Development of a questionnaire module to supplement the EORTC
QLQ-C30 to assess quality of life in patients with
hepatocellular carcinoma, the EORTC QLQ-HCC18
Jane M. Blazeby
a,b,*, Elspeth Currie
c, Benny C.Y. Zee
d, Wei-Chu Chie
e,
Ronnie T. Poon
f, O. James Garden
c, On behalf of the EORTC Quality of Life Group
a
Department of Social Medicine, Canynge Hall, University of Bristol, Whiteladies Road, Bristol BS8 2PR, UK
b
Clinical Sciences at South Bristol (Surgery), Bristol Royal Infirmatory, University of Bristol, Bristol BS2 8HW, UK
c
Clinical and Surgical Sciences (Surgery), University of Edinburgh, Edinburgh, UK
d
Comprehensive Cancer Trials Unit, Department of Clinical Oncology, Chinese University of Hong Kong, Hong Kong
e
Department of Public Health and Graduate Institute of Preventative Medicine, College of Public Health and Department of Family Medicine, College of Medicine, National Taiwan University, Taiwan
f
Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong Received 17 June 2004; accepted 22 June 2004
Available online 11 September 2004
Abstract
Measurement of quality of life (QoL) in hepatocellular carcinoma (HCC) requires assessment of factors related to chronic liver disease, as well as issues related to the primary tumour and its treatment. This study describes the development of a questionnaire module in patients from Europe, as well as Taiwan and Hong Kong. The questionnaire was developed according to the European Organisation for Research and Treatment of Cancer (EORTC) QoL Group guidelines. Twenty nine QoL issues were identified from a literature search. Semi-structured interviews with patients (n = 32) and health-care professionals (n = 10) reduced the issues to 22 items forming a provisional questionnaire. This was tested in 158 patients from three countries. Descriptive statistics and clinical judgement reduced the module to 18 items conceptualised as containing six scales and two single item. This study recommends the EORTC QLQ-HCC18 to accompany the QLQ-C30 to measure QoL in clinical trials in HCC.
Ó 2004 Elsevier Ltd. All rights reserved.
Keywords: EORTC; Quality of life; Hepatocellular carcinoma; Questionnaire
1. Introduction
Hepatocellular carcinoma (HCC) is one of the worldÕs most common malignancies, with a marked het-erogeneous geographical distribution [1]. In high-risk areas, such as Asia and Africa, annual incidence rates are between 10 and 120 per 100,000 population, whereas in low-risk areas, Northern Europe, United States of
America (USA) and India, annual incidence rates are below 3 per 100,000 population [1]. Over the past dec-ade, advances in diagnostic techniques and multi-disci-plinary management of HCC have led to small improvements in survival, although outcomes remains poor with overall one year survival being less than
20% [2,3]. Trials evaluating systemic chemotherapy
show very little benefit [4]. Local arterial infusion of cytotoxic agents or cytotoxic agents in combination with lipiodol may increase survival in well selected patients
[5,6]. Percutaneous approaches with ethanol, or radio-frequency ablation can be used for unresectable HCC
0959-8049/$ - see front matter Ó 2004 Elsevier Ltd. All rights reserved. doi:10.1016/j.ejca.2004.06.033
* Corresponding author. Tel.: +44 117 9283495; fax: +44 117
9252736.
E-mail address:j.m.blazeby@bristol.ac.uk(J.M. Blazeby).
www.ejconline.com European Journal of Cancer 40 (2004) 2439–2444
Journal of
Cancer
and there is currently interest in the role of octreotide
[7]. Potentially curative treatments, including resection, percutaneous ablation or liver transplantation can be of-fered to patients with small lesions confined to the liver and five year survival may reach 50% [5]. Treatment strategies are aimed at incorporating prognosis estima-tion with potential treatment advancements [8]. Although survival data and information about the side-effects of treatment are widely available, much less is known about how treatment for HCC impacts upon patientsÕ health-related quality of life (QoL). Self-reported QoL data includes measures of physical, social and emotional well-being and this is valuable for pa-tients and clinicians in decision-making[9]. Self-reported health data may also predict survival in patients with cancer and in other populations[10].
The most widely used instruments in assessing QoL in cancer patients within the context of clinical trials are the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and the Functional Assessment of Cancer Therapy (FACT) generic ques-tionnaire [11,12]. These both use a general core ques-tionnaire and may be supplemented by disease-specific modules. A module for hepatobiliary cancers has been published by the FACT group [13]. This is designed for patients with cancer of the head of pancreas, colorec-tal liver metastases, primary liver cancer and cholangio-carcinoma. It has 18 items that may be aggregated to produce a FACT-Hep scale. The EORTC group has ta-ken a more focused approach, by developing separate modules for pancreatic cancer, colorectal hepatic meta-stases and primary liver cancer [14,15]. This approach was followed because these cancers have different aetiol-ogies, epidemiology, clinical problems, treatments and disease progression. This paper describes the develop-ment of the EORTC questionnaire, module to
accom-pany the EORTC QLQ-C30 to comprehensively assess QoL in patients with HCC.
2. Patients and methods 2.1. Study design
The development of the provisional module was per-formed according to the EORTC Quality of Life Group (QLG) published guidelines for questionnaire develop-ment[16–18]. These guidelines are summarised inTable 1. The final part of module development (Phase 4) con-sists of psychometric testing and is not part of this paper.
