行政院國家科學委員會專題研究計畫 成果報告
新化學鍍活化液合成與電化學特性之研究(II)(第 2 年)
研究成果報告(完整版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 98-2221-E-151-033-MY2 執 行 期 間 : 99 年 08 月 01 日至 100 年 07 月 31 日 執 行 單 位 : 國立高雄應用科技大學化學工程與材料工程系 計 畫 主 持 人 : 李建良 報 告 附 件 : 出席國際會議研究心得報告及發表論文 處 理 方 式 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢中 華 民 國 100 年 09 月 17 日
行政院國家科學委員會補助專題研究計畫
■ 成 果 報 告
□期中進度報告
新化學鍍活化液合成與電化學特性之研究(Ⅱ)
計畫類別:■個別型計畫 □整合型計畫
計畫編號: NSC 98-2221-E-151-033-MY2
執行期間: 98 年 8 月 1 日至 100 年 7 月 31 日
執行機構及系所:國立高雄應用科技大學 化學工程與材料工程系
計畫主持人:李建良
共同主持人:
計畫參與人員:碩士班研究生- 吳振中、張坤全、許秋美、許嘉傑、邱雪萍
陳致豪、楊昊霖、黃俊翰
成果報告類型(依經費核定清單規定繳交):□精簡報告 ■完整報告
本計畫除繳交成果報告外,另須繳交以下出國心得報告:
□赴國外出差或研習心得報告
□赴大陸地區出差或研習心得報告
■出席國際學術會議心得報告
□國際合作研究計畫國外研究報告
處理方式:
除列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年■二年後可公開查詢
中 華 民 國 100 年 8 月 1 日
Abstract
1. Synthesis of Highly Active Ag/Pd Nanorings for Activating Electroless Copper Deposition
A new and simple method for preparing Ag/Pd nanorings is reported. Energy-dispersive X-ray element mapping results showed a random distribution of Ag and Pd atoms over the nanoring, indicating that the nanorings were made of an alloy of Ag and Pd. These nanorings were then successfully used as catalysts in electroless copper deposition. Results of quartz crystal microbalance measurements revealed that the catalytic activity of prepared Ag/Pd nanorings was better than that of monometallic Ag and Pd nanoparticles.
2. Porous Ag-Pd Triangle Nanoplates with Tunable Alloy Ratio for Catalyzing Electroless Copper Deposition
Porous triangular Ag-Pd nanoplates of various bimetallic ratios were prepared by a galvanic displacement reaction in which Pd(OAc)2 reacted with Ag nanoplates. In order to reduce the interaction of free hexadecyltrimethylammonium bromide (CTAB) with the synthesized Ag-Pd nanoplates during this reaction, Ag nanoplate/CTAB solution was precipitated by high-speed centrifugation before synthesizing the porous bimetallic nanotemplates. The kinetics of electroless copper deposition (ECD) catalyzed by these bimetallic nanoplates are analyzed by using a quartz crystal microbalance (QCM). The results reveal that Ag18Pd1 nanoplates show better catalytic activity than Ag18Pd1.5 and Ag18Pd2 nanoplates. Further, the catalytic activity of the porous nanoplates in the ECD bath can be controlled by varying their alloy ratios.
3. Electrochemical synthesis of Pd–NiO nanoparticles in water-in-oil microemulsions for activating electroless Ni deposition
Dispersions of Pd–NiO nanoparticles were electrochemically synthesized in a water-in-oil microemulsion, with fine control by appropriate adjustment of the concentration ratio of H2O to surfactant. The feasibility of using these Pd–NiO nanoparticles as a new activator for electroless Ni deposition (END) was examined. In this study, fast electroless deposition was achieved using Pd–NiO nanoparticles as a catalyst. A systematic study of the deposition kinetics and the mass activity by electrochemical quartz-crystal microgravimetry indicated that adding NiO to active colloids can shorten the induction time, and that reducing the particle diameter can accelerate END reactions because steady-state deposition is achieved more quickly.
