Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK
Journal of Toxicology and Environmental Health, Part
A: Current Issues
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/uteh20
Nitrates in Drinking Water and the Risk of Death from
Childhood Brain Tumors in Taiwan
Hsu-Huei Weng abc , Shang-Shyue Tsai d , Trong-Neng Wu ef , Fung-Chang Sung g & Chun-Yuh Yang fh
a
Department of Diagnostic Radiology, Chang Gung Memorial Hospital at Chiayi, Chang Gung University College of Medicine, Taiwan
b
Department of Respiratory Care, Chang Gung Institute of Technology, Chiayi, Taiwan c
Department of Psychology, National Chung Cheng University, Chiayi, Taiwan d
Department of Health Care Administration, I-Shou University, Kaohsiung County, Taiwan e
Graduate Institute of Environmental Health, China Medical, University, Taichung, Taiwan f
Division of Environmental Health and Occupational Medicine, National Health Research Institute, Miaoli, Taiwan
g
Department of Public Health, China Medical University, Taichung, Taiwan h
Faculty of Public Health, College of Health Sciences, Kaohsiung Medical University, Kaohsiung, Taiwan
Version of record first published: 02 May 2011.
To cite this article: Hsu-Huei Weng, Shang-Shyue Tsai, Trong-Neng Wu, Fung-Chang Sung & Chun-Yuh Yang (2011): Nitrates in Drinking Water and the Risk of Death from Childhood Brain Tumors in Taiwan, Journal of Toxicology and Environmental Health, Part A: Current Issues, 74:12, 769-778
To link to this article: http://dx.doi.org/10.1080/15287394.2011.567951
PLEASE SCROLL DOWN FOR ARTICLE
Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Journal of Toxicology and Environmental Health, Part A, 74:769–778, 2011 Copyright © Taylor & Francis Group, LLC
ISSN: 1528-7394 print / 1087-2620 online DOI: 10.1080/15287394.2011.567951
NITRATES IN DRINKING WATER AND THE RISK OF DEATH FROM CHILDHOOD BRAIN TUMORS IN TAIWAN
Hsu-Huei Weng1,2,3, Shang-Shyue Tsai4, Trong-Neng Wu5,6, Fung-Chang Sung7,
Chun-Yuh Yang6,8
1Department of Diagnostic Radiology, Chang Gung Memorial Hospital at Chiayi, Chang Gung
University College of Medicine, Taiwan
2Department of Respiratory Care, Chang Gung Institute of Technology, Chiayi, Taiwan 3Department of Psychology, National Chung Cheng University, Chiayi, Taiwan
4Department of Health Care Administration, I-Shou University, Kaohsiung County, Taiwan 5Graduate Institute of Environmental Health, China Medical, University, Taichung, Taiwan 6Division of Environmental Health and Occupational Medicine, National Health Research
Institute, Miaoli, Taiwan
7Department of Public Health, China Medical University, Taichung, Taiwan
8Faculty of Public Health, College of Health Sciences, Kaohsiung Medical University, Kaohsiung,
Taiwan
The objective of this study was to (1) examine the relationship between nitrate (NO3-N) levels
in public water supplies and risk of death from childhood brain tumors (CBT) and (2) deter-mine whether calcium (Ca) and magnesium (Mg) levels in drinking water might modify the effects of NO3-N on development of CBT. A matched cancer case-control study was used
to investigate the relationship between the risk of death attributed to CBT and exposure to NO3-N in drinking water in Taiwan. All CBT deaths of Taiwan residents from 1999 through
2008 were obtained from the Bureau of Vital Statistics of the Taiwan Provincial Department of Health. Controls were deaths from other causes and were pair-matched to the cases by gen-der, year of birth, and year of death. Information on the levels of nitrate-nitrogen (NO3-N), Ca,
and Mg in drinking water were collected from Taiwan Water Supply Corporation. The munici-pality of residence for CBT cases and controls was presumed to be the source of the subject’s NO3-N, Ca, and Mg exposure via drinking water. Relative to individuals whose NO3-N
expo-sure level was≤0.31 ppm, and the adjusted odds ration (OR) (95% confidence interval [CI]) for CBT occurrence was 1.4 (1.07–1.84) for individuals who resided in municipalities served by drinking water with a NO3-N exposure>0.31 ppm. No significant effect modification was
observed by Ca and Mg intake via drinking water. Data suggest that exposure to NO3-N in
drinking water is associated with a higher risk of CBT development in Taiwan.
The U.S. Environmental Protection Agency established a maximal contaminant level (MCL) in drinking water of 10 mg/L as nitrate-N to protect infants from developing methe-moglobinemia (Ward et al., 2005). However, the effectiveness of this regulatory threshold limit for preventing other adverse health risks Received 1 October 2010; accepted 30 November 2010.
