Dissociation of energy-selected c - C 2 H 4 S + in a region 10.6–11.8 eV: Threshold
photoelectron—photoion coincidence experiments and quantum-chemical calculations
Yung-Sheng Fang, I-Feng Lin, Yao-Chang Lee, and Su-Yu Chiang
Citation: The Journal of Chemical Physics 123, 054312 (2005); doi: 10.1063/1.1993589 View online: http://dx.doi.org/10.1063/1.1993589
View Table of Contents: http://scitation.aip.org/content/aip/journal/jcp/123/5?ver=pdfcov Published by the AIP Publishing
Articles you may be interested in
Exploring the dynamics of reaction N + SiH 4 with crossed molecular-beam experiments and quantum-chemical calculations
J. Chem. Phys. 129, 174304 (2008); 10.1063/1.3005652
Dynamics of the reaction C ( P 3 ) + SiH 4 : Experiments and calculations J. Chem. Phys. 129, 164304 (2008); 10.1063/1.3000005
Production of methyl-oxonium ion and its complexes in the core-excited ( H C ( O ) O C H 3 ) n clusters: H H + transfer from the carbonyl
J. Chem. Phys. 123, 124309 (2005); 10.1063/1.2044727
UV photodissociation of the van der Waals dimer ( C H 3 I ) 2 revisited: Pathways giving rise to ionic features J. Chem. Phys. 122, 204301 (2005); 10.1063/1.1909083
Laser desorption time-of-flight mass spectrometry of fluorocarbon films synthesized by C 4 F 8 / H 2 plasmas J. Vac. Sci. Technol. A 21, 866 (2003); 10.1116/1.1577135
Dissociation of energy-selected c-C
2H
4S
+in a region 10.6–11.8 eV:
Threshold photoelectron—photoion coincidence experiments
and quantum-chemical calculations
Yung-Sheng Fang
Department of Applied Chemistry, National Chiao Tung University, 1001, Ta Hsueh Road, Hsinchu 30010, Taiwan
I-Feng Lin, Yao-Chang Lee, and Su-Yu Chianga兲
National Synchrotron Radiation Research Center, 101, Hsin Ann Road, Hsinchu Science Park, Hsinchu 30076, Taiwan
共Received 20 January 2005; accepted 13 June 2005; published online 9 August 2005兲
Dissociation of energy-selected c-C2H4S+ was investigated in a region of 10.6–11.8 eV with a
threshold photoelectron-photoion coincidence technique and a synchrotron as a source of vacuum ultraviolet radiation. Branching ratios and average releases of kinetic energy in channels of formation of c-C2H4S+, CH3CS+, and HCS+were obtained from well-resolved time-of-flight peaks
in coincidence mass spectra. Measured average releases of kinetic energy for channel CH3CS+
+ H of least energy are substantial and much greater than calculated with quasiequilibrium theory; in contrast, small releases of kinetic energy near the appearance onset for channel HCS++ CH
3agree
satisfactorily with statistical calculations. Calculations of molecular electronic structures and energetics of c-C2H4S+and C
2H3S+isomers and various fragments and transition states were also
performed with Gaussian 3 method to establish dissociation mechanisms. A predicted dissociation energy of 11.05 eV for c-C2H4S→HCS++ CH
3 agrees with a linearly extrapolated threshold at
10.99± 0.04 eV and a predicted dissociation mechanism that c-C2H4S+ isomerizes to CH3CHS+
before dissociating to HCS++ CH
3 supports the experimental results. The large releases of kinetic
energy for channel CH3CS++ H might result from a dissociation mechanism according to which
c-C2H4S+isomerizes to a local minimum CH3CSH+and then dissociates through a transition state
to form CH3CS++ H. © 2005 American Institute of Physics.关DOI: 10.1063/1.1993589兴
I. INTRODUCTION
Photodissociation 共PD兲 and photoionization 共PI兲 of thi-irane共c-C2H4S兲 are important topics from both experimental
and theoretical perspectives because the large ring strain fa-cilitates the ring-opening processes and isomerization, and also because they offer a powerful means of obtaining infor-mation about the structures and energetics of important radicals and ions.1–8 For example, photodissociation of
c-C2H4S to form C2H4+ S has been investigated with
exci-tation at 193 nm;3,9–11 understanding of energetics of triplet C2H4共3B1u兲 was thus obtained with
photofragment-translational spectroscopy共PTS兲 using a tunable synchrotron light to probe products selectively.3Photodissociation to pro-duce important species H2S, C2H2, CH4, H2, and S pertinent
to combustion and atmospheric chemistry was also reported with excitation in the region of 180–240 nm.12
Butler and Baer investigated the dissociative photoion-ization of c-C2H4S to form fragment ions HCS+and C
2H3S+
with photoionization mass spectroscopy共PIMS兲 and derived their heat of formation according to the determined appear-ance energies 共AEs兲 and existing thermochemical data, but the derived heat of formation 245± 2 kcal mol−1 for HCS+is
greater than 233± 2 kcal mol−1 obtained from dissociative
photoionization of c-C3H6S and c-C4H8S.1They also
deter-mined an average release of 0.03 eV of kinetic energy for the formation of HCS+ at the dissociation threshold from time-of-flight共TOF兲 peaks in photoelectron-photoion coincidence 共PEPICO兲 spectra of c-C2H4S and proposed an angular
structure of HCS+ on a basis that the experimental value is
significantly less than the statistically expected 0.1 eV.1,2 We have measured photoionization mass spectra of
c-C2H4S in a region of⬃9–20 eV using synchrotron radia-tion as an ionizaradia-tion source.13 The experimental ionization energy 共IE兲 at 90.51±0.003 eV agrees satisfactorily with a theoretical prediction of 9.07 eV for the formation of
c-C2H4S+, reflecting a small structural alternation upon
ion-ization. Five major fragment ions—C2H3S+, C2H2S+, HCS+,
H2S+, and C2H3+—were observed with their respective AEs
determined from the onset of photoionization efficiency 共PIE兲 curves and six dissociation channels—c-C2H4S+
→CH3CS++ H, HCS++ CH3, H2S++ C2H2, C2H3++ HS,
CH2CS++ H2, and CHCSH++ H2—are established based on
the comparison of experimental AE values and theoretically predicted dissociation energies.