目錄
Abstract...I 目錄... II
1. Synthesis of Highly Active Ag/Pd Nanorings for Activating Electroless Copper Deposition... 1
1.1 Introduction... 1
1.2 Experimental ... 1
1.3 Results and discussion ... 2
1.4 Conclusion ... 5
1.5 Acknowledgement ... 5
1.6 References ... 5
2. Porous Ag-Pd Triangle Nanoplates with Tunable Alloy Ratio for Catalyzing Electroless Copper Deposition ... 7
2.1 Introduction... 7
2.2 Experimental ... 7
2.3 Results and discussion ... 8
2.4 Conclusion ... 12
2.5 Acknowledgement ... 12
2.6 References ... 12
3. Electrochemical synthesis of Pd–NiO nanoparticles in water-in-oil microemulsions ... 13
for activating electroless Ni deposition... 13
3.1 Introduction... 13
3.2 Experimental ... 14
3.3 Results and discussion ... 15
3.4 Conclusion ... 21
3.5 Acknowledgements ... 21
1. Synthesis of Highly Active Ag/Pd Nanorings for Activating Electroless Copper
Deposition
1.1 Introduction
Metallic nanoparticles of different shapes have the potential to be used as catalysts in organic 1-3 and electrochemical reactions 1,4. For example, Pt nanoparticles of various shapes (size: 5 nm) are used as catalysts in the electron-transfer reactions between [Fe(CN)6]3– and S2O32– 5. Transmission electron microscopy (TEM) analysis shows that the percentage of atoms located at the corners and edges in tetrahedral, cubic, and truncated octahedral Pt nanocrystal are approximately 35%, 13%, and 6% of the total number of surface atoms, respectively. The catalytic rate constants increase exponentially with the percentage of corner and edge atoms 5. The other reaction involved shape-dependent catalysis is hydrogenation reaction. The conversion percentages of ethylene to ethane in the presence of cubic, cuboctahedral, and porous Pt nanoparticles as catalysts are compared, and it is found that the porous nanoparticles are more active than the cubic and cuboctahedral nanoparticles 6. Metal nanoparticles are often used as catalysts in electrochemical reactions. Ag nanowires 7 show excellent catalytic activities and go toward the four electron oxygen reduction path, which can enhance significantly the working efficiency of the fuel cell. Hollow Ag/Pd nanoshells have been found to show unique catalytic activities when used as catalysts for electroless copper deposition (ECD) 8,9. ECD has gained considerable significance in the field of nanocircuits 10. The mechanism underlying ECD involves activation, which is a catalytic reaction triggered by active colloids on the surface of the substrates dipped into the electroless bath 11,12. The active catalyst acts as an electron carrier for the transfer of electrons from the reducing agent to the copper ions. Hence, the structure and composition of the catalyst can alter the deposition rate and reaction kinetics 13. In a previous study, the authors prepared spherical Pd nanoparticles and used them as activators for ECD 11,13,14. Additionally, for the electroless reaction, the authors synthesized solid nanocatalysts with Pd-rich shells from a Ag/Pd alloy by adding Ag particles to the microstructures of Pd nanoparticles; the nanocatalysts thus prepared showed enhanced catalytic activity 15.
In the present study, Ag/Pd nanorings were prepared via a displacement reaction; in this reaction, a small amount of palladium acetate (Pd(OAc)2) dissolved in acetate acid (CH3COOH) was allowed to galvanically react with Ag nanoparticles. A small amount of the deposited Pd alloyed with Ag. The displacement process resulted in the formation of bimetallic nanorings. Energy-dispersive X-ray (EDX) element mapping was employed to measure the relative atomic distribution of Ag to Pd in the microstructure of the synthesized nanoring. The synthesized Ag/Pd nanorings were used as activators for ECD. Ag/Pd nanorings, pure Pd, and Ag nanoparticles were used as catalysts, and their effects on the kinetics of ECD were compared using results from quartz crystal microbalance (QCM) measurements.