This study was supported by a grant from the National Science Council, Executive Yuan, Taiwan (NSC-97-2314-B-037-006-MY3). Address correspondence to Chun-Yuh Yang, PhD, MPH, Institute of Public Health, College of Health Sciences, Kaohsiung Medical University, 100 Shih-Chuan 1st RD, Kaohsiung, Taiwan. 80708. E-mail: chunyuh@kmu.edu.tw
such as cancer has not been adequately studied (De Roos et al., 2003).
Nitrates may act as procarcinogens, interacting with amines and amides in the stomach to form a variety of N-nitroso com-pounds (NOC) (nitrosation), most of which are potent animal carcinogens (Tricker and
769
Preussmann 1991) following reduction of dietary nitrate to nitrite in saliva (Walker 1990). The generated nitrite is postulated to produce methemoglobinema and potentially carcino-genic processes. There is evidence of a dose-response increase in urinary nitrate secretion (Kleinjans et al. 1991), and elevated salivary nitrate and nitrite levels among those individ-uals exposed to higher levels of drinking water nitrates, relative to those subjects with low amounts of these compounds (Van Maanen et al. 1996).
Several studies support a direct relation-ship between nitrate intake and endogenous formation of NOC. High nitrate levels in drink-ing water are associated with increased excre-tion of N-nitrosoproline in urine (Mirvish et al. 1992; Moller et al. 1989). Nitrate administered via drinking water was shown to be directly correlated with concentration of total NOC in feces (Rowland et al. 1991). In addition, pop-ulations with high rates of esophageal, gastric, and nasopharyngeal cancer excrete high levels of N-nitrosoproline (Kamiyama et al. 1987; Lu et al. 1986; Yi et al. 1993). These results indi-cate a contribution of drinking-water nitrates toward nitrosation and suggest that nitrate intake may be used as a surrogate biomarker for exposure of target tissues to NOC (De Roos et al. 2003).
Brain tumors are the most common solid tumors occurring in children. The etiology of childhood brain tumors (CBT) is largely unknown. The postulation that exposure to NOC and their precursors is related to the risk of CBT is supported by observations in animal studies where Rice et al. (1989) demonstrated that N-alkylnitrosoureas induced brain tumors in the offspring of pregnant rodents and mon-keys. Most epidemiological studies that exam-ined the role of maternal consumption of cured meats during pregnancy found a significant positive association between maternal intake of cured meats and the risk of development of CBT (Dietrich et al. 2005; Preston-Martin et al. 1996; Kuijten et al. 1990; Sarasua and Savitz 1994; McCredie et al. 1994). Cured meats are a major dietary source of preformed NOC and their precursors (Dietrich et al. 2005). In
contrast, no significant association with cured meats consumption was noted in some other studies (Cordier et al. 1994; Bunin et al. 1993; 1994; Lubin et al. 2000).
Given the widespread exposure of pop-ulations to nitrate, there are surprisingly few epidemiologic studies concerning the possible association of nitrates in drinking water with development of CBT. Mueller et al. (2001) determined nitrate levels in water supplies using dipstick measurements, often several years after women’s pregnancies, and reported no significant correlation between CBT occur-rence with levels of nitrate and nitrite. However, an increased risk of CBT occurrence was observed in western Washington State, one of the three study centers, among off-spring of women who used private wells as their drinking-water source during pregnancy. In a SEARCH International Childhood Brain Tumor study (Mueller et al., 2004), risk of CBT development did not increase with rising nitrate levels measured in tap water. However, the risk of astrocytoma was associated with increasing nitrite levels measured in tap water of residences of pregnant women.
Several epidemiologic studies reported no significant association or inverse association between dietary nitrate intake and human cancers (Ward et al. 1996; 2003; Forman 1987), which may be attributed to antioxidants and nitrosation inhibitors in nitrate-containing foods (Bartsch et al. 1988). Antioxidants that inhibit endogenous nitrosation include vitamin C and alpha-tocopherol (Ward et al. 2005). Our previous studies showed that the associ-ation between NO3-N exposure and risk of colorectal cancers was influenced by water hardness (calcium [Ca] and magnesium [Mg]) (Chiu et al. 2010; Chang et al. 2010). No apparent previous studies explored whether Ca and Mg levels in drinking water might mod-ify the association between NO3-N exposure and development of CBT. If a significant effect modification by Ca and Mg levels in drinking water exists, the true magnitude of the asso-ciation between NO3-N exposure and CBT occurrence may be obscured. Furthermore, knowledge of the modifying factors may help in
NITRATE AND CHILDHOOD BRAIN TUMORS 771
initiation of public policy, risk assessment, and setting threshold standards.
