DOI: 10.1126/science.1234369
, 174 (2013);
340
Science
et al.
Yu-Te Su
OO
2
CH
Infrared Absorption Spectrum of the Simplest Criegee Intermediate
This copy is for your personal, non-commercial use only.
clicking here.
colleagues, clients, or customers by
, you can order high-quality copies for your
If you wish to distribute this article to others
here.
following the guidelines
can be obtained by
Permission to republish or repurpose articles or portions of articles
):
April 25, 2014
www.sciencemag.org (this information is current as of
The following resources related to this article are available online at
http://www.sciencemag.org/content/340/6129/174.full.html
version of this article at:
including high-resolution figures, can be found in the online
Updated information and services,
http://www.sciencemag.org/content/suppl/2013/04/10/340.6129.174.DC1.html
can be found at:
Supporting Online Material
http://www.sciencemag.org/content/340/6129/174.full.html#related
found at:
can be
related to this article
A list of selected additional articles on the Science Web sites
http://www.sciencemag.org/content/340/6129/174.full.html#ref-list-1
, 2 of which can be accessed free:
cites 41 articles
This article
http://www.sciencemag.org/content/340/6129/174.full.html#related-urls
1 articles hosted by HighWire Press; see:
cited by
This article has been
http://www.sciencemag.org/cgi/collection/chemistry
Chemistry
subject collections:
This article appears in the following
registered trademark of AAAS.
is a
Science
2013 by the American Association for the Advancement of Science; all rights reserved. The title
Copyright
American Association for the Advancement of Science, 1200 New York Avenue NW, Washington, DC 20005.
(print ISSN 0036-8075; online ISSN 1095-9203) is published weekly, except the last week in December, by the
Science
on April 25, 2014
www.sciencemag.org
Downloaded from
on April 25, 2014
www.sciencemag.org
Downloaded from
on April 25, 2014
www.sciencemag.org
Downloaded from
on April 25, 2014
www.sciencemag.org
Downloaded from
Infrared Absorption Spectrum of the
Simplest Criegee Intermediate CH
2
OO
Yu-Te Su,1Yu-Hsuan Huang,1Henryk A. Witek,1* Yuan-Pern Lee1,2*
The Criegee intermediates are carbonyl oxides postulated to play key roles in the reactions of ozone with unsaturated hydrocarbons; these reactions constitute an important mechanism for the removal of unsaturated hydrocarbons and for the production of OH in the atmosphere. Here, we report the transient infrared (IR) absorption spectrum of the simplest Criegee intermediate CH2OO, produced from CH2I + O2in a flow reactor, using a step-scan Fourier-transform
spectrometer. The five observed bands provide definitive identification of this intermediate. The observed vibrational frequencies are more consistent with a zwitterion rather than a diradical structure of CH2OO. The direct IR detection of CH2OO should prove useful for kinetic
and mechanistic investigations of the Criegee mechanism.
T
he gaseous reactions of ozone (O3) withunsaturated hydrocarbons have been ex-tensively investigated, given their roles in atmospheric depletion of these molecules and as-sociated build-up of OH free-radical and partic-ulate material in the troposphere (1–3). In summer, the dominant OH-production channel in the at-mosphere includes photolysis of ozone to produce O(1D), which subsequently reacts with H2O to
produce OH. During the winter season, the effi-ciency of ozone photolysis drops by 50% or more, and the atmospheric production of OH via ozon-olysis of alkenes was proposed to account for the difference in the photolytic production of OH be-tween summer and winter (4).
