Plant Pathology (2004) 53, 96 –102 Doi: 10.1046/j.1365-3059.2003.00948.x
Blackwell Publishing, Ltd.
Detection by PCR of
Candidatus
Liberibacter asiaticus, the
bacterium causing citrus huanglongbing in vector psyllids:
application to the study of vector–pathogen relationships
T.-H. Hung
a*†, S.-C. Hung
bc, C.-N. Chen
b, M.-H. Hsu
band H.-J. Su
aaDepartment of Plant Pathology and Microbiology, National Taiwan University, Taipei 106; bDepartment of Entomology, National Taiwan
University, Taipei 106; and c
Department of Plant Protection, Chiayi Agricultural Experiment Station, Taiwan Agricultural Research Institute, Chiayi 600, Taiwan
Citrus huanglongbing (HLB), previously called greening, is a serious citrus disease in Asia, eastern and southern Africa. It is caused by Candidatus Liberibacter asiaticus (Las), a phloem-limited, nonculturable bacterium transmitted by the Asian citrus psyllid (Diaphorina citri) in Asia. A PCR-based assay was developed for monitoring Las in vector psyllids using a rapid DNA extraction from psyllid bodies and PCR amplification. The entire procedure for Las detection in psyllids can be completed within 5 h. Using this method, Las can be accurately detected in psyllid adults as well as nymphs in different instar stages. The assay is sensitive enough for Las detection in single-psyllid extract from adult, fifth, fourth and third instars. In a transovarial transmission experiment, Las was not detected in eggs or in offspring produced by Las-carrying psyllid females. In a retention test, the Las-carrying psyllids remained Las-positive for 12 weeks after they were moved to common jasmine orange, a Las-immune plant. From these experimental results it was concluded that Las persists in the Asian citrus psyllid vector, but is not transovarially transmitted by the vector. These data help in under-standing epidemiological characteristics of Las and psyllids in citrus HLB.
Keywords: Asian citrus psyllid, Candidatus Liberibacter asiaticus, citrus huanglongbing, mycoplasma, phytoplasma
Introduction
Citrus huanglongbing (HLB), also known as citrus green-ing, is a severe citrus disease, especially in Asian countries. It is caused by nonculturable bacteria of the Candidatus genus Liberibacter (Garnier et al., 2000) that inhabit citrus phloem, retarding growth of the plant and causing the incomplete colouring of mature citrus fruits (da Graca, 1991). In Asia the pathogen of HLB was categorized as the Candidatus species Liberibacter asiaticus (Las) (Jagoueix et al., 1997; Garnier et al., 2000). Las infects most citrus cultivars and causes substantial economic losses by shortening the lifespan of infected trees (Miyakawa, 1980). It is spread by vegetative propagation and insect vectors, two reasons why HLB has become such a difficult disease to control. HLB can be categorized into two types, Asian and African, based on the influence of temperature on host symptoms. The Asian type, in which HLB symptoms
can occur at temperatures above 30°C, is heat-tolerant; and the African type, in which no symptoms appear above 30°C, is heat-sensitive (Bové et al., 1974). Although the fastidious bacterium causing HLB has not yet been successfully cultured in vitro, it has been characterized by molecular methods and tentatively assigned to the genus Candidatus Liberobacter (Jagoueix et al., 1994; Murray & Schleifer, 1994). By comparison of the 16S/23S rDNA sequences and ribosomal protein genes, strains compos-ing Ca. Liberobacter were further categorized as two different species, Ca. Liberobacter asiaticum for the Asian pathogen and Ca. Liberobacter africanum for the African pathogen (Jagoueix et al., 1997). Following the rules of the International Code of Nomenclature of Bacteria, the two bacterial species have now been renamed Ca. Liberi-bacter asiaticus and Ca. Liberibacter africanus (Garnier et al., 2000). The Asian citrus psyllid, Diaphorina citri, is the vector of Ca. Liberibacter asiaticus (Capoor et al., 1967; Chen et al., 1973) and the African citrus psyllid, Trioza erytreae, for Ca. Liberibacter africanus (McClean & Oberholzer, 1965). HLB has been the most important factor limiting citrus production in Asia.