As the dissociation channel c-C2H4S+→CH3CS++ H of
least energy likely involves structural alternation during dis-sociation, substantial kinetic energy is expected to be re-leased on dissociation if reverse activation barriers exist. The a兲Author to whom correspondence should be addressed. Fax:
共886兲3-578-3813. Electronic mail: schiang@nsrrc.org.tw
共2005兲
0021-9606/2005/123共5兲/054312/7/$22.50 123, 054312-1 © 2005 American Institute of Physics
kinetic energy released after dissociative photoionization is not, however, taken into account in determination of AE from PIMS experiments.14,15The previous proposition of for-mation of an angular structure of HCS+from dissociation of
c-C2H4S+through a tight and early transition state also needs
more experimental and theoretical studies to understand the dissociation mechanisms. Our aim in this work is to investi-gate the dissociation properties of c-C2H4S+to CH3CS++ H
and HCS++ CH3with threshold photoelectron-photoion
coin-cidence共TPEPICO兲 experiments and quantum-chemical cal-culations. We measured well-resolved TPEPICO mass spec-tra of c-C2H4S excited at photon energies in a region of
10.6–11.8 eV. From the area and the full width at half maxi-mum共FWHM兲 of c-C2H4S+, CH3CS+, and HCS+TOF peaks
in coincidence mass spectra, we obtained branching ratios and average releases of kinetic energies at various photon energies, and discuss plausible dissociation mechanisms on this basis and from calculations.
II. EXPERIMENTS
Our apparatus is described in detail elsewhere.16,17 Briefly, we performed coincidence measurements with a molecular-beam/threshold-photoelectron-photoion-coinci-dence 共MB/TPEPICO兲 system on the Seya-Namioka beam-line at the National Synchrotron Radiation Research Center 共NSRRC兲 in Taiwan. Photon energies with resolution of 30 meV and photon flux⬎109photons s−1 in a region of 10.6–
11.8 eV were selected with Seya-Namioka monochromator 共1 m; 1200 grooves mm−1; slit width of 0.15 mm兲. Absolute
photon energies were calibrated within ±0.003 eV on the measurement of Rydberg peaks in the threshold photoelec-tron spectra 共TPES兲 of Ar and Kr.
c-C2H4S and He in a mixture at a total stagnation
pres-sure of 280 Torr and with a seed ratio⬃15% were expanded to form a molecular beam. The monochromatic vacuum ul-traviolet 共VUV兲 radiation intersected perpendicularly with the beam to ionize cooled c-C2H4S molecules in the
ioniza-tion chamber. The threshold electrons and ions produced were then analyzed with a threshold photoelectron spectrom-eter and a TOF mass spectromspectrom-eter, respectively; dual micro-channel plates served as detectors in both spectrometers.
A dc field of 1.0 V cm−1 in the interaction region was
used to extract threshold electrons and a pulsed field of 21 V cm−1 with a duration of 30s was applied to extract
ions on detecting a threshold electron. Signals of detected threshold electrons and ions were fed into a time-to-digital converter共TDC兲 to record flight durations of ions that were detected within 30s of each cycle triggered with a thresh-old electron. To eliminate a large contribution from uncorre-lated ions piling up in the interaction region, a second coin-cidence spectrum triggered with a signal generated randomly relative to the preceding threshold electron signal was accu-mulated following each threshold electron-triggered dence cycle. Subtraction of the randomly generated coinci-dence spectrum from the electron-triggered coincicoinci-dence spectrum yielded a true coincidence spectrum. All data ac-quisition was controlled with a computer via a computer
as-sisted measurement and control 共CAMAC兲 interface, and output from the TDC converter was transferred to the com-puter for further processing.
c-C2H4S 共Aldrich, ⬃98%兲 was degassed with several
freeze-pump-thaw cycles before use and was kept in an ice bath at 0 °C during experiments. He 共⬎99.999%兲, Kr 共⬎99.99%兲, and Ar 共⬎99.99%兲 were used without further purification.