Decades of research suggest the initiation of the ozonolysis reactions involves the cycloaddi-tion of ozone to the C=C double bond to form a cyclic trioxolane intermediate (ozonide) with a C−C single bond. The large exothermicity of this reaction leads to a rapid cleavage of this C−C bond and one O−O bond of the ozonide to form a car-bonyl molecule and a carcar-bonyl oxide that is com-monly referred to as the Criegee intermediate, which was first postulated by Criegee in 1949 (5). The simplest ozone-alkene reaction involves ethene (C2H4); the products from fragmentation of ethene
ozonide (C2H4O3) are formaldehyde (H2CO) and
formaldehyde oxide (or peroxymethylene, CH2OO),
which is hence the simplest Criegee intermediate. The structure and reactions of gaseous Criegee intermediates have been extensively investigated and debated (6–9). Four isomers of CH2O2include
formaldehyde oxide, dioxirane, methylenebis(oxy), and formic acid (HCOOH), as shown in Fig. 1. The large exothermicity of the reaction of O3+ C2H4
might lead to isomerization among these isomers and the decomposition of these species to produce H, OH, CH3, CO, CO2, and other products (1–3).
The highly reactive Criegee intermediates have until recently eluded detection in the gaseous phase. Taatjes and co-workers produced CH2OO from the
reactions of CH3SOCH2+ O2(10) and CH2I + O2
(11) in a flow cell and detected its cation with vac-uum ultraviolet photoionization. They confirmed that the Criegee intermediate, rather than other isomers, was observed because the observed pho-toionization threshold near 10 eV conforms to theoretical predictions of 9.98 eV (12), which is much smaller than the values of 10.82 eV pre-dicted for dioxirane (12) and 11.3 eV determined for formic acid (13). Beames et al. used the CH2I +
O2reaction to prepare CH2OO in a supersonic jet
and reported that a broad ultraviolet (UV) spec-trum of CH2OO peaked near 335 nm; the
spec-trum was obtained through UV-induced depletion of the ion signal of CH2OO produced upon
pho-toionization (14). The infrared (IR) absorption spectrum of gaseous CH2OO would supply more
detailed structural information as well as an alter-native means for performing kinetic measurements. Theoretical investigations of the structure and reactivity of CH2OO have been extensive
(12, 15–18), but predictions of the enthalpy of formation, electronic structure, and vibrational wave numbers vary considerably. The reported enthalpy of formation of CH2OO at 298 K,DHf0, varies
from 26 to 48 kcal mol−1but settles toward the smaller value when more sophisticated methods are used. Earlier theoretical calculations indicated CH2OO to have a planar, singlet biradical structure,
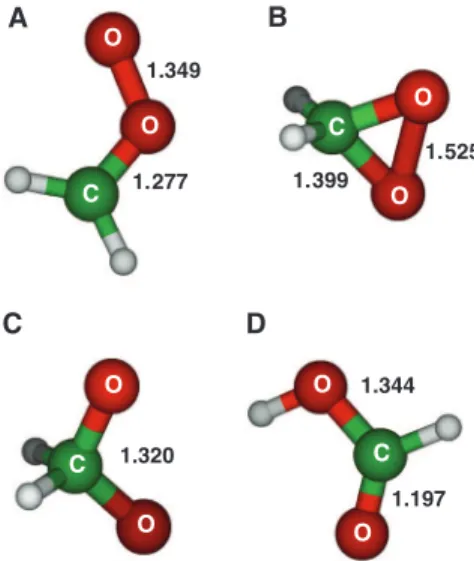
with nearly equally long O−O and C−O bonds of about 1.34 Å (19), whereas coupled-cluster the-ory CCSD(T) and multiconfigurational complete active space self-consistent field (CASSCF) calcu-lations predicted that CH2OO is better described as a
zwitterion with a shorter C−O bond (~1.28 Å) and a longer O−O bond (~1.35 Å) (Fig. 1A) (12, 16, 17). The vibrational frequencies predicted for CH2OO
also vary considerably. For example, predictions of the wave number of the O−O stretching mode ranged from 849 to 1077 cm−1, and of the C−O stretching mode from 1269 to 1407 cm−1.