Detection of Las is difficult because of its low con-centration and uneven distribution in its citrus hosts
*To whom correspondence should be addressed. †E-mail: thhung@ccms.ntu.edu.tw
Detection of the HLB bacterium in psyllids 97
(McClean, 1970; Huang, 1979). A rapid and sensitive assay based on the polymerase chain reaction (PCR) has been developed for Las detection in citrus plants (Jagoueix et al., 1996; Hocquellet et al., 1999; Hung et al., 1999b). However, detailed research associated with monitoring Las in the Asian citrus psyllid is rare (Bové et al., 1993). In this study, a simple method sufficient for Las detection in psyllids was developed by combining rapid preparation of DNA extracts from psyllid bodies and a PCR assay. This method is sensitive enough to detect Las in a single adult psyllid. Use of this sensitive Las-detection method in psyllids enabled research to be conducted on vector–pathogen relationships. Detection of Las in psyllids reported in this paper is applicable for monitoring Las in adult and nymphal psyllids, and was also a useful tool for studying transovarial transmission and Las retention in adults. The results reported here complement earlier psyllid–Las relationships that were carried out by traditional bioassays and electron microscopy (Xu et al., 1988). Understanding the vector–pathogen relationship of psyllids should facilitate control of HLB disease.
Materials and methods
Sources of psyllids and plant materials
The original source of psyllids (D. citri) in this study was from a common jasmine orange (CJO, Murraya panicu-lata var. paniculata). According to previous studies (Miyakawa, 1980; Garnier & Bové, 1993; Hung et al., 2000), CJO is a host for psyllids but is immune to Las. Collected psyllids were transferred to another CJO in an insect-proof cage at 25°C for rearing. The CJO plant was trimmed monthly to stimulate new growth, based on a previous report (Lin et al., 1973) that the sprouting of CJO induces the oviposition of psyllids to produce many offspring.
A Las-infected Luchen sweet orange (LSO, Citrus
sinensis, 50 cm tall, 1 year old) was used as the source of Las. A healthy LSO (50 cm tall, 1 year old) obtained by the shoot-tip grafting technique (Murashige et al., 1972) was used as negative control in this study.
Acquisition of Las by adult psyllids
A total of 50 Las-free adult psyllids were transferred to the Las-infected LSO and placed in an insect-proof cage at 25°C for acquisition feeding. Because adult psyllids tend to feed on the very young parts of hosts where the level of Las is low (McClean, 1970; Huang, 1979), young shoots of the Las-infected LSO were removed, forcing the insects to feed on mature leaves and enhance acquisi-tion. An aspirator was used to collect and transfer psyl-lids. After 2 weeks the psyllids were collected for DNA extraction and Las detection. As a negative control, a further 50 adult psyllids were transferred from a CJO to a healthy LSO and subjected to the same treatments and tests.
Collection of nymphal psyllids for Las detection
One hundred adult psyllids reared on the CJO plant were transferred to a Las-infected LSO plant with abundant young shoots to accelerate egg production. The adult psyllids were removed after 4 weeks. Nymphs that hatched from these eggs on the diseased LSO plant were collected for Las detection. The five nymphal instars of Asian citrus psyllid can be differentiated by their distinct morphol-ogical characteristics (Lin et al., 1973; Yang, 1984). Nymphs were collected with a fine-tip brush and placed in an Eppendorf tube for DNA extraction. As a negative control, another 100 adult psyllids from the healthy CJO plant were transferred to a healthy LSO plant. Nymphs from eggs on the healthy LSO plant were collected and tested as the corresponding negative controls.
Transovarial passage tests
Two experiments were devised for the transovarial passage tests. The objective of the first experiment was to determine if Las could be detected in eggs. After a 2 week acquisition feeding period on the Las-infected LSO plant, 50 adult females were moved to a CJO plant with many young shoots to facilitate oviposition. Twenty adult females were collected for Las detection from this egg-laying population. Their eggs were then collected from shoots of the CJO plant and used for PCR assay. Eggs were combined and used for DNA extractions to counteract the low efficiency of DNA extraction from single eggs. Total DNA was extracted from one egg or from batches of five, 10, 25, 50 and 100 eggs, and the amount of DNA for each sample was deter-mined using a spectrophotometer (GeneQuant II RNA/ DNA Calculator, Pharmacia Biotech, Cambridge, UK). The DNA extracted from single and multiple egg samples was analysed by PCR. The experiment was replicated four times. The objective of the second experiment was to monitor the presence of Las in psyllid offspring. The offspring were produced by the Las-carrying psyllids as described above. After oviposition, all the Las-carrying adult females were removed from the CJO plant. The eggs were kept on CJO shoots for hatching to produce psyllid offspring. After developing into adults, they were subjected to the Las test. The experiment was replicated four times.