III. THEORETICAL CALCULATIONS
We calculated the molecular structures and energies of
c-C2H4S and species pertinent to this work with the
Gauss-ian 3共G3兲 method using the GAUSSIAN 2003program.18The equilibrium structure of each species was fully optimized at the MP2共full兲/6-31G共d兲 level and single-point calcula-tions of G3 energy were performed at levels MP4 / 6-31G共d兲, QCSID共T兲/6-31G共d兲, MP4/6-31+G共d兲, MP4 / 6-31G共2df ,p兲, and MP2 共full兲/G3 large; G3 large is a modified 6-311+ G共3df ,2p兲 basis set. Additional energies include a spin-orbit correction for atomic species, higher-level corrections for atoms and molecules, and a zero-point vibrational energy; the HF/6-31G共d兲 vibrational frequencies are scaled by 0.8929 and applied for the correction of zero-point vibrational energy. All identified structures were veri-fied to have only real vibrational frequencies at the MP2共full兲/6-31G共d兲 level, except transition structures that have one imaginary vibrational frequency. Transition struc-tures for dissociation channels c-C2H4S+→CH3CS++ H and
HCS++ CH3were located in this work; each dissociation path
was confirmed with intrinsic reaction coordinate 共IRC兲 cal-culations.
IV. RESULTS AND DISCUSSION
A. Coincidence TOF spectra in 10.6–11.8 eV
Coincidence mass spectra of c-C2H4S having
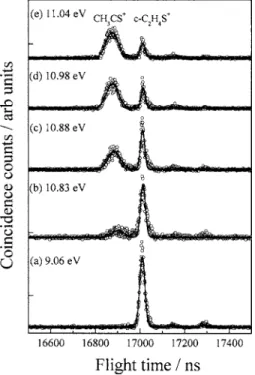
well-resolved TOF peaks were recorded at selected photon ener-gies in a region of 10.6–11.8 eV with 1-ns resolution and corrected for a false coincidence background. Figures 1共a兲–1共e兲 show the corrected coincidence TOF spectra of
c-C2H4S excited at 9.06, 10.83, 10.88, 10.98, and 11.04 eV; the solid lines indicate the TOF peaks fitted to Gaussian shapes and all spectra are normalized to 7000 total ion counts. Ion signals at m / z = 59, 60, 61, and 62, corresponding to isomeric structures of C2H3S+, c-C2H4S+, c-13C2H4S+,
and c-C2H434S+, respectively, were identified according to
ion flight durations calculated with an equation To/ ns
= 2175.2共m/z兲1/2+ 231.1 obtained from coincidence TOF
spectra of He, Ar, and Kr excited at photon energies near their ionization thresholds.
In Fig. 1共a兲, only signals attributed to parent ions
c-C2H4S+ and their two isotopic variants are observed; no
attempt was made to fit the ion signals of those variants at greater photon energies because they were not abundant. The TOF peak of c-C2H4S+ fitted to a Gaussian profile has a
FWHM of 26 ns; accordingly the transverse temperature of the molecular beam is calculated to be 10 K.19 This result indicates a small transverse velocity of the skimmed molecu-lar beam along the detection axis and negligible c-C2H4S+
054312-2 Fang et al. J. Chem. Phys. 123, 054312共2005兲
signals resulting from thermal 共298 K兲 background that would broaden a TOF peak. In Figs. 1共b兲–1共e兲, a well-resolved and symmetrically broadened TOF peak of frag-ment ion C2H3S+is also observed; the broadened TOF peak
indicates the substantial release of kinetic energy upon dis-sociation and its bandwidth and intensity increase with in-creasing photon energy.
Figures 2共a兲–2共e兲 show corrected coincidence TOF spec-tra of c-C2H4S excited at 11.13, 11.31, 11.50, 11.71, and
11.81 eV; all spectra are normalized to 7000 total ion counts. In these figures, a broadened TOF peak of fragment ion HCS+ is observed in addition to those of c-C
2H4S+ and
C2H3S+. The broadening also reflects the release of kinetic
energy upon dissociation and was fitted well to a Gaussian shape; moreover, its bandwidth and intensity increase with increasing photon energy. Also discernible in the figures,
c-C2H4S+ signals become less distinct but remain nearly
constant at greater photon energies. The constantly small
c-C2H4S+ signals are attributable to coincidences with hot
electrons due to the hot-electron tail function of our thresh-old electron analyzer, because a continuous background through the Franck-Condon gap region of 10.0–10.7 eV is observed in our TPES and this continuum could extend to a band that is associated with removal of a a electron in the
region of 10.9–11.8 eV.20–22We observed also a small TOF peak at m / z = 53, not shown in Figs. 1 and 2, at photon en-ergies smaller than 11.71 eV, but its formation as a result of dissociation of c-C2H4S+ is excluded because its TOF peak
is narrow,⬃20 ns; its source is unclear, but likely an impu-rity in the sample.