Because CH2OO is unstable, its detection with
a conventional Fourier-transform IR (FTIR) spec-trometer is difficult. We have demonstrated that coupling a step-scan FTIR spectrometer with a multipass absorption cell enables the recording of temporally resolved IR absorption spectra of gas-eous reaction intermediates such as ClCO (20)
and CH3OO (21); distinct absorption bands of
various isomers of CH3SO2(22), CH3SOO (23), and
CH3OSO (24) were recorded to provide
defini-tive structural identification. Here, we report a fur-ther application of this technique to characterize the IR absorption spectra of gaseous CH2OO species.
A step-scan FTIR [Vertex 80v (Bruker Optik, Ettlingen, Germany)] spectrometer coupled with a multireflection White cell was used to record the IR spectra of transient species. The laser beam, of wavelength 248 nm, passed through the White cell and was reflected six times with two external mir-rors so as to photodissociate a flowing mixture of CH2I2in N2/O2and thereby produced CH2I that
subsequently reacted with O2to form CH2OO. The
derivation of conventional time-resolved difference absorption spectra from the temporal profiles recorded at each scan step has been established (20, 25).
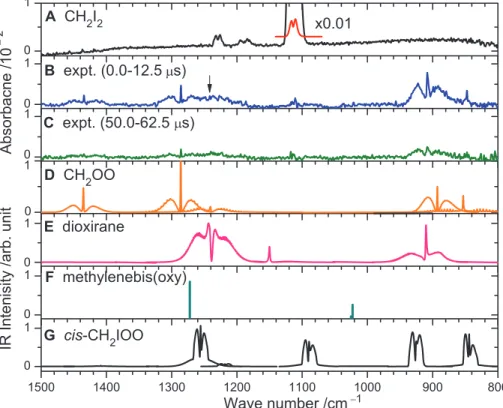
The partial IR absorption spectrum (800 to 1500 cm−1) of the flowing mixture of CH2I2/N2/O2
(1/20/760, 94 torr) at 340 K (Fig. 2A) exhibits absorption lines of CH2I2near 1229, 1188, and
1113 cm−1. Upon irradiation with light at 248 nm, the absorption of CH2I2decreased owing to
pho-tolysis, whereas new bands near 1435, 1286, 908, and 848 cm−1appeared, as shown in the difference spectrum recorded at 0- to 12.5-ms delays (Fig. 2B); a band with a weak Q-branch near 1241 cm−1 (indicated by an arrow) might belong to the same group but is interfered with by absorption of parent or other products. The intensities of these new lines decreased rapidly with time and diminished after ~100ms; a spectrum recorded 50 to 62.5 ms after photo-irradiation is shown in Fig. 2C. The decay of CH2OO resulted in increased absorption of H2CO.
Because photolysis of CH2I2produces mainly
the CH2I radical (26) and because this radical
re-acts readily with excess O2in the system (27, 28), the
possible carriers of the observed new bands in-clude CH2IOO and isomers of CH2O2. Most
pre-1
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, 1001, Ta-Hsueh Road, Hsinchu 30010, Taiwan.2Institute of Atomic and Molecular Sci-ences, Academia Sinica, Taipei 10617, Taiwan.
*Corresponding author. E-mail: yplee@mail.nctu.edu.tw (Y.-P.L.); hwitek@mail.nctu.edu.tw (H.W.) A 1.349 1.277 B 1.525 1.399 D 1.344 1.197 C 1.320 O O O O O O O O C C C C
Fig. 1. Geometries of possible isomers of CH2O2.
(A) Formaldehyde oxide CH2OO, (B) dioxirane, (C)
methylenebis(oxy), and (D) formic acid predicted with the NEVPT2/aVTZ method [(A) to (C)] and the B3LYP/aVTZ method (D). The O–O and C–O bond distances are given in angstroms.