1.2 Experimental
Ag/Pd nanorings were prepared in the following manner: initially, 25 μmoles of silver nitrate was gradually dissolved in 3 ml of a neutral aqueous solution of 0.1 M sodium n-dodecyl sulphate (SDS). Approximately 25 μl of 0.1 M NaBH4 was added dropwise to the abovementioned solution in order to reduce the Ag ions to metallic Ag. The solution temperature was maintained at 30 °C. After 10 min, Ag nanoparticles were obtained. Approximately 2.496 mg of Pd(OAc)2 was gradually dissolved in 1 ml of pure
CH3COOH to afford an acidic Pd2+ solution, which was used for the subsequent galvanic displacement reaction. Approximately 50 µl of this solution was added to 3 ml of a well-stirred solution of Ag nanotemplates at a constant temperature of 30 °C. After 70 min, the dispersed nanorings were collected. For a fair comparison of the catalytic properties, Ag and Pd nanoparticles were produced by the reduction of 25 μmoles of Ag ions and 0.56 μmoles of Pd ions, respectively, in 0.1 M SDS solution (3 ml).
The prepared nanoparticle solutions were placed on a carbon-coated copper grid and allowed to dry naturally; the characteristic shape and composition of the nanorings were then analyzed by a TEM (JEOL JEM-3000F) and EDX element mapping. X-ray diffraction (XRD) patterns of the prepared Ag/Pd nanorings were obtained using XRD spectroscopy (Shimadzu XD-3A apparatus equipped with a Cu anode).
The prepared Ag/Pd nanorings and solid Ag and Pd nanoparticles were then used as activators for ECD. In order to reduce the interaction of free SDS with the obtained nanoparticles during the ECD reaction, 1 ml of the nanoparticle solution was precipitated by high-speed centrifugation (15000 rpm) and redispersed in 1 ml of H2O; this process helped eliminate the free SDS. The electroless bath for the QCM measurements was prepared from 0.44 M formaldehyde, 0.1 M EDTA, and 0.05 M CuSO4. The pH of the electroless bath was adjusted to 12.3 using NaOH powder. Prior to the measurements, the electroless baths were maintained at 30 °C and bubbled with N2 for 15 min. The working electrode used for the QCM (Seiko EG&G QCA922) measurement was prepared by coating 0.159 cm2 of the Au surface of the QCM substrate with a uniform layer comprising of the nanoparticle solution (2 µl). Au was sputtered on both sides of a Ti film (thickness: 10 nm) in the QCM substrate (Seiko EG&G QA20-A9M-Au), which was connected to an oscillator manufactured in-house. A Luggin capillary filled with saturated KCl solution separated the reference electrode (Hg/Hg2Cl2) from the compartment that housed the electrolyte solution.
1.3 Results and discussion
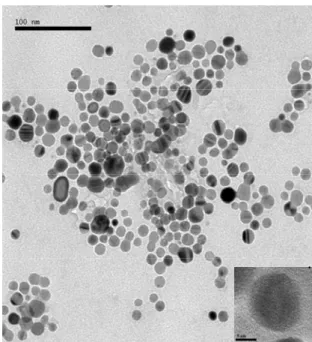
Figure 1A shows a typical TEM image of the morphorlogy of the as-synthesized nanomaterials. The nanorings were formed after Pd(OAc)2 was displaced with Ag nanoparticles, whose TEM image is shown in Figure 1B. The TEM observations showed that Ag/Pd nanorings were successfully prepared by using the proposed method.
Figure 2 shows the XRD spectra of the prepared nanorings. The four peaks at 38.72°, 44.65°, 65.18°, and 78.18° were assigned to (111), (200), (220), and (311) diffraction planes, respectively, of the face-centered cubic (fcc) structure. The (111) diffraction peak of the Ag/Pd nanorings prepared by the displacement method was located between the (111) diffraction peaks of Ag and Pd in the standard spectrum. However, discrete and independent Ag and Pd peaks were not observed in the spectra of the nanorings. This implied that the nanorings prepared by using the proposed method were made of an alloy of Ag and Pd.