B. Theoretical calculations of dissociation energies and vibrational frequencies
We obtained fully optimized structures 关MP2共full兲/ 6-31G共d兲兴 of the five most stable isomers of
c-C2H4S+– CH3CHS+共1兲, cis-CH2CHSH+共2a兲,
trans-CH2CHSH+共2b兲, c-C2H4S+共3兲, and CH2SCH2+共4兲, three
isomers of C2H3S+– CH3CS+共5兲, CH2CSH+共6兲,
c-C2H3S+共7兲, HCS+, CH3, H, and species at local minimum
and transition states that occur in isomerization processes of
c-C2H4S+. Predicted structural parameters for c-C2H4S+,
CH2SCH2+, CH3CS+, CH2CSH+, and c-C2H3S+ agree with
previous calculations using varied methods in the literature.23,24
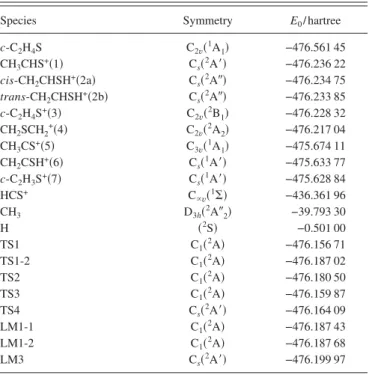
Table I lists the calculated G3 energies共E0/ hartree兲 for
species pertinent to this work and Table II lists the predicted ionization energies and dissociation energies共⌬E/eV兲 calcu-lated from Table I. According to Table II, predicted IE for the formation of CH3CHS+共1兲, cis-CH2CHSH+共2a兲,
trans-CH2CHSH+共2b兲, c-C2H4S+共3兲, and CH2SCH2+共4兲 are 8.85,
8.89, 8.91, 9.07, and 9.37 eV, respectively; CH3CHS+is the
most stable structure among the five isomers of c-C2H4S+,
but cis- and trans-CH2CHSH+have energies only 0.04 and
0.06 eV greater than that for CH3CHS+, respectively. A
pre-diction of 9.07 eV for the formation of c-C2H4S+ agrees
satisfactorily with literature values IE= 9.04± 0.01, 9.051± 0.003, or 9.051± 0.006 eV determined in various experiments;2,13,15this result reflects no significant structural alternation from c-C2H4S to c-C2H4S+and is associated with
removal of a nonbonding electron on the S atom upon ion-ization.
Predicted dissociation energies for c-C2H4S
→CH3CS+共5兲+H, CH2CSH+共6兲+H, and c-C2H3S+共7兲+H
are 10.51, 11.61, and 11.74 eV, respectively. CH3CS+has the
least energy among three isomers of C2H3S+; unstable
CH2CHS+, unlike CH2CHSH+共2a,2b兲, with one imaginary
frequency corresponds to no local minimum. In contrast to FIG. 1. Coincidence TOF spectrum of c-C2H4S excited at photon energies
共a兲 8.89, 共b兲 10.83, 共c兲 10.88, 共d兲 10.98, and 共e兲 11.04 eV.
FIG. 2. Coincidence TOF spectrum of c-C2H4S excited at photon energies
共a兲 11.13, 共b兲 11.31, 共c兲 11.50, 共d兲 11.71, and 共e兲 11.81 eV.
dissociation c-C2H4O+→c-C2H3O++ H reported
previously,25 the formation of c-C2H3S+ is excluded as its
predicted dissociation energy of 11.74 eV is much greater than the experimental AE value of 10.71± 0.01 eV deter-mined in PIMS experiments;2,13 CH3CS+is the most likely
structure on energetic grounds despite a small difference of 0.2 eV between predicted and experimental values.
A predicted dissociation energy of 11.05 eV for
c-C2H4S→HCS++ CH3 is near previously reported AE
val-ues of 11.13± 0.04 and 11.13± 0.01 eV for the formation of HCS+.2,13This small discrepancy supports an observation of a small release of kinetic energy near the dissociation thresh-old reported from a previous PEPICO investigation and in
this work, which are discussed later. Especially noteworthy is our predicted structure of HCS+ at the threshold that is
linear, not angular as reported previously;1a previous propo-sition of a tight and early tranpropo-sition state for this dissociation channel to form a bent HCS+is thus excluded.
Vibrational wave numbers of CH3CHS+for eight normal
modes and of HCS+for four normal modes in their ground
states calculated at the HF/6-31G共d兲 level and scaled by 0.8929 are listed in Table III and used for subsequent quasi-equilibrium theory 共QET兲 calculations. Scaled vibrational wave numbers 3122 and 788 cm−1 for HCS+ are consistent
with the experimental values of 3141 and 766 cm−1.26
C. Branching ratios and average releases of kinetic energy
Figure 3 shows the branching ratios of c-C2H4S+ and
fragment ions CH3CS+and HCS+in the region of 10.6–11.8
eV obtained from the area ratios of TOF peaks in coinci-dence spectra. Fragment ion CH3CS+ appears at threshold
⬃10.76 eV and then increases to a crossing point with c-C2H4S+ at 10.87 eV before reaching a maximum at
⬃11.18 eV, after which it diminishes toward greater photon energies because of the formation of HCS+. Fragment ion
HCS+ appears at an onset ⬃11.13 eV that lies between the
onset at⬃10.7 eV and the maximum at 11.37 eV of the first excited electronic state of c-C2H4S+; formation of HCS+is
thus likely due to a new dissociation channel opening as the TABLE I. Calculated G3 energies共E0兲 for species pertinent to dissociation
channels c-C2H4S+→CH3CS++ H and HCS++ CH3.