Retention tests of psyllids for Las carrying
After a 2-week acquisition-feeding period, 220 Las-carrying adult psyllids on the diseased LSO plant were collected for the Las-retention tests. Psyllids were transferred to a CJO plant in an insect-proof growth chamber at 25°C. Immediately before they were transferred, 20 adults were tested for Las to determine the percentage of the popula-tion carrying Las. All CJO shoots were removed to avoid offspring production. To determine whether citrus psyl-lids retain Las in a persistent, semipersistent or nonpersist-ent manner, 20 adults were taken from the population and tested for Las at 2, 4, 6, 8, 10 and 12 weeks after transfer. Four experimental replicates were carried out.
98 T.-H. Hung et al.
DNA extraction from psyllid bodies and citrus tissues
For the detection of Las in psyllids, a simple protocol was designed to extract DNA from psyllids for the PCR test. Each psyllid was put in a 1·5 mL Eppendorf tube contain-ing 300 µL DNA extraction buffer (0·1 m Tris-HCl pH 8·0, 0·05 m EDTA, 0·5 m NaCl, 1% N-lauroylsarcosine), homogenized with a plastic rod, and incubated at 55°C for 1 h. After phenol/chloroform/isoamyl alcohol extraction, the DNA was precipitated by mixing 200 µL of the supernatant and 500 µL 100% ethanol followed by centrifugation at 12 000 g at 4°C for 10 min. The pellet was dried and resuspended in 15–50 µL volume of dis-tilled water to serve as template for PCR amplification. The amount of extracted DNA was measured using a spectrophotometer for quantification of the DNA tem-plate. DNA extracts from citrus tissues were prepared using the method described by Hung et al. (1999b).
Primers and thermal cycles
A primer pair (5′-CAC CGA AGA TAT GGA CAA
CA-3′; 5′-GAG GTT CTT GTG GTT TTT CTG-3′) for PCR-based detection of Las was derived from the sequence of a cloned Las-specific DNA fragment (Hung et al., 1999a) and designed to amplify a Las-specific DNA fragment (226 bp) by PCR. The assay has proven to be Las-specific and sensitive for Las detection in citrus tissues (Hung et al., 1999b). PCR was performed using 25 µL reaction mixture containing 20 mm Tris-HCl pH 8·4, 50 mm KCl, 4 mm MgCl2, 0·2 mm each dATP, dTTP, dCTP and dGTP,
50 ng of each primer, 0·75 units Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 250 ng template DNA. Thermal cycling conditions were: one cycle at 94°C for 3 min; 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min; followed by a 72°C extension for 10 min. Reactions were carried out in a DNA Thermal Cycler 2400 (Perkin Elmer, Norwalk, CT, USA).
Analysis of PCR products by electrophoresis
PCR products were analysed by gel electrophoresis using 1·4% agarose in TAE buffer (40 mm Tris-acetate, 1 mm EDTA pH 8·0). After electrophoresis (100 V for 30 min), products in the gel were stained with ethidium bromide (0·5 µg mL−1
), visualized and analysed using the alphaimager 2000 Documentation & Analysis System (Alpha Innotech Co., San Leandro, CA, USA). A 100 bp DNA ladder set (Promega, Madison, WI, USA) was included to determine fragment size.
Results
Las detection in single and multiple adult psyllids
Forty-four adult psyllids on the Las-infected LSO plant were collected for DNA extraction as well as Las detection after a 2-week acquisition-feeding period. Las-carrying psyllids were detected by PCR assay using the Las-specific
primer pair with our DNA extraction procedure. DNA extracts were prepared from single or multiple (three, five, 10, 25) adults. The results of Las detection in single and multiple psyllid adults are shown in Fig. 1. A PCR prod-uct of correct size was amplified from all psyllids from Las-infected plants, whereas no fragment was obtained from psyllids exposed to healthy plants. Las detection in an individual adult showed an equal signal to that in multiple adults when the DNA template of PCR is stand-ardized (Fig. 1). Signal strength from PCR-amplified fragments on the gel did not appear to differ among Las detections in one, three, five, 10 and 25 adults.