A. B.
. .
Figure 1. (A) TEM image of Ag/Pd nanorings prepared by this displacement reaction. (B) TEM image of Ag nanoparticles (the insert: high resolution TEM image).
30 40 50 60 70 80 220 200 111 Pd In te nsit y ( a rb itr a l unit ) 2-Theta (degrees) 088-2335 311 220 200 111 Ag 87-0597 78.188 65.176 44.653 38.718 Nanoring
Figure 2. XRD pattern of Ag/Pd nanorings, and standard XRD patterns of Ag and Pd.
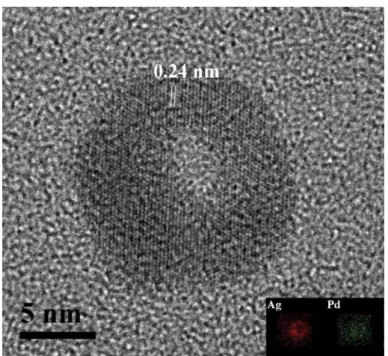
Additional supporting information on composition architecture and atomic distribution of the nanorings was obtained by the EDX element mapping. The inset image in Figure 3 presents the EDX patterns obtained from the element mapping of the nanorings while the high-resolution TEM (HR-TEM) image of the nanorings
(Fig. 3) was captured. Two signals with uniform distribution were obtained from the nanomaterials, and these were identified to be due to Ag and Pd. The exact composition and alloy components of the synthesized nanorings were thus determined.
HR-TEM was employed to provide deeper insights into the mechanism underying the formation of the Ag/Pd nanorings. Figure 3 shows a HR-TEM image in which the value of one lattice fringe spacing was detected to be approximately 0. 24 nm. This value was slightly lower than the lattice fringe spacing of the Ag (100) face. The deposition of Au on Ag nanoparticles during the displacement reaction 16 occurred initially and preferentially on high-energy {100} facets, and simultaneously, Ag dissoluted from the {111} facets. The Au atoms depsoited on the {100} facets quickly alloyed with the underlying Ag atoms. The alloy composition was obtained by EDX element mapping; from the EDX results, the atomic distribution in the nanoring was found to be uniform. The low lattice spacing of 0. 24 nm corresponding to the Ag (100) plane was plausibly due to alloy formation between Pd and Ag.
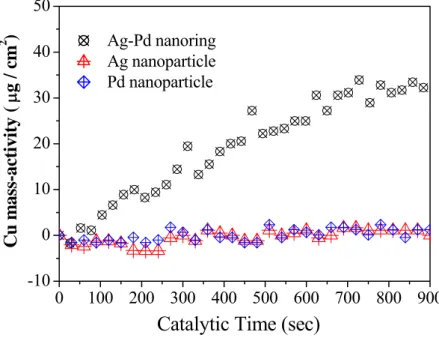
Figure 3. High resolution TEM image, and element mapping EDX patterns of Ag/Pd nanorings The prepared Ag/Pd nanorings were tested for their effectiveness as activators for ECD. Figure 4 shows a comparison of the results of QCM analyses carried out after in situ measurement of the catalytic activities of the Ag/Pd nanorings and the Ag and Pd nanoparticles in ECD. The deposition rates shown in Fig. 4 were recalculated from the changes in the electrode frequency by using Sauerbrey’s equation 11. Ag nanoparticles and Pd nanoparticles were found to be inactive for ECD.
The average ECD deposition rate observed during the preparation of the Ag/Pd nanorings was approximately 0.0357 μg⋅cm-2⋅s-1. The catalytic activity of the nanocatalysts was calculated from to the following relation:
catalytic activity = deposition rate/(weight of the alloy catalyst).