12 APRIL 2013 VOL 340 SCIENCE www.sciencemag.org
174
vious experimental attempts to generate and detect CH2OO used either the reaction of CH2+ O2or
C2H4+ O3, but the large exothermicity of these
reactions makes the stabilization of CH2OO
dif-ficult. In contrast, in the reaction of CH2I + O2→
CH2OO + I chosen here, the stabilization of CH2OO
is possible because of the small exothermicity of ~13 kJ mol−1.
The observed vibrational wave numbers and relative intensities are compared with theoretical predictions for CH2OO in Table 1. Previous
in-vestigations (12, 18) reported only harmonic vibrational frequencies, so we performed new calculations in order to derive the anharmonic frequencies. The potentially zwitterionic charac-ter of the CH2OO molecule requires appropriate
multireference treatment. The harmonic and an-harmonic vibrational frequencies have been com-puted by using a quadratic force field obtained with then-electron valence state perturbation the-ory (NEVPT2) method (29) implemented in the Molpro quantum chemistry package (30) by using
the CASSCF(8,8) reference wave function. No symmetry has been used in these calculations so as to avoid numerical problems. The anharmonic frequencies (31) have only approximate charac-ter because the effects of three- and four-mode couplings have been neglected owing to high com-putational complexity. Nevertheless, the computed NEVPT2/aVDZ anharmonic frequencies corre-spond well to the observed experimental bands.
The IR spectra of CH2OO, dioxirane,
methylenebis(oxy), and cis-CH2IOO simulated
according to the geometries and anharmonic vibrational frequencies that were predicted with quantum-chemical calculations are shown in Fig. 2, D to G, respectively. A compilation of anhar-monic vibrational levels and IR intensities for the isomers of CH2OO and other possible
interme-diate structures used in the simulations is given in tables S1 and S2. The rotational constants used in the simulation are compiled in table S3. For CH2IOO and CH2OO, the characteristic
OO-stretching modes have wave numbers near 900 cm−1. For dioxirane, two intense features near 1238 and 911 cm−1are characteristic of symmetric and antisymmetric CO-stretching modes (17). For methylenebis(oxy), only an anharmonic NEVPT2 (2,2) stick spectrum is shown because the B3LYP (Becke, three-parameter, Lee-Yang-Parr) rotational constants for each fundamental mode could not be assessed (supplementary text). A comparison of the observed new spectral features with these simulated spectra indicates that the best agree-ment in terms of relative intensities and positions is obtained for the predicted spectrum of CH2OO.
The Criegee intermediate is predicted to have in-tense lines at 1458 (52), 1302 (100), 1220 (33), 892 (100), and 853 (31) cm−1; the relative IR in-tensities are listed in parentheses. The observed features are at 1435 (33), 1286 (42), 1241 (39), 908 (100), and 848 (24) cm−1, with typical devia-tions of 5 to 23 cm−1from predicted anharmonic vibrational wave numbers. The predicted spectra of other candidate species disagree with the ob-served spectrum. For example, although dioxirane is predicted to have two intense lines near 1238 and 911 cm−1, near the observed features at 1286 and 848 cm−1 the relative intensities and the rota-tional contours do not match. The widths of the rotational contours of bands of CH2IOO are
predicted to be much smaller than the observed widths because the massive I atom induces small rotational parameters; the possibility that the ob-served new features are due to CH2IOO is
positive-ly eliminated. In contrast, the predicted rotational contours of each vibrational band of CH2OO
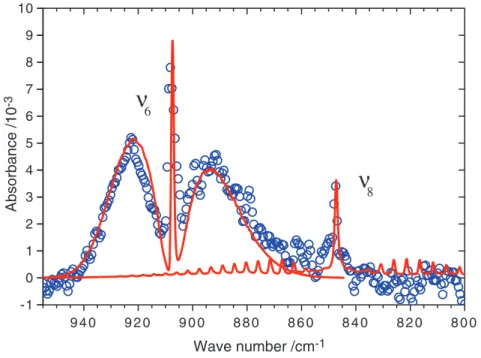
agree well with observations, except for a weak Q-branch near 1241 cm−1that might experience interference from absorption of the precursor or other product. A comparison of observed and simulated rotational contours for then6andn8
modes is shown in Fig. 3; those for then3andn4/n5
modes are shown in figs. S1 and S2, respectively. Most bands of CH2OO have a mixeda/b-type
con-tour with P-, Q-, and R-branches, whereas the out-of-plane CH2-wagging (n8) mode near 848 cm−1
Fig. 2. Comparison of observed spectra with simulated spectra of possible species. (A) IR absorption spectrum of a flowing mixture of CH2I2/N2/O2(1/20/760, 94 torr) before photolysis. (B) Difference spectra
recorded 0 to 12.5ms and (C) 50 to 62.5 ms after irradiation of the sample at 248 nm; the spectrum at 300 to 312.5ms was subtracted for background correction. IR spectra of (D) CH2OO, (E) dioxirane, (F)
methylenebis(oxy), and (G) cis-CH2IOO were simulated by using the predicted rotational constants,
vibrational frequencies, and IR intensities listed in tables S1 and S3.