The objective of this study was thus to (1) examine the relationship between NO3-N lev-els in public water supplies and the risk of death attributed to CBT and (2) determine whether Ca and Mg levels in drinking water might modify the effects of NO3-N on development of CBT.
MATERIALS AND METHODS Study Area
Taiwan is divided into 361 administrative districts, which will be referred to herein as municipalities. These are the units that will be subjected to statistical analysis. Excluded from the analysis were 30 aboriginal townships and 9 islets which had different life-styles and liv-ing environments (the diets of subjects in these municipalities are generally rich in fiber, antiox-idants, and nitrosation inhibitors, which may yield beneficial properties and act in a way against brain carcinogenesis occurrence). This elimination of unsuitable municipalities yielded 322 municipalities.
Socioeconomic Factors
Each municipality in Taiwan was assigned to a degree-of-urbanization category from 1 to 8 based on the urban–rural classification of Tzeng and Wu (1986), which takes into account variables such as population density, age, economic activity and family income, educational level, environment, and health service-related facilities. A municipality with the highest urbanization score, such as the Taipei metropolitan area, was classified in cat-egory 1, whereas mountainous areas with the lowest score were assigned to category 8. The urbanization index used in this study serves as a proxy for a large number of explana-tory variables such as socioeconomic status and differential exposures to environmental con-ditions, which contributed to the etiology of mortality. For the analyses, the urban–rural clas-sification was further divided into four levels: I, metropolitan (categories 1 and 2); II, city
(categories 3 and 4); III, town (categories 5 and 6); and IV, rural (categories 7 and 8).
Subject Selection
Data on all deaths of Taiwan residents from 1999 through 2008 were obtained from the Bureau of Vital Statistics of the Taiwan Provincial Department of Health, which is responsible for the death registration system in Taiwan. For each death, detailed demographic information including gender, year of birth, year of death, cause of death, place of death (municipality), and residential district (munici-pality) were recorded. The cancer case group consisted of all eligible malignant tumors of the brain (International Classification of Disease, 9th rev. [ICD-9], code 191) or cranial nerves (code 192) occurring in individuals between 0 and 19 yr of age. In all, 457 deaths due to CBT with complete records satisfied this criterion.
Controls were drawn from all other deaths excluding deaths due to neoplasms. Control subjects were pair-matched to the cases by gender, year of birth, and year of death. Each matched control was selected randomly from the set of possible controls for each case.
The most frequent causes of death among the controls were motor vehicle traffic dents of unspecified nature (17.1%), acci-dental drowning and submersion (11.2%), ill-defined and unknown causes of morbidity and mortality (7.9%), congenital cardiac anoma-lies (3.1%), paralytic syndromes (2.8%), and accidents caused by unspecified fire (2.8%).
Nitrate-Nitrogen (NO3-N), Ca, and Mg Levels in Drinking Water
Information on the levels of NO3-N, Ca, and Mg in each municipality’s treated drinking-water supply was obtained from the Taiwan Water Supply Corporation (TWSC) (TWSC/ROC, 1991), to which each water-works is required to submit drinking-water quality data including the levels of NO3-N, Ca, and Mg. Four treated water samples, one for each season, were collected from each waterworks. The samples were analyzed by
the waterworks laboratory office using stan-dard methods (cadmium reduction method for NO3-N and spectrophotometric method for Ca and Mg, respectively). Since the laboratory office examines NO3-N, Ca, and Mg levels on a routine basis using standard methods, it was presumed that analytical variability was mini-mal. Among the 322 municipalities, 70 were excluded as these had more than one sup-ply of drinking water and the exact population served by each could not be determined with details provided in earlier publications (Yang et al. 1998; Yang 1998). The final complete data comprised NO3-N, Ca, and Mg data from 252 municipalities. Calcium and Mg concen-trations remain reasonably constant for long periods of time and are a quite stable character-istic of a municipality’s water supply (Bell and Doege 1984). Data collected were the annual mean levels of NO3-N, Ca, and Mg for the year 1990. The municipalities of residence for all cancer cases and controls were identified from death certificates and it was presumed that municipal drinking water was the source of the subjects’ NO3-N, Ca, and Mg exposure via drinking water. The levels of NO3-N, Ca, and Mg of each municipality were used as indica-tors of exposure to NO3-N, Ca, and Mg for an individual residing in that municipality.
Statistics
In the analysis, the subjects were catego-rized into one of the two NO3-N exposure categories: low (less than or equal to median among controls;≤0.31 ppm) and high (greater than median among controls; >0.31 ppm). Conditional logistic regression was used to esti-mate the association between NO3-N levels present in drinking water and risk of death attributed to CBT. Odds ratios (OR) and their 95% confidence intervals (95% CI) were calcu-lated using the low-exposure group as the refer-ent group (Breslow and Day 1980). The associ-ation between drinking-water NO3-N and risk of CBT occurrence was stratified by Ca and Mg levels in drinking water. The analyses were performed using the SAS software (version 8.2; SAS Institute, Inc., Cary, NC). All statistical tests
were two-sided and values of p < .05 were considered statistically significant.
