% of this population. The frequency distribution of
the activation index [HA/free NNAL] was 10.2F 13.42
(n = 126) and was >1.5 in f10% of this population (Fig. 2B).
Discussion
Cigarette smoking and exposure to ETS are important risk factors for many cancers (17). NNK is one of the major carcinogens in cigarette smoke (11, 13). However, carcinogenic effects of NNK are greatly modulated by its metabolic activation and detoxification. In humans, NNK from cigarette smoke is completely converted into metabolites after activation or detoxification, including
free NNAL, NNAL-Gluc, and HA.N7-MeG is one of the
DNA adducts induced by NNK metabolites (12, 24). It is well known that CYP2A catalyzes the conversion of NNAL to HA (24) and UDPglucuronyl transferase catalyzes the conversion of free NNAL to NNAL-Gluc (total NNAL-free NNAL; ref. 25). Thus, the [HA/total NNAL] and [(total NNAL-free NNAL)/free NNAL] indexes may, respectively, represent individual varia-tions in CYP2A and UDP glucuronyl transferase activ-ities. In the present study, we showed that both NNK activation [HA/total NNAL] and detoxification [(total NNAL-free NNAL)/free NNAL] indices correlated with
N7-MeG levels in a human. It appears that N7-MeG
levels are modulated by CYP2A and UDP glucuronyl transferase activities. Thus, these indices could be used to investigate the role of individual variations in cigarette smoking-associated cancers in the future.
N7-MeG is a major DNA adduct (70-90%) produced by
methylation in biological systems (26), but it has little
effect on DNA duplex structure (27). However,N7-MeG
is readily excised spontaneously or by methylpurine
DNAN-glycosylase, converting it into mutagenic abasic
sites (28). Because N7-MeG is also formed by other
endogenous methylation reactions, it is plausible that the
N7-MeG levels were f100 times the levels of NNK
metabolites in the urine. However, we still observed that the levels ofN7-MeG were well correlated with the levels
of NNK metabolites and metabolism indices. Thus,
N7-MeG might be associated with the carcinogenic risk
of ETS exposure. Recently, urinary N7-MeG excretion
was shown to be associated with lung cancer risk in smokers and subjects with null genotype of glutathione
Figure 2. A, frequency distribution of total NNAL-free NNAL/ free NNAL in human urine. B, frequency distribution of HA/ total NNAL in human urine.
Table 3. Correlation among biomarkers analyzed in this study Total NNAL
(Ag/g creatinine) (Ag/g creatinine)Free NNAL (Ag/g creatinine)HA N 7
-MeG (mg/g creatinine) Total NNAL
Pearson correlation coefficients 1.00
P Free NNAL
Pearson correlation coefficients 0.83 1.00
P <0.0001
HA
Pearson correlation coefficients 0.70 0.68 1.00
P <0.0001 <0.0001
N7-MeG
Pearson correlation coefficients 0.26 0.38 0.47 1.00
S-transferase M1 (29). The association between N7-MeG and cancer risk may be modulated by individual variations in metabolism capability. In the future, we
shall combine the metabolic indices of NNK andN7-MeG
for assessing cancer risks.
The metabolic indices of activation [HA/total NNAL] and detoxification [(total NNAL-free NNAL)/free NNAL] were highly variable in this population. Theo-retically, individuals with either high [HA/total NNAL] or low [(total NNAL-free NNAL)/free NNAL] are at higher risk of cigarette smoking-associated cancers. Indeed, [HA/total NNAL] was positively correlated but [(total NNAL-free NNAL)/free NNAL] negatively
correlated with N7-MeG in this population. We also
noticed that majority of individuals were low in both [HA/total NNAL] and [(total NNAL-free NNAL)/free NNAL]. In other words, majority of individuals carry a low risk of [HA/total NNAL] index and high risk of [(total NNAL-free NNAL)/free NNAL] index. Because cancer incidence is relatively low in general population, majority of individuals are at low cancer risk. Therefore, the [HA/total NNAL], but not the [(total NNAL-free NNAL)/free NNAL], distribution was consistent with individual susceptibility to cigarette smoke-associated cancers. As mentioned in the previous paragraph, CYP2A converts NNAL to HA. Hepatic CYP2A6 and pulmonary CYP2A13 were reported to be involved in metabolic activation of NNK (24). Genetic polymorphism of CYP2A6 was not associated with cancer risks (30-32). Recently, we showed that arsenic increased hepatic CYP2A expression and activity, subsequently enhancing
urinary HA, keto acid, and N7-MeG levels in
NNK-treated mice (21). We believe that some environmental factors or foods could also modulate metabolic activation or detoxification of NNK. By measuring the metabolic indices and corresponding genetic polymorphisms, we should be able to study the interaction between metab-olism capability, environmental factors, and cigarette smoking on cancer risks in future.