Table 1. Comparison of experimentally observed wave numbers (per centimeter) and intensities with the vibrational wave numbers (per centimeter) and IR intensities of CH2OO predicted with various methods.
Sym., symmetry.
Mode Sym. Experiment NEVPT2/aVDZ CAS(14,12) CCSD(T) Description† Harmonic Anharmonic /VDZ* /aVTZ*
n1 Aʹ 3370 3149 (5)‡ 3215 3290 a-CH str. n2 Aʹ 3197 3030 (1) 3065 3137 s-CH str. n3 Aʹ 1435 (33)§ 1500 1458 (52) 1465 1483 CH2scissor/CO str. n4 Aʹ 1286 (42) 1338 1302 (100) 1269 1306 CO str./CH2scissor n5 Aʹ 1241 (39) 1235 1220 (33) 1233 1231 CH2rock n6 Aʹ 908 (100) 916 892 (100) 849 935 OO str. n7 Aʹ 536 530 (1) 537 529 COO deform n8 Aʺ 848 (24) 856 853 (31) 793 862 CH2wag n9 Aʺ 620 606 (2) 618 632 CH2twist
Reference This work This work This work (18) (12)
*Harmonic wave numbers. †Approximate mode description. a, asymmetric; s, symmetric; str., stretch. ‡Relative IR
intensities are normalized to the most intense line (n4) with intensity 124 km mol−1. §Integrated IR intensities relative to
n6are listed in parentheses.
www.sciencemag.org SCIENCE VOL 340 12 APRIL 2013 175
has a characteristicc-type structure with a promi-nent Q-branch because the dipole moment oscil-lates mainly along thec axis (perpendicular to the molecular plane) upon vibrational excitation. This uniquec-type feature of a planar molecule near 848 cm−1further supports the assignment of the observed features to CH2OO.
The observed wave number of the OO-stretching mode of CH2OO near 908 cm−1is much
smaller than that of the corresponding modes of CH3OO at 1117 cm−1(21), CH3C(O)OO (32) at
1102 cm−1, and C6H5C(O)OO at 1108 cm−1(33)
determined with a similar technique. The observed wave number of the CO-stretching mode near 1286 cm−1is much larger than that of the cor-responding mode of CH3OO at 902 cm−1 (34)
observed in a matrix, indicating some double-bond character. These trends strongly support a zwitterionic, rather than singlet biradical, struc-tural description of CH2OO because of a
strength-ened C−O bond and a weakstrength-ened O−O bond. The ~50-ms lifetime of CH2OO observed in
our experiment is much shorter than that (~2 ms) reported by Welzet al. (11), who used [CH2I]0≅
9×1011molecules cm−3in their experiments. Be-cause the sensitivity of IR absorption is not as good as that of mass detection, a higher concentra-tion of [CH2I]0≅ 4×1013molecules cm−3is needed
in our experiments in order to record a satisfactory spectrum of CH2OO. In some preliminary
low-resolution experiments, we varied [CH2I]0from
1.0 × 1013to 2.8 × 1014molecules cm−3 and found that the lifetime of CH2OO decreased from
~150 to 15ms, indicating that the bimolecular reaction—either CH2OO + I or CH2OO + CH2OO—
might be responsible for this rapid decay. More detailed kinetic measurements are in progress.