RESULTS
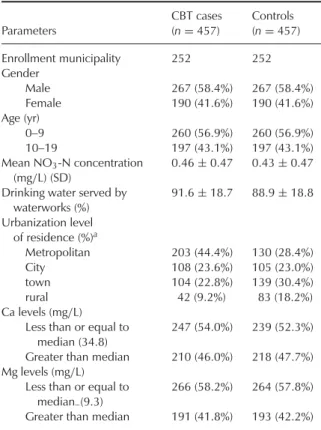
In total, 457 CBT cases with complete records were collected for the period 1999– 2008. Of the 457 cases, 267 were males and 190 females (Table 1). The mean NO3-N con-centration in the drinking water of the CBT cases was 0.46 mg/L (SD = 0.47). Controls had a mean NO3-N exposure of 0.43 mg/L (SD = 0.47). CBT cases lived in municipal-ities in which 91.6% of the population was served by waterworks. For controls this num-ber was 88.9%. CBT cases had a higher rate (44.4%) of residing in metropolitan munici-palities than controls (28.4%). CBT cases had a numerically lower rate (46%) of living in municipalities served by drinking water with high levels (>34.8 mg/L) of Ca than controls (47.7%). CBT cases had a similar rate (41.8%)
TABLE 1. Characteristics of the Study Population
Parameters CBT cases (n= 457) Controls (n= 457) Enrollment municipality 252 252 Gender Male 267 (58.4%) 267 (58.4%) Female 190 (41.6%) 190 (41.6%) Age (yr) 0–9 260 (56.9%) 260 (56.9%) 10–19 197 (43.1%) 197 (43.1%) Mean NO3-N concentration (mg/L) (SD) 0.46± 0.47 0.43± 0.47
Drinking water served by waterworks (%) 91.6± 18.7 88.9± 18.8 Urbanization level of residence (%)a Metropolitan 203 (44.4%) 130 (28.4%) City 108 (23.6%) 105 (23.0%) town 104 (22.8%) 139 (30.4%) rural 42 (9.2%) 83 (18.2%) Ca levels (mg/L)
Less than or equal to median (34.8)
247 (54.0%) 239 (52.3%)
Greater than median 210 (46.0%) 218 (47.7%)
Mg levels (mg/L)
Less than or equal to
median–(9.3)
266 (58.2%) 264 (57.8%)
Greater than median 191 (41.8%) 193 (42.2%)
aThe urbanization level of each municipality was based on the
urban–rural classification scheme of Tzeng and Wu (1986).
NITRATE AND CHILDHOOD BRAIN TUMORS 773
TABLE 2. Odds Ratios (OR) and 95% Confidence Intervals (CI)
for Childhood Brain Tumors (CBT) Death in Relation to Nitrate Levels in Drinking Water, 1999–2008
NO3-N levels
(mg/L) CBT cases Controls OR (95% CI)a
≤0.31 190 (41.6%) 229 (50.1%) 1.00
>0.31 267 (58.4%) 228 (49.9%) 1.40 (1.07–1.84)
aAdjusted for age, gender, and urbanization level of residence.
of residing in municipalities served by drink-ing water with high levels (>9.3 mg/L) of Mg compared to controls (42.2%) (Table 1).
Table 2 shows the distribution of CBT cases and controls and OR with respect to the levels of NO3-N in drinking water. Relative to indi-viduals whose NO3-N levels were≤0.31 mg/L, the adjusted OR (95% CI) for CBT was 1.4 (1.07–1.84) for subjects who resided in munici-palities served by drinking water with a NO3-N levels>0.31 mg/L.
The association between NO3-N levels in drinking water and risk of CBT among those with high (greater than median) and low (less than or equal to median) Ca intake via drink-ing water is shown in Table 3. There was no evidence of a significant interaction between drinking water NO3-N levels and low Ca intake via drinking water.
The association between NO3-N levels in drinking water and CBT risk was evaluated among those with high (great than median) and low (less than or equal to median) Mg intake via drinking water (Table 4). There
TABLE 3. Odds Ratios for Childhood Brain Tumors (CBT) by
Levels of Nitrate and Ca in Drinking Water
NO3-N levels (mg/L) ≤0.31 >0.31 Ca levels (mg/L) CBT cases Controls ORa (95% CI) CBT cases Controls ORa (95% CI) >34.8 58 86 1.00 152 132 1.30 (0.84– 1.99) ≤34.8 132 143 0.93 (0.60– 1.45) 115 96 1.41 (0.90– 2.19)
aAdjusted for age, gender, and urbanization level of residence.