Here, the weight of the Ag/Pd nanorings used as catalysts on the QCM electrode was estimated to be approximately 8 μg. The catalytic activity of the nanorings was 4.46 × 10–3 cm-2⋅s-1, thereby indicating that the
nanorings prepared by the proposed method can be successfully employed as catalysts for ECD. The enhanced activity was plausibly from an increase on the reactive area of the nanoring with a pore.
0 100 200 300 400 500 600 700 800 900 -10 0 10 20 30 40 50 Ag-Pd nanoring Ag nanoparticle Pd nanoparticle C u m a ss -acti v ity ( μ g / cm 2 )
Catalytic Time (sec)
Figure 4. Comparative QCM curves of ECD kinetic catalyzed with Ag-Pd nanorings, Ag nanoparticles, and Pd nanoparticles.
1.4 Conclusion
Ag/Pdnanorings were successfully prepared by a galvanic displacement reaction, in which a small amount of Pd(OAc)2 dissolved in CH3COOH was allowed to react slowly with previously synthesized Ag nanoparticles, which acted as templates. The EDX element mapping showed that the prepared nanorings were alloys of Ag and Pd; these nanorings showed better catalytic activity for ECD than did Ag and Pd nanoparticles. These Ag/Pd nanorings thus constitute a novel class of catalysts for ECD.
1.5 Acknowledgement
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 98-2221-E-151-033-MY2.
1.6 References
1 Y. J. Xiong, B. Wiley, and Y. N. Xia, Angew. Chem.-Int. Edit. 46, 7157 (2007). 2 R. Xu, D. S. Wang, J. T. Zhang, and Y. D. Li, Chem.- Asian J. 1, 888 (2006). 3 A. R. Tao, S. Habas, and P. D. Yang, Small 4, 310 (2008).
4 N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding, and Z. L. Wang, Science 316, 732 (2007). 5 R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B 108, 5726 (2004).
6 K. M. Bratlie, H. Lee, K. Komvopoulos, P. D. Yang, and G. A. Somorjai, Nano Lett. 7, 3097 (2007). 7 K. Ni, L. Chen, and G. X. Lu, Electrochem. Commun. 10, 1027 (2008).
8 C. L. Lee, C. M. Tseng, S. C. Wu, R. B. Wu, and K. R. Yang, Electrochim. Acta 53, 5905 (2008). 9 C. L. Lee, C. M. Tseng, S. C. Wu, and R. B. Wu, Electrochem. Solid State Lett. 11, D27 (2008).
10 H. Akahoshi, M. Kawamoto, T. Itabashi, O. Miura, A. Takahashi, S. Kobayashi, M. Miyazaki, T. Mutoh, M. Wajima, and T. Ishimaru, Ieee Trans. Compon. Packaging Manufacturing Technol. A 18, 127 (1995).
11 C. L. Lee, Y. C. Huang, and L. C. Kuo, J. Solid State Electrochem. 11, 639 (2007).
12 C. L. Ma, W. C. Ye, X. Z. Shi, Y. L. Chang, Y. Chen, and C. M. Wang, Appl. Surf. Sci. 255, 3713 (2009).
13 C. L. Lee, Y. C. Huang, C. C. Wan, Y. Y. Wang, Y. J. Ju, L. C. Kuo, and J. C. Oung, J. Electrochem.
Soc. 152, C520 (2005).
14 C. L. Lee and Y. C. Huang, Electrochem. Solid State Lett. 9, C196 (2006). 15 C. L. Lee, Y. C. Huang, and L. C. Kuo, Electrochem. Commun. 8, 1021 (2006).
2.