With our detection method, we can probe CH2OO directly in a reaction. The advantage is
demonstrated in the investigation of the yield of CH2OO from the reaction CH2I + O2. Solar
photolysis of CH2I2, one major source of iodine
in the marine boundary layer, generates CH2I and
I. The reaction of CH2I + O2is important in the
atmosphere partly because this reaction releases the second I atom to form IO, which can affect O3,
HOx, and NOxlevels and also lead to the formation
of particulates in the atmosphere (35). Huang et al. detected I atom as a product of the reaction of CH2I with O2by probing the IR absorption of I
atom at 7603.138 cm−1. These authors reported that O2stabilizes CH2IOO with remarkable
effi-ciency (13 times that of N2), hence decreasing the
yield of I atoms (28). The yield of CH2OO from
CH2I + O2was estimated to be 0.04 in air at 760 torr
because CH2IOO is expected to be readily
stabil-ized. However, because these authors probed only I atoms, their measurements could not distinguish between the stabilization of CH2IOO and other
secondary reactions. With our new detection meth-od, we can probe CH2OO directly to provide direct
measurements of the yield of CH2OO. Contrary to
their predictions, in our experiment with O2at 90
torr we observed no CH2IOO, and the yield of
CH2OO was estimated to be at least 35% of CH2I
with the assumption that the predicted IR inten-sities of CH2OO are correct. Whether this
dis-crepancy is due to the difference in photolysis wavelengths [355 nm in experiments of Huanget al. (28) and 248 nm in this work]—in which the total available energy of CH2I + I (118 and 263 kJ mol−1,
respectively) might affect the efficiency of stabi-lization of CH2IOO and CH2OO produced from
CH2I + O2—requires further investigation.
References and Notes
1. D. Johnson, G. Marston, Chem. Soc. Rev. 37, 699 (2008).
2. J. G. Calvert et al., The Mechanisms of Atmospheric Oxidation of the Alkenes (Oxford University Press, Oxford,
UK, 2000), pp. 172−335.
3. O. Horie, G. Moortgat, Acc. Chem. Res. 31, 387 (1998). 4. R. M. Harrison et al., Sci. Total Environ. 360, 5 (2006). 5. R. Criegee, G. Wenner, Chem. Ber. 9, 564 (1949). 6. W. Sander, Angew. Chem. Int. Ed. Engl. 29, 344 (1990). 7. W. H. Bunnelle, Chem. Rev. 91, 335 (1991). 8. S. Hatakeyama, H. Akimoto, Res. Chem. Intermed. 20,
503 (1994).
9. G. Marston, Science 335, 178 (2012).
10. C. A. Taatjes et al., J. Am. Chem. Soc. 130, 11883 (2008).
11. O. Welz et al., Science 335, 204 (2012).
12. M. T. Nguyen, T. L. Ngyuen, V. T. Ngan, H. M. T. Ngyuen, Chem. Phys. Lett. 448, 183 (2007) and references therein. 13. T. A. Cool, J. Wang, K. Nakajima, C. A. Taatjes, A. McIlroy,
Int. J. Mass Spectrom. 247, 18 (2005).
14. J. M. Beames, F. Liu, L. Lu, M. I. Lester, J. Am. Chem. Soc. 134, 20045 (2012).
15. J. M. Anglada, J. González, M. Torrent-Sucarrat, Phys. Chem. Chem. Phys. 13, 13034 (2011). 16. L. Vereecken, J. S. Francisco, Chem. Soc. Rev. 41, 6259
(2012).