Las detection in nymphal psyllids
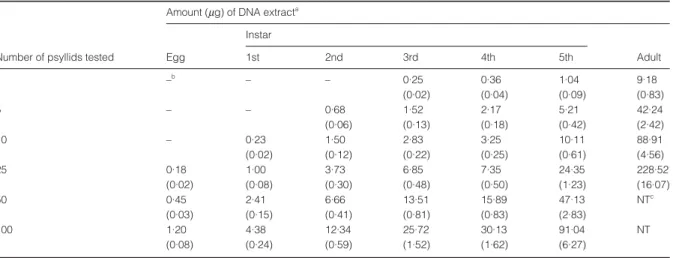
The different nymphal instars collected from a Las-infected citrus plant were separated and tested for the presence of Las. Before PCR was conducted, the quantity of DNA extract from different instars was estimated by a spectrophotometer (Table 1). Detectable amounts of DNA were obtained from individual nymphs of the third, fourth or fifth instars. According to the average results of 10 single-nymph extractions, approximately 250 ng DNA could be obtained from a third instar; 360 ng from a fourth instar; and 1040 ng from a fifth instar. However, DNA from individuals of the first and second instars was undetectable. To gather more DNA from the first two instars, DNA was extracted from multiple nymphs. A minimum of either five second instars or 10 first instars was necessary to obtain enough DNA (≈200 ng) for spec-trophotometer monitoring.
Las was detected in individual third, fourth and fifth instars (Fig. 2). Ten nymphs of each different instar were collected for the individual tests. Seven, six and four individuals in tests of fifth, fourth and third instars, respectively, were Las-positive. Las was not detected in individuals of the second or first instars. The total DNA extracted from individual first and second instars may not be enough to serve as PCR templates. The results in Fig. 3
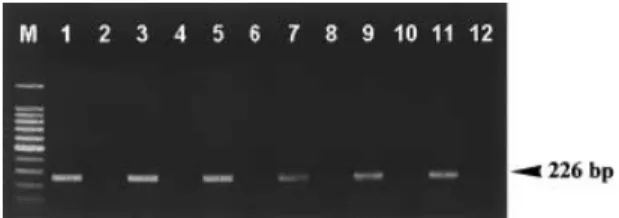
Figure 1 Detection of Las in single and multiple adult psyllids by PCR using a Las-specific primer pair. The template was 250 ng DNA extracted from 1, 3, 5, 10 and 25 adults. PCR products were analysed by electrophoresis in a 1·4% agarose gel, and positive results were recognized by the appearance of the Las-specific 226 bp band. DNA samples extracted from one, three, five, 10 and 25 adults inhabiting the Las-infected citrus plant were Las-positive (lanes 1, 3, 5, 7 and 9, respectively), whereas those from one, three, five, 10 and 25 adults inhabiting the healthy citrus plant were Las-negative (lanes 2, 4, 6, 8 and 10). Tissues from Las-infected and healthy citrus leaves were included in this test (lanes 11 and 12) for comparison. Lane M, 100 bp DNA ladder.
Detection of the HLB bacterium in psyllids 99
show that Las could be detected in the second instar when 10 or more nymphs were combined for DNA extraction. However, samples of first instars were always negative for Las, even when 100 nymphs were extracted for PCR. Standardization of PCR template prepared from
25 nymphs resulted in Las detection in different instars (Fig. 4). Las was detected from the second to fifth instars, but the signal strength of the PCR band observed in the gel varied among the four different instars. The signal was faint for the second instar, moderate for the third, robust for the fourth, and the strongest for the fifth instar. Com-pared to detection in the adult sample, the fifth instar had an equally strong signal.
Tests for transovarial passage of Las
To determine whether transovarial passage of Las occurs in D. citri, the eggs oviposited by Las-carrying females
Table 1 Amount of DNA obtained from individual or multiple eggs, nymphs and adults of Asian citrus psyllid (Diaphorina citri Kuwayama) using a rapid DNA extraction method
Amount (µg) of DNA extracta
Instar
Number of psyllids tested Egg 1st 2nd 3rd 4th 5th Adult
1 –b – – 0·25 0·36 1·04 9·18 (0·02) (0·04) (0·09) (0·83) 5 – – 0·68 1·52 2·17 5·21 42·24 (0·06) (0·13) (0·18) (0·42) (2·42) 10 – 0·23 1·50 2·83 3·25 10·11 88·91 (0·02) (0·12) (0·22) (0·25) (0·61) (4·56) 25 0·18 1·00 3·73 6·85 7·35 24·35 228·52 (0·02) (0·08) (0·30) (0·48) (0·50) (1·23) (16·07) 50 0·45 2·41 6·66 13·51 15·89 47·13 NTc (0·03) (0·15) (0·41) (0·81) (0·83) (2·83) 100 1·20 4·38 12·34 25·72 30·13 91·04 NT (0·08) (0·24) (0·59) (1·52) (1·62) (6·27) a
Means (SD) of 10 replicates of eggs, nymphs or adults. The amount of DNA extract was calculated from the OD260. b
–, not detectable.