TABLE 4. Odds Ratios for Childhood Brain Tumors (CBT) by
Levels of Nitrate and Mg in Drinking Water
NO3-N levels (mg/L) ≤0.31 >0.31 Mg levels (mg/L) CBT cases Controls ORa (95% CI) CBT cases Controls ORa (95% CI) >9.3 77 61 1.00 130 116 1.24 (079– 1.95) ≤9.3 129 152 0.83 (0.53– 1.29) 137 112 1.21 (0.77– 1.9)
aAdjusted for age, gender, and urbanization level of residence.
was no evidence of a significant interaction between drinking-water NO3-N levels and low Mg intake via drinking water.
DISCUSSION
Our findings are in contrast to results reported in previous epidemiologic studies (Mueller et al. 2001; 2004). The basis for these inconsistencies is not known. All these stud-ies, including our investigation, have common problems concerning exposure assessment methods. Instead of using a real individual exposure to NO3-N, our data estimated past NO3-N levels by linking each study subject’s residence to the subject’s individual water source while not taking into account resi-dential histories. Data on individual exposure were thus characterized by a lack of precision. A more basic reason for the inconsistent results may be that there is no causality between NO3 -N in drinking water and development of CBT. It is also possible that high level of NO3-N is a proxy for some other contaminants, such as pesticides or metals, in the water for which no information is available (Mueller et al., 2001).
The biological mechanisms underlying NOC exposure and correlation to increase risk of CBT remain unknown. It is possible that, due to a decreased capacity for DNA repair and a high rate of neural cell division, the fetal brain is particularly susceptible to potential car-cinogenic effects following exposures to NOC (Rice and Ward 1982; Berleur and Cordier 1995; Mueller et al. 2001). Furthermore,
an increased hypoxanthine-guanine phospho-ribosyltransferase (HPRT) variant frequency in peripheral lymphocytes of subjects exposed to high levels of drinking-water NO3-N was noted, suggesting the occurrence of genotoxic effects (Van Maanen et al. 1996).
Although there appears to be a con-sistent association between intake of high drinking-water NO3-N and endogenous nitro-sation capacity, intake of dietary NO3-N is not likely to increase nitrosation due to the presence of nitrosation inhibitors in vegeta-bles (Bartsch et al. 1988). Antioxidants that inhibit endogenous nitrosation include vitamin C and alpha-tocopherol, which reduce nitrite to NO (Bartsch et al., 1988). Previous findings suggested that Ca and Mg may act like vita-min C and alpha-tocopherol, which inhibited endogenous nitrosation produced by intake of NO3-N in drinking water, and therefore indi-viduals who had low levels of Ca or Mg intake via drinking water may be at increased risk for exposure to NOC and colorectal cancer (Chiu et al. 2010; Chang et al. 2010). It was further postulated that the effect of NO3-N in drink-ing water on risk of CBT development might be modified by intake of Ca and Mg in drinking water. To our knowledge, this is the first to study the effect modification by Ca and Mg intake in drinking water in the correlation between NO3-N exposure and CBT occurrence.
The completeness and accuracy of the death registration system needed to be evalu-ated before any conclusion based on mortality analysis is reached. In Taiwan, it is mandatory to register death certificates at local household registration offices, and the death registration in Taiwan is complete. Although causes of death may be misdiagnosed and/or misclassi-fied, the problem has been minimized through the improvement in the verification and classifi-cation of causes of death in Taiwan since 1972. Furthermore, malignant neoplasms, including CBT, were reported to be one of the most unequivocally classified causes of death in Taiwan (Chen and Wang 1990). Because of a fatal outcome, it is believed that all CBT cases from exposure to high or low levels of NO3-N in drinking water had access to medical care
regardless of geographical location in recent years.
Inherent in this study design were the assumptions that CBT cases and controls were exposed to NO3-N in drinking water attributed to the “usual place of residence” recorded on the death certificate and that individuals spent most of their daily life in this residential munic-ipality. The probability that these assumptions were true among the subjects aged 0-19 yr was based on the fact that the majority of these study subjects (1) spent more time at home, (2) were studying in their usual residential munic-ipality, and (3) were less likely to have resided in other locations.
Migration from a municipality of high NO3 -N exposure to one of low exposure or vice versa may have introduced misclassification bias and bias in OR estimates (Gladen and Rogan 1979; Polissar 1980). Our study popu-lation was stable in terms of mobility compared with populations in most industrialized coun-tries, probably because of cultural factors and age distribution (Yu et al. 2006). Further, any misclassification of exposure is most likely to be nondifferential, which would reduce the estimated magnitude of association rather than introduce a positive bias in the estimation.