Species Symmetry E0/ hartree
c-C2H4S C2v共1A1兲 −476.561 45 CH3CHS+共1兲 Cs共2A⬘兲 −476.236 22 cis-CH2CHSH+共2a兲 Cs共2A⬙兲 −476.234 75 trans-CH2CHSH+共2b兲 Cs共2A⬙兲 −476.233 85 c-C2H4S+共3兲 C 2v共2B1兲 −476.228 32 CH2SCH2+共4兲 C2v共2A2兲 −476.217 04 CH3CS+共5兲 C 3v共1A1兲 −475.674 11 CH2CSH+共6兲 Cs共1A⬘兲 −475.633 77 c-C2H3S+共7兲 Cs共1A⬘兲 −475.628 84 HCS+ C ⬀v共1⌺兲 −436.361 96 CH3 D3h共2A⬙2兲 −39.793 30 H 共2S兲 −0.501 00 TS1 C1共2A兲 −476.156 71 TS1-2 C1共2A兲 −476.187 02 TS2 C1共2A兲 −476.180 50 TS3 C1共2A兲 −476.159 87 TS4 Cs共2A⬘兲 −476.164 09 LM1-1 C1共2A兲 −476.187 43 LM1-2 C1共2A兲 −476.187 68 LM3 Cs共2A⬘兲 −476.199 97
TABLE II. Predicted ionization energies and relative energies共⌬E兲 for spe-cies pertinent to this work.
Species ⌬E/eV c-C2H4S→CH3CHS+共1兲 8.85 →cis-CH2CHSH+共2a兲 8.89 →trans-CH2CHSH+共2b兲 8.91 →c-C2H4S+共3兲 9.07 →CH2SCH2 +共4兲 9.37 →CH3CS+共5兲+H 10.51 →CH2CSH+共6兲+H 11.61 →c-C2H3S+共7兲+H 11.74 →HCS++ CH 3 11.05 →TS1 11.01 →TS1-2 10.19 →TS2 10.37 →TS3 10.93 →TS4 10.81 →LM1-1 10.18 →LM1-2 10.17 →LM3 9.84
TABLE III. Vibrational wave numbers of ground-state CH3CS+and HCS+
calculated at the HF level and scaled by 0.8929.
Ion Modes Vibrational wave numbers/cm−1 CH3CS+ v1共e兲 2937 v2共a1兲 2852 v3共a1兲 1585 v4共e兲 1381 v5共a1兲 1365 v6共e兲 991 v7共a1兲 715 v8共e兲 336 HCS+ v 1共兲 3122 v2共兲 788 v3共兲 1421
FIG. 3. Branching ratios of c-C2H4S+, CH
3CS+, and HCS+in a region
10.6–11.8 eV; fractional abundances of these three ions were obtained from their total signals.
054312-4 Fang et al. J. Chem. Phys. 123, 054312共2005兲
internal energy of the parent ion c-C2H4S+ increases. The
small signals of c-C2H4S+remain constant at greater photon
energies likely because of coincidences with hot electrons, as mentioned above.
The broadened TOF peak for each fragment ion is well fitted with a Gaussian shape. In general, for a distribution of this type, the average release of kinetic energy 共KE兲 is re-lated to the FWHM of the TOF peak and calcure-lated from that FWHM according to the Maxwellian equation19,27
具KE典 = 3 15 ln 2 2共FWHM兲2 Mp Mf共Mp− Mf兲 −3 2RT Mf 共Mp− Mf兲 , 共1兲
in which 具KE典 is the average release of kinetic energy, = 21 V cm−1is the strength of the pulsed electric field for ion
extraction, FWHM is obtained from the fitted TOF peak of a fragment ion, Mpand Mf are masses of parent and fragment
ions, and T = 10 K is the transverse temperature of the mo-lecular beam. Obtained FWHM and calculated average re-lease of kinetic energy for both dissociation channels at se-lected photon energies in the region of 10.6–11.8 eV are listed in Table IV. As a pulsed delay extraction might narrow a TOF peak that is broadened because kinetic energy is re-leased, we calculated this effect on our derived具KE典 based on a delay of ⬃231 ns between a threshold photoelectron detection and a pulsed extraction field from the equation
To/ ns= 2175.2共m/z兲1/2+ 231.1. Within the delay, fragment
ions CH3CS+move⬃0.04–0.06 mm away from the
interac-tion region in the weak field 1 V cm−1according to average releases ⬃0.4–0.88 eV of kinetic energy obtained in this work for channel CH3CS++ H, and fragment ions HCS+
move⬃0.08–0.1 mm with average releases ⬃0.04–0.17 eV of kinetic energy for channel HCS++ CH3. Because of the
small displacements of both fragment ions CH3CS+ and
HCS+, the effect on narrowing a TOF peak before a pulsed high-voltage applied is expected to be negligible.