c
NT, no test.
Figure 2 PCR detection of Las in individual psyllids of the third, fourth and fifth instars. Approximately 250 ng of DNA extract was used for the PCR template of each sample. PCR products were analysed by electrophoresis in a 1·4% agarose gel, and positive results were recognized by the appearance of the Las-specific 226 bp (arrowed) band. Ten nymphs of each different instar inhabiting the Las-infected citrus plant were collected for the individual tests. Lane M, 100 bp DNA ladder; lanes 1–10, 10 randomly sampled nymphs of the third instar; lanes 11– 20, 10 randomly sampled nymphs of the fourth instar; lanes 21– 30, 10 randomly sampled nymphs of the fifth instar; lane D, a Las-infected citrus sample as positive control.
Figure 3 PCR detection of Las in single and multiple first and second instar psyllids. The template DNA for PCR was extracted from one, five, 10, 50 and 100 nymphs colonizing the Las-infected citrus plant. All the DNA was used for PCR templates in the samples of one, five and 10 first instars as well as single second instars. Approximately 250 ng DNA was used for PCR templates in the other samples. PCR products were analysed by electrophoresis in a 1·4% agarose gel, and positive results were recognized by the appearance of the Las-specific 226 bp (arrow) band. In the first instar the samples of one, five, 10, 50 and 100 nymphs were Las-negative (lanes 1–5, respectively). In the second instar, Las was not detected in the samples of one and five nymphs (lanes 6 and 7), whereas Las was detected in samples of 10, 50 and 100 nymphs (lanes 8–10). Lane D, a Las-infected citrus sample. Lane M, 100 bp DNA ladder for size markers.
100 T.-H. Hung et al.
were tested by the PCR method described above. Twenty adult females were sampled and tested for Las before the egg test. Fifteen (75%) females tested positive for Las, but Las was not detected in their eggs. Las detection in an individual or five, 10, 25, 50 and 100 egg batches was negative. Spectrophotometric measurement indicated that the DNA extracts from 25 or more eggs were enough to obtain the detectable value of OD260 (Table 1).
Approxi-mately 180 ng of DNA extracts was obtained from 25 eggs; 450 ng from 50 eggs; and 1200 ng from 100 eggs. However, the PCR fragment of Las could not be amplified from DNA derived from multiple eggs.
Another experiment for transovarial passage was the offspring test. The eggs produced by Las-carrying females on a CJO plant were kept in an insect-proof chamber to produce offspring after removing all the parental psyllids. Six weeks later, a total of 45 adults emerged. Las was not detected by PCR in any of the offspring (data not shown).
Las-carrying retention test of adult psyllids
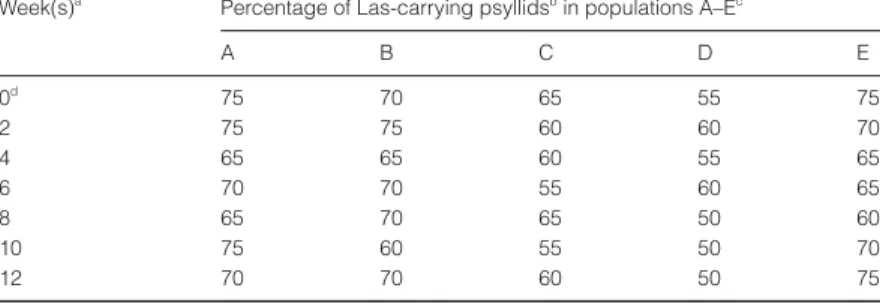
Results of the retention test are shown in Table 2. Twenty adult psyllids collected from the Las-carrying population (220 adults) were tested for Las immediately before the retention test, and 15 of 20 (75%) adults were Las-positive (population A). The percentage of Las-carrying adults did not differ significantly over the 12 weeks. The propor-tion of Las-carrying adults in populapropor-tion A, for example, was 65–75% during the 12-week experimental period. The experiments were repeated four times (psyllid popu-lations B–E), with similar results. Adult psyllids had Las in their bodies for more than 12 weeks even though they were moved to a Las-immune plant (CJO).