Since the measure of effect in this study is mortality rather than incidence, migration during the interval between CBT diagnosis and death must also be considered. During this period, CBT diagnosis may have influ-enced parental decision to migrate and may have possibly introduced bias. If there was a trend toward migration to more urban areas or municipalities with higher levels of NO3-N in drinking water because of proximity to medical care, a spurious association between NO3-N exposure from drinking water and death due to CBT would have resulted. Two aspects of this study presumably minimize this possibility. First, migration precipitated by a CBT diagnosis would have been less likely for the parents of the subjects, because for this cohort of dece-dents, the parental occupational status would weigh against a move requiring a job change. Second, urbanization level was included as a control variable in the analysis.
NITRATE AND CHILDHOOD BRAIN TUMORS 775
Intake of NO3-N from drinking water and dietary sources may result in increased expo-sure to NOC through endogenous nitrosation (Mirvish et al. 1992; Moller et al. 1989). The principal dietary NO3-N sources are veg-etables. Vegetables also contain vitamin C and other nitrosation inhibitors (Bartsch et al. 1988), and therefore, high intakes may not necessarily result in high rates of formation of NOC (Coss et al. 2004). Dietary intakes of red and processed meat are of particu-lar importance in the formation of fecal NOC (Bingham 1999; Bingham et al. 2002). There is unfortunately no information available for assessing dietary NO3-N sources from vegeta-bles and meat for individual study subjects in this investigation. However, there is no reason to believe that there would be any correla-tion between the sources of dietary NO3-N and the levels of NO3-N in drinking water. Furthermore, Chilvers et al. (1984) indicated that when the concentration of waterborne NO3-N is high, drinking water contributes sub-stantially to total NO3-N intake and the poten-tial for nitrite and NOC formation may be increased. It is thus proposed that individuals with higher daily NO3-N intake from drinking water and lower intakes of nitrosation inhibitors may be at an elevated risk of CBT develop-ment.
There are other risk factors, not included in this study, that may play a role in the etiol-ogy of CBT, such as ionizing radiation, parental smoking, and certain heredity and genetic fac-tors (Little 1999). There is unfortunately no information available on these variables for an individual study subject and thus it could not be adjusted for directly in the analysis. However, there is no reason to believe that there would be any correlation between these confounders and the levels of NO3-N in drinking water. It is also unlikely that there would be a direct relationship between other risk factors and the levels of NO3-N in drinking water.
The nitrate concentration in drinking water in Taiwan is below the guideline value 10 mg/L recommended by the World Health Organization (1984). This guideline was not based on estimates of cancer risk. In addition,
there is no scientific evidence to justify firm conclusions about the safety of any concentra-tion of NO3-N in water with regard to cancer risk occurrence. Formam (1989) noted that although environmental NO3-N exposure may play a role in the development of cancer, it does not show a rate-limiting effect.
In summary, our data suggest that expo-sure to NO3-N in drinking water at levels in this study is associated with higher risk of death attributed to CBT. Future studies need to increase the precision of the estimation of the individual’s intake of NO3-N, through both food and water, and control for confounding factors such as radiation.
REFERENCES
Bartsch, H., Ohshima, H., and Pignatelli, B. 1988. Inhibitors of endogenous nitrosation. Mechanisms and implications in human can-cer prevention. Mutat. Res. 202:307–324. Bell, J. A., and Doege, T. C. 1984. Drinking
water and human health. Chicago: American
Medical Association.
Berleur, M. P., Cordier, S. 1995. The role of chemical, physical, or viral exposures and health factors in neurocarcinogene-sis: Implications for epidemiologic studies of brain tumors. Cancer Causes Control 6: 240–256.
Bingham, S. A. 1999. High-meat diets and cancer risk. Proc. Nutr. Soc. 58:243–248. Bingham, S. A., Hughes, R., and Cross, A. J.
2002. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J. Nutr. 132:3522S–3525S. Breslow, N. E., and Day, N. E. 1980.
Statistical methods in cancer research. Vol. I. The analysis of case-control studies.
Lyon: International Agency for Research on Cancer.
Bunin, G. R., Kuijten, R. R., Boesel, C. P., Rorke, L. B., and Meadows, A. T. 1993. Relation between maternal diet and sub-sequent primitive neuroectodermal brain tumors in young children. N. Engl. J. Med. 329:536–541.
Bunin, G. R., Kuijten, R. R., Boesel, C. P., Buckley, J. D., and Meadows, A. T. 1994. Maternal diet and risk of astrocytic glioma in children: A report from the Children Cancer Group (United States and Canada). Cancer
Causes Control 5:177–187.