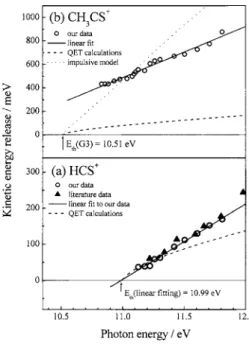
In Figs. 4共a兲 and 4共b兲, calculated average releases of kinetic energy for channels HCS++ CH3and CH3CS++ H are
depicted as circles, respectively. For channel HCS++ CH3, a
solid line for a linear fit to our data, a dashed curve for QET calculations, and solid triangles representing literature data of Butler and Baer1 are also depicted in Fig. 4共a兲 for com-parison. The QET calculations were performed according to an equation formulated by Klots28–30
h− E0=
共r + 1兲
2 具KE典 +
兺
ihi
exp共hi/具KE典兲 − 1, 共2兲 in which h is the photon energy, E0= 10.99± 0.04 eV de-rived from linear extrapolation of our experimental data, is the dissociation threshold for the formation of HCS+ and
CH3, r is the number of rotational degrees of freedom of products 共two for HCS+ and three for CH
3兲, andi are the
vibrational wave numbers of CH3 and HCS+; vibrational
wave numbers 3004.4, 606.5, 3160.8, and 1396 cm−1 for
CH3 are taken from the literature31 and those of HCS+ are
listed in Table III.
As seen in Fig. 4共a兲, our data agree satisfactorily with literature values; our linearly extrapolated threshold at 10.99± 0.04 eV also agrees with a predicted dissociation en-ergy of 11.05 eV for formation of linear HCS+and CH
3from
c-C2H4S. Although significant differences are observed at
greater photon energies, QET results seem to fit well the experimental data near the dissociation threshold; accord-ingly, the average release of kinetic energy is 0.037 eV at the TABLE IV. Calculated average release of kinetic energies from the full
width at half maximum共FWHM兲 at selected photon energies for dissocia-tion channels c-C2H4S+→CH3CS++ H and HCS++ CH3.
CH3CS+ HCS+
PE/ eV FWHM/ ns 具KE典/eV FWHM/ ns 具KE典/eV
10.83 62.4 0.434 - -10.86 62.4 0.434 - -10.88 62.4 0.434 - -10.93 63.9 0.459 - -10.98 64.9 0.475 - -11.04 65.1 0.479 - -11.08 66.3 0.500 - -11.10 67.5 0.520 - -11.11 68.4 0.537 - -11.13 69.5 0.556 59.7 0.037 11.18 69.0 0.548 61.0 0.039 11.22 72.2 0.607 62.0 0.040 11.26 73.5 0.631 73.9 0.059 11.31 74.1 0.642 76.1 0.063 11.42 75.5 0.670 91.8 0.093 11.50 76.3 0.686 100.3 0.112 11.61 78.3 0.727 107.9 0.130 11.71 80.5 0.774 116.6 0.152 11.81 85.2 0.876 122.8 0.170
FIG. 4. Average kinetic energy released into dissociation channels c-C2H4S+→共a兲HCS++ CH3and共b兲 CH3CS++ H with excitation at photon
en-ergies in a region 10.6–11.8 eV. Data, a line fitted to data, QET calculations, and a calculation according to an impulsive model are marked as circles, solid line, dashed line and dotted line, respectively; literature data in共a兲 are marked as solid triangles for comparison.
AE value of 11.13 eV for HCS+ determined in previous
PIMS studies of c-C2H4S. Because formation of HCS+ and
CH3likely involves H migration before dissociation occurs,
the agreement of an extrapolated threshold with a G3 predic-tion and small releases of kinetic energy near the dissociapredic-tion threshold imply that the energy for isomerization of
c-C2H4S+ into the dissociation precursor lies below or near
the dissociation threshold.
For channel CH3CS++ H, a linear solid line fitted to data,
a dashed curve for QET calculations, and a dotted line for calculations based on a pure impulsive model32,33 are de-picted in Fig. 4共b兲 for comparison. In QET calculations, a predicted dissociation energy of 10.51 eV for c-C2H4S+
→CH3CS++ H was taken as the dissociation threshold
be-cause, to our knowledge, no experimental dissociation threshold is available, except the experimental AE of 10.71± 0.01 eV for CH3CS+ determined in previous PIMS
studies. Calculations based on a pure impulsive model were performed with a simple equation
具KE典 = 共b/f兲Eavl, 共3兲
in whichbis the reduced mass of the two atoms共C and H兲
between which a bond is broken, fis the reduced mass of
the two products 共CH3CS+ and H兲, and E
avl= h− E0 is the
difference between photon energies and dissociation thresh-old 共E0兲; E0= 10.51 eV is taken from a G3 prediction, as explained above.