Discussion
The newly devised method described here is efficient for Las detection in psyllids. It consists of three major steps: extraction of total nucleic acids from psyllid bodies; PCR amplification; and analysis of PCR products by electro-phoresis. The entire procedure can be completed within 5 h: 1·5 h for extraction of nucleic acids; 3 h for PCR; 0·5 h for electrophoresis. The process is more efficient than the protocol designed for Las detection in citrus tissues (Hung et al., 1999b). The entire procedure takes 6–7 h for Las detection in citrus tissues. This method is simple, efficient, and can be used on a large number of psyllid samples in a short period of time.
The highly sensitive PCR-based assay can detect Las in a single adult psyllid. The signal strength of the PCR band on gel obtained from the single-adult test is similar to that obtained from multiple-adult tests. Thus this assay should be helpful in determining the proportion of Las-carrying individuals in a field population of psyllids. The method is currently being used to study the seasonal dynamics of Las in psyllid populations on citrus orchards in Taiwan.
Las could be detected in nymphal psyllids inhabiting diseased citrus plants. This shows that both adult and nymphal psyllids can carry Las in their bodies, agreeing
Figure 4 Comparative sensitivity of PCR detection of Las in different instars and adults of the psyllids. DNA was prepared from 25 psyllids, and 250 ng of DNA was used for the PCR template. The Las-specific 226 bp (arrow) band was amplified from samples of the second (lane 3), third (lane 5), fourth (lane 7) and fifth (lane 9) instars, as well as from adults (lane 11) reared on the Las-infected citrus plant. Las could not be detected in the sample of the first instar from the diseased citrus host (lane 1). The samples of nymphal and adult psyllids collected from the healthy citrus plant were the corresponding negative controls (lanes 2, 4, 6, 8, 10, 12 for first, second, third, fourth, fifth instars and adults, respectively). Lane M, 100 bp DNA ladder for size markers.
Week(s)a Percentage of Las-carrying psyllidsb in populations A–Ec
A B C D E 0d 75 70 65 55 75 2 75 75 60 60 70 4 65 65 60 55 65 6 70 70 55 60 65 8 65 70 65 50 60 10 75 60 55 50 70 12 70 70 60 50 75 a
Tests were conducted every 2 weeks after the psyllid population was transferred to a Las-immune common jasmine orange (CJO) plant.
b
Twenty adults were sampled and tested for Las from a population of adult psyllids each time.
c
Five populations (A–E) of adult psyllids were collected from different Las-infected citrus plants for Las.
d
In week 0 the test was conducted immediately before the psyllid population was transferred to a CJO plant.
Table 2 Retention of Las by adult psyllids over time
Detection of the HLB bacterium in psyllids 101
with the previous bioassay results (Xu et al., 1988). Previous research demonstrated that Las-carrying adult psyllids were effective vectors that transmitted Las to host plants. Plants inoculated by Las-carrying adults tested Las-positive by the PCR assays 2 months after inoculation, and showed evident HLB symptoms 8 months after inoculation (Hung et al., 2001). Although the nymphs hardly move, they soon become Las-carrying adults with the ability to fly and transmit Las to other citrus plants. Thus the control period of vector psyllid should include the nymphal stages. The single-psyllid PCR-based test is also suitable for screening third, fourth and fifth instars (Fig. 2). However, Las was not detected using individual first or second instars. The amount of DNA extracted from individuals of the first and second instars was too low to monitor using a spectrophotometer. The multiple-nymph test was therefore adopted for Las detection in the first two instars. Las could be detected when 10 or more second-instar nymphs were combined in one sample for DNA extrac-tion (Fig. 3). This indicates that psyllids acquired few Las through acquisition feeding between the first and second instars. Las was always detected in samples of 10 nymphs of the second instar which were kept on Las-infected citrus shoots, whereas Las was not detected in any of the multiple-nymph first instar samples. Based on these data, it is concluded that psyllids can carry Las in either adult or nymphal stages, except in the first instar.