Porous Ag-Pd Triangle Nanoplates with Tunable Alloy Ratio for Catalyzing
Electroless Copper Deposition
2.1 Introduction
Electroless copper deposition (ECD) has gained considerable importance in view of the advancement in the circuit industry [1–3]. ECD is frequently used to deposit conductive copper on an insulating substrate. Activation, which is a catalytic reaction, is induced by active colloids present on the surface of substrates that are dipped into the ECD bath. An active catalyst acts as an electron carrier and transfers electrons from the reducer to the metal ions. Accordingly, the structure and composition of the activator can influence the deposition rate and the reaction kinetics [4–9]. Porous metallic nanoparticles are promising candidates for catalysis [10, 11]. In a previous study, the present authors prepared mesoporous Pd nanoparticles by chemical reduction and used these nanoparticles as activators for ECD [4, 12]. The activity of Ag-Pd alloy nanocatalysts with Pd-rich shells is improved when Ag is added to the microstructure of Pd nanoparticles [13]. In addition, large triangular Ag-Pd nanoplates show unique chemical characterization when they catalyzed ECD bath [14]. In this study, porous Ag-Pd triangular nanoplates with three alloy ratios were synthesized by a galvanic displacement reaction in which Ag triangular nanoplates reacted with Pd ions. These Ag-Pdnanoplates were then used as potential ECD activators. Quartz crystal microbalance (QCM) measurements were used to compare the ECD activities of (1) three Ag-Pd nanoplates with different compositions, (2) Ag nanoplates, and (3) Pd nanoparticles.
2.2 Experimental
Triangular Ag-Pd nanoplates with different compositions were prepared as follows. Initially, 0.05 ml of 0.05 M AgNO3 aqueous solution was added to 10 ml of 2.5 × 10-4 M aqueous sodium citrate solution. Then, 0.025 ml of 0.1 M NaBH4 was gradually added to a stirred solution of sodium citrate and AgNO3; a light yellow Ag seed solution was obtained. Then, 1 ml of 0.05 M AgNO3 was added to 200 ml of 0.1 M hexadecyltrimethylammonium bromide (CTAB). Next, 10 ml of 0.1 M ascorbic acid and 0.182 ml of the prepared Ag seed solution were slowly added to the aqueous solution of CTAB. Finally, 0.8 ml of 2 M NaOH aqueous solution was added to this CTAB solution to synthesize Ag triangular nanotemplates. A 200-ml solution containing the triangular Ag nanoplates synthesized earlier was precipitated by centrifugation at 4,000 rpm and redispersed in 30 ml of deionized water; this was done to reduce the interaction of free CTAB with the synthesized Ag-Pd nanoplates. Then, to prepare Ag18Pd1 nanoplates, ~1.25 mg of Pd(OAc)2 slowly dissolved in 1 ml of pure acetic acid to obtain 5.56 × 10-3 M Pd2+ solution for the galvanic displacement reaction. At a fixed controlled temperature of 60°C, ~50 µl of 5.56 × 10-3 M Pd(OAc)2 (2.78 × 10-7 moles) solution was added to 3 ml of a stirred solution of Ag nanoplates that did not contain free CTAB. Porous Ag-Pd nanoplates with an Ag–Pd molar ratio of 18:1 were obtained after 60 min. The pathway for this displacement synthesis is shown in Figure 1. Ag18Pd1.5 or Ag18Pd2 nanoplates can be obtained by using 50 µl of Pd(OAc)2 solution with concentrations of 8.33 × 10-3 M (4.17 × 10-7 moles) and 1.112 × 10-2 M (5.56 × 10-7 moles), respectively. Ag-Pd nanoplates were dipped onto the copper grid that was covered with a carbon film and dried naturally. The characteristic size and shape of the nanoplates were observed under a high-resolution transmission electron microscope (HRTEM; JEOL JEM-3000F) and with an energy-dispersive X-ray (EDX) spectroscope.