Chang, C. C., Chen, C. C., Wu, D. C., and Yang, C. Y. 2010. Nitrate in drinking water and the risk of death from rectal cancer: Does hardness in drinking water matter? J. Toxicol.
Environ. Health A 73:1337–1347.
Chen, C.J., Wang, C.J. 1990. Ecological corre-lation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 50:5470–5474. Chilvers, C., Inskip, H., Caygill, C.,
Bartholomew, B., Fraser, P., and Hill, M. 1984. A survey of dietary nitrate in well-water users. Int. J. Epidemiol. 13:324–331. Chiu, H. F., Tsai, S. S., Wu, T. N., and Yang, C. Y.
2010. Colon cancer and content of nitrates and magnesium in drinking water. Magnes.
Res. 23:81–89.
Cordier, S., Iglesias, M. J., Le Goaster, C., Guyot, M. M., Mandereau, L., and Hemon, D. 1994. Incidence and risk factors for child-hood brain tumors in the Ile De France. Int.
J. Cancer 59:776–782.
Coss, A., Cantor, K. P., Reif, J. S., Lynch, C. F., and Ward, M. H. 2004. Pancreatic can-cer and drinking water and dietary sources of nitrate and nitrite. Am. J. Epidemiol. 159:693–701.
De Roos, A. J., Ward, M. H., Lynch, C. F., and Cantor, K. P. 2003. Nitrate in public water supplies and the risk of colon and rectum cancers. Epidemiology 14:640–649.
Dietrich, M., Block, G., Pogoda, J. M., Buffler, P., Hecht, S., and Preston-Martin, S. 2005. A review: Dietary and endogenously formed N-nitroso compounds and risk of child-hood brain tumors. Cancer Causes Control 16:619–635.
Forman, D. 1987. Dietary exposure to N-nitroso compounds and the risk of human cancer. Cancer Surveys 6:719–738.
Forman, D. 1989. Are nitrates a significant risk factor in human cancer? Cancer Surv. 8:443–458.
Gladen, B., and Rogan, W. 1979.
Misclassification and the design of envi-ronmental studies. Am. J. Epidemiol.
109:607–616.
Kamiyama, S., Ohshima, H., Shimada, A., Saito, N., Bourgade, M. C., Ziegler, P., and Bartsch, H. 1987. Urinary excretion of N-nitrosoamino acids and nitrate by inhabi-tants of high- and low-risk areas for stomach cancer in northern Japan. IARC Sci. Publ. 84:479–502.
Kleinjans, J. C., Albering, H. J., Marx, A., van Maanen, J. M., van Agen, B., ten Hoor, F., Swaen, G. M., and Mertens, P. L. 1991. Nitrate contamination of drinking water: Evaluation of genotoxic risk in human populations. Environ. Health Perspect. 94: 189–193.
Kuijten, R. R., Bunin, G. R., Nass, C. C., and Meadows, A. T. 1990. Gestational and famil-ial risk factors for childhood astrocytoma: Results of a case-control study. Cancer Res. 50:2608–2612.
Little, J. 1999. Epidemiology of childhood
can-cer. Lyon:IARC.
Lu, S. H., Ohshima, H., Fu, H. M., Tian, Y., Li, F. M., Blettner, M., Wahrendorf, J., and Bartsch, H. 1986. Urinary excretion of N-nitrosoamino acids and nitrate by inhabitants of high- and low-risk areas for esophageal cancer in northern China: Endogenous for-mation of nitrosoproline and its inhibi-tion by vitamin C. Cancer Res. 46:1485– 1491.
Lubin, F., Farbstein, H., Chetrit, A., Farbstein, M., Freedman, L., Alfandary, E., and Modan, B. 2000. The role of nutritional habits dur-ing gestation and child life in pediatric brain tumor etiology. Int. J. Cancer 86:139–143. McCredie, M., Maisonneuve, P., and Boyle, P.
1994. Antenatal risk factors for malignant brain tumors in New South Wales children.
Int. J. Cancer 56:6–10.
Ministry of Economics, Taiwan. 1989. Census of
manufactures. Taipei: MOE.
Mirvish, S. S., Grandjean, A. C., Moller, H., Fike, S., Maynard, T., Jones, L., Rosinsky, S., and Nie, G. 1992. N-Nitrosoproline excre-tion by rural Nebraskans drinking water of
NITRATE AND CHILDHOOD BRAIN TUMORS 777
varied nitrate content. Cancer Epidemiol.
Biomarkers Prev. 1:455–461.
Moller, H., Landt, J., Pedersen, E., Jensen, P., Autrup, H., and Jensen, O. 1989. Endogenous nitrosation in relation to nitrate exposure from drinking water and diet in a Danish rural population. Cancer
Res. 49:3117–3121.