Whereas the activation index was associated with
N7-MeG in both smokers/ever smokers and never
smokers, the detoxification index was associated with
N7-MeG only in smokers/ever smokers but not in never
smokers. The activation index (CYP2A activity) is
relatively specific to NNK-associated N7-MeG
produc-tion. On the other hand, glucuronyl conjugation widely involves in metabolism of exogenous and endogenous chemicals. The detoxification activity (UDPglucuronyl transferase) may differently modulate other endogenous methylation reactions and detoxification of NNK. There-fore, the detoxification index was not associated with
N7-MeG in never smokers. It further implied that the
activation index might modulate the cigarette smoke-associated cancer risk in both active and passive smokers.
HA is the most abundant NNK metabolite detected in
urine and it is also produced during
N¶-nitrosonornico-tine and nicoN¶-nitrosonornico-tine metabolism (33-35). However, the
formation of HA fromN¶-nitrosonornicotine and nicotine
is not accompanied withN7-MeG generation. It has been shown that 12% of urinary HA is generated from NNK,
31% fromN¶-nitrosonornicotine, 1% from keto acid, and
only 0.1% from nicotine in rats (33). More recently, Stepanov et al. (36) showed that the levels of NNK-derived HA were much higher than the levels of total NNAL in smokers. It may explain why the HA level was higher than total NNAL and free NNAL levels in the present study. Furthermore, urinary HA levels correlated
well with urinary total NNAL and N7-MeG levels,
suggesting that NNAL has significant contribution to HA formation in humans. Keto acids were also detect-able, as low as 25% of HA, in urine of smokers (37). However, we failed to detect keto acids in our specimens perhaps due to lower sensitivity of our analytic con-ditions. The total NNAL and free NNAL levels measured in this study were similar to those reported by others (38, 39). Total NNAL has been used as an exposure biomarker for ETS. In our population, total NNAL and HA were detectable in all cases regardless of smoking status. NNAL and NNAL-Gluc was detectable 281 days after smoking cessation (35). In addition, it appears that never smokers were exposed to ETS via passive smoking. We believe that NNK metabolites could serve as better biomarkers for studying the carcinogenic potential of ETS in humans in future.
Exposure to chemical carcinogens, such as DNA alkylating agent (40) and cigarette smoke, has been associated with urothelial carcinoma risk. A variety of
DNA adducts, including N7
-methyldeoxyguanosine, were detected in the urothelial carcinoma tumor tissues. In our study, urinary NNK metabolites, metabolism
index, and N7-MeG were well correlated in urothelial
carcinoma patients. Previously, we observed an interac-tion between cigarette smoking and arsenic methylainterac-tion profile in the risk of urothelial carcinoma (18). Therefore, these biomarkers could be used to investigate the mechanism of cigarette smoking-increased risk of uro-thelial carcinoma in future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Table 4. Correlation between metabolic indices andN7-MeG
Correlation withN7-MeG (Total NNAL-free NNAL)/free NNAL (n = 126) HA/total NNAL (n = 126)
b P b P
Total 0.17 0.007 0.43 <0.0001
Smokers/ever smokers 0.22 0.033 0.51 0.0003
Never smokers 0.08 0.314 0.27 0.013
References
1. O’Neill IK, Fishbein L. An IARC manual series aimed at assisting cancer epidemiology and prevention. Environmental carcinogens: selected methods of analysis. Int J Environ Anal Chem 1986;26: 229 – 40.
2. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 2004;45 Suppl 2:S3 – 9.
3. Wen CP, Tsai SP, Cheng TY, et al. Uncovering the relation between betel quid chewing and cigarette smoking in Taiwan. Tob Control 2005;14 Suppl 1:i16 – 22.
4. Hukkanen J, Jacob PIII, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57:79 – 115.
5. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 1996;18:188 – 204.
6. de Leon J, Diaz FJ, Rogers T, et al. Total cotinine in plasma: a stable biomarker for exposure to tobacco smoke. J Clin Psychopharmacol 2002;22:496 – 501.
7. Al-Delaimy WK, Crane J, Woodward A. Passive smoking in children: effect of avoidance strategies, at home as measured by hair nicotine levels. Arch Environ Health 2001;56:117 – 22.
8. Jarvis MJ, Primatesta P, Erens B, Feyerabend C, Bryant A. Measuring nicotine intake in population surveys: comparability of saliva cotinine and plasma cotinine estimates. Nicotine Tob Res 2003;5: 349 – 55.
9. Idle JR. Titrating exposure to tobacco smoke using cotinine—a minefield of misunderstandings. J Clin Epidemiol 1990;43:313 – 7. 10. Domino EF, Hornbach E, Demana T. The nicotine content of common
vegetables. N Engl J Med 1993;329:437.
11. Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 1988;9:875 – 84.
12. Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specificN-nitrosamines. Chem Res Toxicol 1998;11:559 – 603. 13. Smokeless tobacco and some tobacco-specificN-nitrosamines. IARC
Monogr Eval Carcinog Risks Hum 2007;89:1 – 592.
14. Lewis SJ, Cherry NM, Niven RM, Barber PV, Povey AC. Associations between smoking, GST genotypes andN7
-methylguanine levels in DNA extracted from bronchial lavage cells. Mutat Res 2004;559:11 – 8. 15. Stillwell WG, Xu HX, Adkins JA, Wishnok JS, Tannenbaum SR. Analysis of methylated and oxidized purines in urine by capillary gas chromatography-mass spectrometry. Chem Res Toxicol 1989;2: 94 – 9.