Electrochemical Synthesis of Pd/Ni Nanoparticles and Their Application as Catalyst for Electroless Ni Deposition
Chen-Chung Wu, Chia-Chieh Syu, Chia-Chen Yang, Chien-Liang Lee*
Department of Chemical and Materials Engineering, National Kaohsiung University of Applied Science,
Kaohsiung 807, Taiwan
cl_lee@url.com.tw
Electroless nickel deposition is a catalytic reaction and an important industrial surface treatment method owing to its good corrosive protection, hardness and wear resistance [1]. Although the performance of an electroless process is influenced by numerous factors, including the composition of the deposition solution [2] and the choice of ligands [3], catalysis is the key factor in controlling the rate and mechanism of
electroless deposition [4]. (C) (B) (E) (A) (D)
In this study, Pd/Ni nanoparticles of various diameters were prepared in an electrochemical cell composed of two anodes and one cathode, at which both of Pd and Ni foil were simultaneously used as anodic
electrodes and Pt foil was used as cathodic foil. Their images observed by transmission electron microscope (TEM) are shown in Figure 1.
These alloy nanoparticles are electroreduced from nickel and palladium ion dissolved from Pd and Ni anodes and their diameters were controlled from 2.87 nm to 7.51 nm by the added H2O amount in a
tetrahydrofuran electrolyte containing micelles of tetraoctylammonium bromide (TOAB). The size of TOAB micelle has to be regulated. As the H2O amount
increased, the structure of the TOABmicelle changed; this caused the Pd/Ni nanoparticles to grow and be easily oxidized to NiO on the surface. This result was confirmed by X-ray photoelectron spectrum. Pd/Ni nanoparticles with oxidized surface were thus obtained.
Then, these alloy nanoparticles were tested as a
novel activator for electroless nickel deposition and the activities were in-situly measured by electrochemical quartz crystal microbalance. In a comparison between
the activities of Pd/Ni nanoparticles, the smallest Figure 1. TEM images of electroreduced Pd/Ni nanoparticles in TOAB/THF solution with various H2O amount. (A) 0 μl; (B) 20 μl; (C) 40 μl; (D) 80
μl; (E) 100 μl nanoparticles had maximum activity, which
reached to 1.84 × 10-2 (1/cm-2s-1).
References [1]. R. L. Zeller, Corrosion 50 (1994) 457.
[3]. G. F. Cui, N. Li, D. Y. Li, M. Li, J. Electrochem. Soc. 152 (2005) C669. [4]. J. F. Hamilton , R. C. Baetzold Science 205 (1979) 1213.
國科會補助計畫衍生研發成果推廣資料表
日期:2011/09/17國科會補助計畫
計畫名稱: 新化學鍍活化液合成與電化學特性之研究(II) 計畫主持人: 李建良 計畫編號: 98-2221-E-151-033-MY2 學門領域: 電化學無研發成果推廣資料
98 年度專題研究計畫研究成果彙整表
計畫主持人:李建良 計畫編號:98-2221-E-151-033-MY2 計畫名稱:新化學鍍活化液合成與電化學特性之研究(II) 量化 成果項目 實際已達成 數(被接受 或已發表) 預期總達成 數(含實際已 達成數) 本計畫實 際貢獻百 分比 單位 備 註 ( 質 化 說 明:如 數 個 計 畫 共 同 成 果、成 果 列 為 該 期 刊 之 封 面 故 事 ... 等) 期刊論文 0 0 100% 研究報告/技術報告 1 1 100% 研討會論文 1 1 100% 篇 論文著作 專書 0 0 100% 申請中件數 0 0 100% 專利 已獲得件數 0 0 100% 件 件數 0 0 100% 件 技術移轉 權利金 0 0 100% 千元 碩士生 6 6 100% 博士生 0 0 100% 博士後研究員 0 0 100% 國內 參與計畫人力 (本國籍) 專任助理 0 0 100% 人次 期刊論文 11 11 100% 研究報告/技術報告 0 0 100% 研討會論文 1 1 100% 篇 論文著作 專書 0 0 100% 章/本 申請中件數 0 0 100% 專利 已獲得件數 0 0 100% 件 件數 0 0 100% 件 技術移轉 權利金 0 0 100% 千元 碩士生 0 0 100% 博士生 0 0 100% 博士後研究員 0 0 100% 國外 參與計畫人力 (外國籍) 專任助理 0 0 100% 人次其他成果