Las detections among five instars showed different signal strength in the PCR-based assays (Fig. 4). The signal strength of the PCR band on the gel increased with each instar. Detection in the second instar had the weakest signal, whereas the fifth instar gave a signal which was as strong as that obtained with adults. Thus the amount of Las DNA appears to increase through vector metamor-phosis. This may be indirect evidence to suggest the prop-agative nature of Las in vector psyllids.
In the Las-retention test, psyllids maintained Las-carrying ability for 12 weeks, even though acquisition feeding of Las had been terminated. According to a previous study (Lin et al., 1973), the Asian citrus psyllid has an adult lifespan of ≈90 days. The retention test was carried out for 84 days, which covered most of the lifespan. Thus it can be inferred that Las persists in the Asian citrus psyllid vector. In general, vectors carrying persistent pathogens need more time than vectors carrying nonpersistent pathogens to acquire the pathogens through acquisition feeding. Vectors carrying persistent pathogens also need a longer period to transmit the pathogens to host plants successfully (Nault & Ammar, 1989). Vector control with insecticides is usually considered feasible for controlling the diseases caused by insect-borne pathogens that are transmitted in a persistent manner.
Although Asian citrus psyllids can carry Las through-out their adult lives, they cannot directly transmit Las to their offspring. A similar phenomenon appears in pear decline, another disease caused by a psyllid-borne phyto-plasma (Hibino et al., 1971; Davies et al., 1995). If an insect vector has the property of transovarial passage, disease control is more difficult. Fortunately, the results
reported here showed that Asian citrus psyllids cannot transovarially transmit Las to their offspring although the pathogen is persistent in the adult vectors.
PCR-based assays have been proven effective for stud-ying other insect-borne phytoplasma-caused diseases such as aster yellows and sugarcane white leaf. The aster yel-lows phytoplasma was detected in nymphs and adults of the leafhopper vector Scaphoideus titanus by PCR, using phytoplasma 16Srl group-specific primers (Alma et al., 1997). Likewise, the phytoplasma causing sugarcane white leaf disease was detected in nymphs and adults of the leafhopper vector Matsumuratettix hiroglyphicus (Hanboonsong et al., 2002). However, unlike the psyllid vectors of Las, both these leafhoppers tested positive for transovarial transmission by PCR.
References
Alma A, Bosco D, Danielli A, Vibio M, Arzone A, 1997. Identification of phytoplasmas in eggs, nymphs and adults of Scaphoideus titanus Ball reared on healthy plants. Insect Molecular Biology 6, 115–21.
Bové JM, Calavin EC, Capoor SP, Cortez RE, Schwarz RE, 1974. Influence of temperature on symptoms of California stubborn, South Africa greening, India citrus decline and Philippine leaf mottling disease. In: Weathers LG, Cohen M, eds. Proceedings of the Sixth Conference of the International Organization of Citrus Virologists, 1972. Swaziland: IOCV, 12–15.
Bové JM, Garnier M, Ahlawat YS, Chakraborty NK, Varma A, 1993. Detection of the Asian strains of the greening BLO by DNA–DNA hybridization in Indian orchard trees and Malaysian Diaphorina citri psyllids. In: Moreno P, de Graca JV, Timmer LW, eds. Proceedings of the 12th Conference of the International Organization of Citrus Virologists, 1992. New Delhi, India: IOCV, 258–63.
Capoor SP, Rao DG, Viswanath SM, 1967. Diaphorina citri Kuwayama, a vector of the Greening disease of citrus in India. Indian Journal of Agricultural Science 37, 572–6.
Chen MH, Miyakawa T, Matsui C, 1973. Citrus Likubin pathogens in salivary glands of Diaphorina citri. Phytopathology 63, 194–5.
Davies D, Barbara DJ, Clark MF, 1995. The detection of MLOs associated with pear decline in pear trees and pear psyllids by polymerase chain reaction. Acta Horticulturae 386, 484–8. Garnier M, Bové JM, 1993. Citrus greening disease and the
greening bacterium. In: Moreno P, de Graca JV, Timmer LW, eds. Proceedings of the 12th Conference of the International Organization of Citrus Virologists, 1992. New Delhi, India: IOCV, 212–9.