16. Shuker DE, Farmer PB. Relevance of urinary DNA adducts as markers of carcinogen exposure. Chem Res Toxicol 1992;5:450 – 60. 17. Hecht SS. Human urinary carcinogen metabolites: biomarkers for
investigating tobacco and cancer. Carcinogenesis 2002;23:907 – 22. 18. Pu YS, Yang SM, Huang YK, et al. Urinary arsenic profile affects the
risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol 2007;218:99 – 106.
19. Saad AA, O’Connor PJ, Mostafa MH, et al. Bladder tumor contains higher N7-methylguanine levels in DNA than adjacent normal
bladder epithelium. Cancer Epidemiol Biomarkers Prev 2006;15: 740 – 3.
20. Byrd GD, Ogden MW. Liquid chromatographic/tandem mass spectrometric method for the determination of the tobacco-specific nitrosamine metabolite NNAL in smokers’ urine. J Mass Spectrom 2003;38:98 – 107.
21. Lee HL, Chang LW, Wu JP, et al. Enhancements of 4-(methylni-trosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism and carci-nogenic risk via NNK/arsenic interaction. Toxicol Appl Pharmacol 2008;227:108 – 14.
22. Zhang F, Bartels MJ, Pottenger LH, Gollapudi BB, Schisler MR. Simultaneous quantitation of 7-methyl- and O6-methylguanine
adducts in DNA by liquid chromatography-positive electrospray
tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2006;833:141 – 8.
23. Lubin JH, Caporaso N, Hatsukami DK, Joseph AM, Hecht SS. The association of a tobacco-specific biomarker and cigarette consump-tion and its dependence on host characteristics. Cancer Epidemiol Biomarkers Prev 2007;16:1852 – 7.
24. Jalas JR, Hecht SS, Murphy SE. Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Chem Res Toxicol 2005;18: 95 – 110.
25. Carmella SG, Le Ka KA, Upadhyaya P, Hecht SS. Analysis ofN- and O -glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chem Res Toxicol 2002;15:545 – 50. 26. Degan P, Montesano R, Wild CP. Antibodies against
7-methyldeox-yguanosine: its detection in rat peripheral blood lymphocyte DNA and potential applications to molecular epidemiology. Cancer Res 1988;48:5065 – 70.
27. Chu BC, Lawley PD. Increased urinary excretion of nucleic acid and nicotinamide derivatives by rats after treatment with alkylating agents. Chem Biol Interact 1975;10:333 – 8.
28. Avkin S, Adar S, Blander G, Livneh Z. Quantitative measurement of translesion replication in human cells: evidence for bypass of abasic sites by a replicative DNA polymerase. Proc Natl Acad Sci U S A 2002;99:3764 – 9.
29. Loft S, Svoboda P, Kasai H, et al. Prospective study of urinary excretion of 7-methylguanine and the risk of lung cancer: Effect modification byA class glutathione-S-transferases. Int J Cancer 2007; 121:1579 – 84.
30. Brown PJ, Bedard LL, Reid KR, Petsikas D, Massey TE. Analysis of CYP2A contributions to metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human peripheral lung microsomes. Drug Metab Dispos 2007;35:2086 – 94.
31. Ribeiro Pinto LF, Teixeira Rossini AM, Albano RM, et al. Mechan-isms of esophageal cancer development in Brazilians. Mutat Res 2003;544:365 – 73.
32. Loriot MA, Rebuissou S, Oscarson M, et al. Genetic polymorphisms of cytochromeP450 2A6 in a case-control study on lung cancer in a French population. Pharmacogenetics 2001;11:39 – 44.
33. Trushin N, Hecht SS. Stereoselective metabolism of nicotine and tobacco-specificN-nitrosamines to 4-hydroxy-4-(3-pyridyl)butanoic acid in rats. Chem Res Toxicol 1999;12:164 – 71.
34. Hecht SS, Hatsukami DK, Bonilla LE, Hochalter JB. Quantitation of 4-oxo-4-(3-pyridyl)butanoic acid and enantiomers of 4-hydroxy-4-(3-pyridyl)butanoic acid in human urine: a substantial pathway of nicotine metabolism. Chem Res Toxicol 1999;12:172 – 9.
35. Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res 1999;59:590 – 6.
36. Stepanov I, Upadhyaya P, Carmella SG, et al. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol Biomarkers Prev 2008;17:1764 – 73.
37. Hecht SS, Carmella SG, Murphy SE. Effects of watercress consump-tion on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev 1999;8:907 – 13.
38. Hecht SS, Carmella SG, Le KA, et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Bio-markers Prev 2006;15:988 – 92.
39. Stepanov I, Hecht SS, Lindgren B, Jacob PIII, Wilson M, Benowitz NL. Relationship of human toenail nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol to levels of these bio-markers in plasma and urine. Cancer Epidemiol Biobio-markers Prev 2007;16:1382 – 6.
40. Kyrtopoulos SA. DNA adducts in humans after exposure to methylating agents. Mutat Res 1998;405:135 – 43.