17. D. Cremer, J. Gauss, E. Kraka, J. F. Stanton, R. J. Bartlett, Chem. Phys. Lett. 209, 547 (1993).
18. D.-C. Fang, X.-Y. Fu, J. Phys. Chem. A 106, 2988 (2002) and references therein.
19. L. B. Harding, W. A. Goddard, J. Am. Chem. Soc. 100, 7180 (1978).
20. S.-H. Chen, L.-K. Chu, Y.-J. Chen, I.-C. Chen, Y.-P. Lee, Chem. Phys. Lett. 333, 365 (2001).
21. D.-R. Huang, L.-K. Chu, Y.-P. Lee, J. Chem. Phys. 127, 234318 (2007).
22. L.-K. Chu, Y.-P. Lee, J. Chem. Phys. 124, 244301 (2006). 23. L.-K. Chu, Y.-P. Lee, J. Chem. Phys. 133, 184303 (2010). 24. J.-D. Chen, Y.-P. Lee, J. Chem. Phys. 134, 094304
(2011).
25. Materials and methods are available as supplementary materials on Science Online.
26. S. L. Baughcum, S. R. Leone, J. Chem. Phys. 72, 6531 (1980). 27. T. J. Gravestock, M. A. Blitz, W. J. Bloss, D. E. Heard,
ChemPhysChem 11, 3928 (2010).
28. H. Huang, A. J. Eskola, C. A. Taatjes, J. Phys. Chem. Lett. 3, 3399 (2012).
29. C. Angeli, R. Cimiraglia, S. Evangelisti, T. Leininger, J. P. Malrieu, J. Chem. Phys. 114, 10252 (2001). 30. H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby,
M. Schütz, WIREs Comput. Mol. Sci. 2, 242 (2012). 31. M. Neff, G. Rauhut, J. Chem. Phys. 131, 124129 (2009). 32. S.-Y. Chen, Y.-P. Lee, J. Chem. Phys. 132, 114303 (2010). 33. B. Golec, J.-D. Chen, Y.-P. Lee, J. Chem. Phys. 135,
224302 (2011).
34. S. Nandi et al., J. Phys. Chem. A 106, 7547 (2002). 35. G. McFiggans et al., Atmos. Chem. Phys. 4, 701 (2004). Acknowledgments: Calculation details and a compilation of anharmonic vibrational levels and IR intensities for possible intermediate structures with additional simulation plots are presented in the supplementary materials. National Science Council of Taiwan (grants NSC102-2745-M009-001-ASP and NSC99-2113-M-009-011-MY3) and the Ministry of Education,
Taiwan (“Aim for the Top University Plan” of National Chiao
Tung University) supported this work. The National Center for High-Performance Computing provided computer time. We thank G. Rauhut for his assistance in the calculations of anharmonic vibrational frequencies.
Supplementary Materials
www.sciencemag.org/cgi/content/full/340/6129/174/DC1 Materials and Methods
Supplementary Text Figs. S1 and S2 Tables S1 to S3
References (36–44)
20 December 2012; accepted 1 March 2013 10.1126/science.1234369 9 4 0 9 2 0 9 0 0 8 8 0 8 6 0 8 4 0 8 2 0 8 0 0 -1 0 1 2 3 4 5 6 7 8 9 10 Wave number /cm-1 Absorbance /10 -3
ν
8ν
6Fig. 3. Comparison of observed and simulated spectra of CH2OO in the region of 800 to 955 cm−1
at resolution 1.0 cm−1. Simulated absorption bands for then6(n0= 908 cm−1anda-/b-type = 8.2/1)
andn8(n0= 848 cm−1andc-type) modes are shown with red lines, and the observed spectrum is shown
with open circles. Spectral width = 1 cm−1,Jmax= 150, andT = 340 K.
12 APRIL 2013 VOL 340 SCIENCE www.sciencemag.org
176