As seen in Fig. 4共b兲, unlike channel HCS++ CH 3,
mea-sured releases of kinetic energy for channel CH3CS++ H are
substantial, but results of QET calculations lie far below ex-perimental values; moreover, a linearly extrapolated thresh-old at 9.87 eV for this channel is much smaller than a G3 prediction of 10.51 eV and the experimental AE of 10.71± 0.01 eV.2,13A nonstatistical distribution of measured substantial kinetic energies and the discrepancies at the dis-sociation threshold indicate that disdis-sociation occurs through a dissociative excited state or involves substantial exit barri-ers. The presence of substantial exit barriers for formation of CH3CS+is likely due to H migration and structural
alterna-tions during dissociation. On the other hand, the existence of a dissociative excited state in a region of 10.51–10.71 eV is excluded because there is no electronic state but a
Franck-Condon gap in a region of ⬃10.0–10.7 eV according to a previously reported photoelectron spectrum and our thresh-old photoelectron spectrum.20–22 Moreover, an impulsive model is generally applied to the dissociation that proceeds rapidly from a repulsive dissociative potential-energy sur-face, but our calculations based on an impulsive model do not fit well the experimental data.
D. Dissociation mechanisms
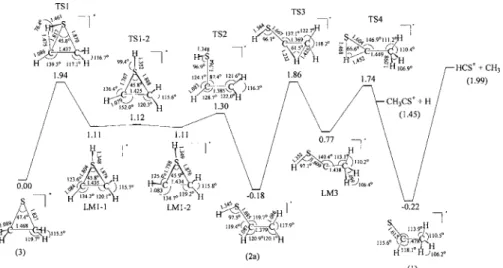
Figure 5 shows relative energies of a feasible dissocia-tion mechanism for channels c-C2H4S+→CH3CS++ H and
HCS++ CH
3predicted with the G3 method; structural
param-eters for the stationary states are labeled in the figure. In Fig. 5, c-C2H4S+ first undergoes H migration to form a local
minimum c-CHCH2SH+共LM1-1兲 via a transition state TS1;
a G3 barrier for this process is 1.94 eV. Next, the H atom of the CH group of c-CHCH2SH+共LM1-1兲 rotates with nearly
no barrier to form its trans-isomer c-CHCH2SH+ 共LM1-2兲,
which subsequently proceeds through ring opening and C-S bond breaking to form cis-CH2CHSH+ 共2a兲 via TS2 with a
G3 barrier at 1.30 eV. In further formation of CH3CSH+
共LM3兲 and the most stable isomer CH3CHS+ 共1兲, the
inter-mediate cis-CH2CHSH+共2a兲 proceeds through H migrations
from the CH group to the CH2group and from the SH group to the central C atom via TS3 and TS4 with predicted barri-ers at 1.86 and 1.74 eV, respectively. As dissociation of CH3CHS+ 共1兲 to form CH
3CS++ H involves only direct
cleavage of the C-H bond, one would expect to find a com-pletely statistical distribution of energy, which is not ob-served in our experiments. In contrast, if dissociation of CH3CSH+共LM3兲 to form CH3CS++ H takes place via TS4,
substantial kinetic energies are expected to be released dur-ing dissociation, compatible with experimental observations. The predicted barrier of TS4 at 1.74 eV agrees with a differ-ence of 1.66 eV between the experimental AE of 10.71 eV and IE of 9.05 eV determined in previous PIMS studies.13,15 Although a predicted barrier of 1.94 eV for isomerization of
c-C2H4S+ to the dissociation precursor CH3CSH+ 共LM3兲 is
slightly greater than the predicted barrier of TS4 at 1.74 eV, but still within an uncertainty of theoretical calculations for transition states. We sought to break the C-S bond directly to form a ring-opened structure CH2CH2S+, which can proceed
FIG. 5. Theoretical predictions of relative energies in eV for isomeriza-tion of c-C2H4S+into CH3CHS+
be-fore dissociation into channels CH3CS++ H and HCS++ CH3; bond
lengths in angstroms and interbond angles in degrees are indicated for mo-lecular structures optimized at the MP2共full兲/6-31G共d兲 level.
054312-6 Fang et al. J. Chem. Phys. 123, 054312共2005兲
through H migrations from one CH2group to the other CH2
group to form CH3CHS+, but located no transition state for
this process. Although BelBruno predicted a smaller activa-tion barrier of 1.87 eV for isomerizaactiva-tion of c-C2H4S+ into
CH2SCH2+, 23
this dissociation mechanism is unexpected be-cause of the weakness of the C-S bond relative to the C-C bond.
For channel HCS++ CH
3, Fig. 5 shows that dissociation
of CH3CHS+ 共1兲 to form HCS++ CH3 proceeds through
di-rect cleavage of the C-C bond without an exit barrier and would result in a statistical energy distribution in the disso-ciation; a predicted G3 barrier of 1.94 eV for isomerization of c-C2H4S+ to CH3CHS+ 共1兲 is nearly the same as a
dicted dissociation energy of 1.99 eV. The results of a pre-dicted negligible barrier and a statistical distribution of en-ergy in the dissociation agree satisfactorily with experimental observations.