Garnier M, Jagoueix-Eveillard S, Cronje PR, Roux HF, Bové JM, 2000. Genomic characterization of a liberobacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape Province of South Africa. Proposal of ‘Candidatus Liberibacter africanus subsp. capensis’. International Journal of Systematic and Evolutionary Microbiology 50, 2119–25.
da Graca JV, 1991. Citrus Greening disease. Annual Review of Phytopathology 29, 109–36.
Hanboonsong Y, Choosai C, Panyim S, Damak S, 2002. Transovarial transmission of sugarcane white leaf
102 T.-H. Hung et al.
phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura). Insect Molecular Biology 11, 97–103.
Hibino H, Kaloostian GH, Schneider H, 1971. Mycoplasma-like bodies in the pear psylla vector of pear decline. Virology 43, 34–40.
Hocquellet A, Toorawa P, Bové JM, Garnier M, 1999. Detection and identification of the two Candidatus Liberobacter species associated with citrus huanglongbing by PCR amplification of ribosomal protein genes of the beta operon. Molecular and Cellular Probes 13, 373–9.
Huang CH, 1979. Distribution of likubin pathogen in likubin-affected citrus plant. Journal of Agricultural Research of China 28, 29–33.
Hung TH, Wu ML, Su HJ, 1999a. Detection of fastidious bacteria causing citrus greening disease by non-radioactive DNA probes. Annals of the Phytopathological Society of Japan 65, 140–6.
Hung TH, Wu ML, Su HJ, 1999b. Development of a rapid method for the diagnosis of citrus greening disease using the polymerase chain reaction. Journal of Phytopathology 147, 599–604.
Hung TH, Wu ML, Su HJ, 2000. Identification of alternative hosts of the fastidious bacterium causing citrus greening disease. Journal of Phytopathology 148, 321–6.
Hung TH, Wu ML, Su HJ, 2001. Identification of the Chinese box orange (Severinia buxifolia) as an alternative host of the bacterium causing citrus Huanglongbing. European Journal of Plant Pathology 107, 183–9.
Jagoueix S, Bové JM, Garnier M, 1994. The phloem-limited bacterium of Greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. International Journal of Systematic Bacteriology 44, 379–86.
Jagoueix S, Bové JM, Garnier M, 1996. PCR detection of the two ‘Candidatus’ Liberobacter associated with greening disease of citrus. Molecular and Cellular Probes 10, 43–50. Jagoueix S, Bové JM, Garnier M, 1997. Comparison of the 16S/
23S ribosomal intergenic regions of ‘Candidatus Liberobacter asiatium’ and ‘Candidatus Liberobacter africanum’, the two species associated with citrus huanglongbing (greening) disease. International Journal of Systematic Bacteriology 47, 224–7.
Lin SJ, Ke YF, Tao CC, 1973. Bionomics observation and integrated control of citrus psylla, Diaphorina citri Kuwayama. Journal of the Chinese Society for Horticultural Science 19, 234–42.
McClean APD, 1970. Greening disease of sweet orange: its transmission in propagative parts and distribution in partially diseased trees. Phytophylactica 2, 263–8.
McClean APD, Oberholzer PCJ, 1965. Citrus psylla, a vector of the greening disease of sweet orange. South African Journal of Agricultural Science 8, 297–8.
Miyakawa T, 1980. Experimentally-induced symptoms and host range of citrus likubin (greening disease) in Taiwan, mycoplasma-like organisms, transmitted by Diaphorina citri. Annals of the Phytopathological Society of Japan 46, 224–30. Murashige T, Bitters WP, Naver EM, Roistacher CN, Holiday PB, 1972. A technique of shoot tip grafting and its utilization towards recovering virus-free citrus clones. Hortscience 7, 118–9.
Murray RGE, Schleifer KH, 1994. Taxonomic notes: a proposal for recording the properties of putative taxa of prokaryotes. International Journal of Systematic Bacteriology 44, 174–6. Nault LR, Ammar ED, 1989. Leafhopper and planthopper
transmission of plant viruses. Annual Review of Entomology 34, 503–29.
Xu CF, Xia YH, Li KB, Ke C, 1988. Further study of the transmission of citrus huanglungbin by a psyllid, Diaphorina citri Kuwayama. In: Timmer LW, Garnsey SM, Navarro L, eds. Proceedings of the 10th Conference of the International Organization of Citrus Virologists, 1986. Valencia, Spain: IOCV, 243–8.
Yang CT, 1984. Psyllidae of Taiwan. Special Publication Series 3. Taipei: Taiwan Museum.