Mueller, B. A., Newton, K., Holly, E. A., and Preston-Martin, S. 2001. Residential water source and the risk of childhood brain tumors. Environ. Health Perspect. 109: 551–556.
Mueller, B. A., Nielsen, S. S., Preston-Martin, S., Holly, E. A., Cordier, S., Filippini, G., Peris-Bonet, R., and Choi, N. W. 2004. Household water source and the risk of childhood brain tumors: Results of the SEARCH international brain tumor study. Int.
J. Epidemiol. 33:1209–1216.
Preston-Martin, S., Pogoda, J. M., Mueller, B. A., Holly, E. A., Lijinsky, W., and Davis, R. L. 1996. Maternal consumption of cured meats and vitamines in relation to pediatric brain tumors. Cancer Epidemiol. Biomarkers Prev. 5:599–605.
Polissar, L. 1980. The effect of migration on comparison of disease rates in geo-graphic studies in the United States. Am. J.
Epidemiol. 111:175–182.
Rice, J. M., and Ward, J. M. 1982. Age depen-dence of susceptibility to carcinogenesis in the nervous system. Ann. NY Acad. Sci. 381:274–289.
Rice, J. M., Rehm, S., Donovan, P. J., and Perantoni, A. O. 1989. Comparative transplacental carcinogenesis by directly act-ing and metabolism-dependent alkylatact-ing agents in rodents and nonhuman primates.
IARC Sci. Publ. 96:17–34.
Rowland, I. R., Granli, T., Bockman, O. C., Key, P. E., and Massey, R. C. 1991. Endogenous N-nitrosation in man assessed by measure-ment of apparent total N-nitroso compounds in feces. Carcinogenesis 12:1395–1401. Sarasua, S., and Savitz, D. A. 1994. Cured
and broiled meat consumption in relation to childhood cancer, Denver, Colorado. Cancer
Causes Control 5:141–148.
Taiwan Water Supply Corporation. 1991. The
statistical data of water quality. Taipei:
Taiwan Water Supply Corporation.
Tricker, A. R., and Preussmann, R. 1991. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 259: 277–289.
Tzeng, G. H., and Wu, T. Y. 1986. Characteristics of urbanization levels in Taiwan districts. Geogr. Res. 12:287–323. Van Maanen, J. M., Welle, I. J., Hageman, G.,
Dallinga, J. W., Mertens, P. L., and Kleinjans, J. C. 1996. Nitrate contamination of drink-ing water: Relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N-nitrosamines. Environ. Health
Perspect. 104:522–528.
Walker, R. 1990. Nitrates, nitrites and N-nitroso compounds: A review of the occur-rence in food and diet and the toxicolog-ical implications. Food Addit. Contam. 7: 717–768.
Ward, M. H., Mark, S. D., Cantor, K. P., Weisenburger, D. D., Correa-Villasenore, A., and Zahm, S. H. 1996. Drinking water nitrate and the risk of non-Hodgkin lym-phoma. Epidemiology 7:465–471.
Ward, M. H., Cantor, K. P., Riley, D., Merkle, S., and Lynch, C. F. 2003. Nitrate in pub-lic water supplies and risk of bladder cancer.
Epidemiology 14:183–190.
Ward, M. H., deKok, T. M., Levallois, P., Brender, J., Gulis, G., Nolan, B. T., and VanDerslice, J. 2005. Workgroup report: Drinking-water nitrate and health—Recent findings and research needs. Environ. Health
Perspect. 113:1607–1614.
World Health Organization. 1984. Guidelines
for drinking water quality: Vol. 1.
Recommendations. Geneva: WHO.
Yang, C. Y. 1998. Calcium and magnesium in drinking water and risk of death from cere-brovascular disease. Stroke 29:411–414. Yang, C. Y., Cheng, M. F., Tsai, S. S., and
Hsieh, Y. L. 1998. Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Jpn. J. Cancer Res. 89: 124–130.
Yi, Z., Ohshima, H., Bouvier, G., Roy, P., Zhong, J., Li, B., Brouet, I., de The, G., and Bartsch, H. 1993. Urinary excretion of N-nitrosoamino acids and nitrate by inhabitants of high- and low-risk areas for nasopha-ryngeal cancer in southern China. Cancer
Epidemiol. Biomarker Prev. 2:195–200.
Yu, C. L., Wang, S. F., Pan, P. C., Wu, M. T., Ho, C. K., Smith, T. J., Li, Y., Pothier, L., and Christiani, D. C. 2006. Residential exposure to petrochemicals and the risk of leukemia: Using geographical information system tools to estimate individual-level residential expo-sure. Am. J. Epidemiol. 164:200–207.