V. CONCLUSION
Dissociation of energy-selected c-C2H4S+into channels
CH3CS++ H and HCS++ CH3was investigated in a region of
10.6–11.8 eV with a TPEPICO technique in pulsed mode and quantum-chemical calculations. Branching ratios for c-C2H4S+, CH3CS+, and HCS+and average releases of kinetic
energy for CH3CS+ and HCS+ were derived from
well-resolved TOF peaks in coincidence spectra. Small releases of kinetic energy for formation of HCS+agree with QET calcu-lations near the appearance threshold, but substantial releases of kinetic energy for formation of CH3CS+ differ
signifi-cantly from statistical calculations. A detailed energy profile for dissociation mechanisms of c-C2H4S+ to form CH
3CS+
+ H and HCS++ CH
3 is reported based on the calculations
with the Gaussian 3 method. A large kinetic energy released on the formation of CH3CS++ H is probably due to a reverse
activation barrier required for dissociation of CH3CSH+共LM3兲 through a transition state; in contrast, the
statistical energy distribution for formation of HCS++ CH 3is
due to dissociation of the most stable isomer CH3CHS+
with-out an exit barrier. ACKNOWLEDGMENTS
The National Synchrotron Radiation Research Center and the National Science Council of Taiwan 共Contract No. NSC92-2113-M-213-001兲 provided financial support, and
the National Center for High-Performance Computing pro-vided computing time for theoretical calculations.
1J. J. Butler and T. Baer, J. Am. Chem. Soc. 104, 5016共1982兲. 2J. J. Bulter and T. Baer, Org. Mass Spectrom. 18, 248共1983兲. 3F. Qi, O. Sorkhabi, and A. G. Suits, J. Chem. Phys. 112, 10707共2000兲. 4J. W. Gardiner, Combustion Chemistry共Springer, New York, 1984兲. 5M. B. Robin, Higher Excited States of Polyatomic Molecules共Academic,
New York, 1985兲.
6A. S. Bodke, D. A. Olschki, L. D. Schmidt, and E. Ranzi, Science 285,
712共1999兲.
7M. C. Pirrung, Acc. Chem. Res. 32, 711共1999兲.
8T. R. Younkin, E. F. Connor, J. I. Henderson, S. K. Friedrich, R. H.
Grubbs, and D. A. Bansleben, Science 287, 460共2000兲.
9H. L. Kim, S. Satyapal, P. Brewer, and R. Bersohn, J. Chem. Phys. 91,
1047共1989兲.
10P. Felder, E. A. J. Wannenmacher, I. Wiedmer, and J. R. Huber, J. Phys.
Chem. 96, 4470共1992兲.
11F. Qi, O. Sorkhabi, A. G. Suits, S.-H. Chien, and W.-K. Li, J. Am. Chem.
Soc. 123, 148共2001兲.
12E. M. Lown, K. S. Sidhu, A. W. Jackson, A. Jodhan, M. Green, and O. P.
Strausz, J. Phys. Chem. 85, 1089共1981兲.
13S.-Y. Chiang and Y.-S. Fang, J. Electron Spectrosc. Relat. Phenom. 144,
223共2005兲.
14B. P. Tsai, T. Baer, A. S. Werner, and S. F. Lin, J. Phys. Chem. 79, 570
共1975兲.
15H. Shiromaru, Y. Achiba, and Y. T. Lee, J. Phys. Chem. 91, 17共1987兲. 16S.-Y. Chiang and C.-I Ma, J. Phys. Chem. A 104, 1991共2000兲. 17S.-Y. Chiang, Y.-C. Lee, and Y.-P. Lee, J. Phys. Chem. A 105, 1226
共2001兲.
18M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian, Inc.,
Pitts-burgh, PA, 2003.
19R. Stockbauer, Int. J. Mass Spectrom. Ion Phys. 25, 89共1977兲. 20D. H. Aue, H. M. Webb, W. R. Davidson, et al., J. Am. Chem. Soc. 102,
5151共1980兲.
21D. C. Frost, F. G. Herring, A. Katrib, and C. A. McDowell, Chem. Phys.
Lett. 20, 401共1973兲.
22A. Schweig and W. Thiel, Chem. Phys. Lett. 21, 541共1973兲. 23J. J. BelBruno, Chem. Phys. Lett. 254, 321共1996兲.
24S.-W. Chiu, K.-C. Lau, and W.-K. Li, J. Phys. Chem. A 104, 3028
共2000兲.
25F. Liu, F. Qi, H. Gao, L. Sheng, Y. Zhang, S. Yu, K.-C. Lau, and W.-K.
Li, J. Phys. Chem. A 103, 4155共1999兲.
26P. J. Linstrom and W. G. Mallard, NIST Chemistry Webbook, NIST Stan-dard Reference Database No. 69, 2005 Release共http://webbook.nist.gov兲. 27T. Baer, G. D. Whillett, D. Smith, and J. S. Phillips, J. Chem. Phys. 70,
4076共1979兲.
28C. E. Klots, J. Chem. Phys. 58, 5364共1973兲. 29C. E. Klots, Adv. Mass Spectrom. 6, 969共1974兲. 30C. E. Klots, J. Chem. Phys. 64, 4269共1976兲. 31M. E. Jacox, J. Phys. Chem. Ref. Data 32, 157共2003兲. 32G. E. Busch and K. R. Wilson, J. Chem. Phys. 56, 3626共1972兲. 33K. E. Holdy, L. C. Klotz, and K. R. Wilson, J. Chem. Phys. 52, 4588
共1970兲.