2.2. Subjects
Patients for the interviews carried out in Phase 1 were recruited from the United Kingdom (UK), The Chinese University of Hong Kong and Egypt. In Phase 3 of the study, patients from the UK, Taiwan, the Chinese Uni-versity of Hong Kong and Queen Mary Hospital Hong Kong were included. Eligible patients were required: (a) to have been diagnosed with HCC; (b) to have no other concurrent malignancy; (c) to speak and understand the respective language of the questionnaire; (d) to give full informed consent. Ethical committee permission was obtained.
2.3. Data analysis
Descriptive statistics were used to analyse results of the interviews in Phases 1 and 3. Mean scores of <2.0 for the professionals and patients were used as cut-off points for consideration of deletion of an issue in
Table 1
Guidelines for development of an EORTC disease-specific QL module
Phase Aim Process
1 Generation of QoL issues relevant to the selected group of patients
1. Literature search
2. Semi-structured interviews with health-care professionals and patients 3. Analysis of qualitative and quantitative data
4. Combination of results from interviews to produce a list of issues 2 Construction of a provisional
questionnaire
1. Consultation of the EORTC QoL group item database 2. Construction of new items
3. Translation of provisional questionnaire 3 Pretesting the questionnaire for
acceptability and relevance
1. Patients complete module with interview 2. Analysis of quantitative and qualitative data 3. Modification of questionnaire
4. Formal development report reviewed by EORTC QoL group
4 International field testing Psychometric testing of the reliability, validity and sensitivity of the module EORTC, European Organisation for Research and Treatment of Cancer; QoL, quality of life.
Phase 1. In Phase 3, mean scores <1.5 were used for consideration for deletion from the final module. Items scores were considered in conjunction with qualitative comments made during the interviews
[19,20].
3. Results
3.1. Phase 1: Generation of QoL issues 3.1.1. Literature search
Literature searches were performed in three dat-abases: MEDLINE (1966–June 2002), EMBASE (1980–June 2002), and CINAHL (1982–April 2002). The searches were limited to the English language. The major subject heading, HCC was combined with sur-gery, QoL, questionnaires, chemotherapy, alcohol ablation, chemoembolisation, physical distress, psycho-logical distress, psychosocial distress and physical symp-toms. This identified 2055 articles. Nine papers described QoL questionnaires used with patients with HCC, but only two had supporting psychometric data and may be used for all patients with HCC [13,21]. These articles produced a list of 33 potentially relevant QoL issues.
3.1.2. Interviews with health-care professionals
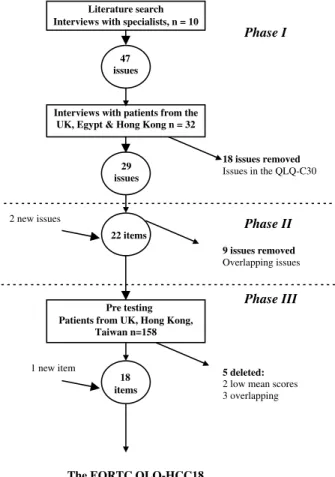
Ten health-care professionals (two surgeons, four specialist nurses, three gastroenterologists and an oncol-ogist) were interviewed. Mean scores were above 2.0 for all QoL issues except six. An additional 14 issues were suggested and 47 issues were therefore presented to pa-tients in Phase 1.Fig. 1summarises the module develop-ment process.
3.1.3. Interview with patients
Patients were recruited from six hospitals in the UK, Taiwan, Egypt and Hong Kong. Socio-demographic and clinical details are shown in Table 2. Analyses of mean relevance scores, discussion of qualitative inter-views and scrutiny for overlapping issues resulted in exclusion of 18 issues (29 issues remaining).
3.2. Phase 2: production of the provisional questionnaire The 29 QoL issues were discussed in detail with the EORTC QLG. Issues that overlapped with items in the core questionnaire or with each other were merged. There were two new issues that were added: Ôthe need to take pain killersÕ and Ôworry about nutritionÕ. This proc-ess resulted in 22 items (Fig. 1). Eight items were ob-tained from QLG Item Bank and 14 new items developed. The provisional module, the EORTC QLQ-HCC22, was reviewed and approved by two members of the EORTC QoL Group and subsequently translated
according to the strict translating guidelines into Tai-wanese and Chinese[22].
3.3. Phase 3: pre-testing in the EORTC QLQ-HCC22 Pre-testing was performed in 158 patients (Table 2). On the basis of descriptive statistics and interviews, two single items were deleted (need to take pain-killers and swollen ankles/legs). Three other items had low mean scores: ÔHave you had pain in your upper abdo-men?Õ, ÔHave you had problems with your sense of taste?Õ and ÔDid you have night sweats?Õ After discussion and because of clinical importance, it was decided to re-tain these items. The wording of the item addressing abdominal pain was changed to synchronise with EORTC modules. The item ÔDid you have night sweatsÕ was split into two items, ÔHave you had feversÕ and ÔHave you had chills?Õ.
All six fatigue items in Phase 3 had high patient mean scores (>1.78). These were considered alongside the three fatigue items in the QLQ-C30 and three were de-leted because of perceived content overlap. The resultant questionnaire consisted of 18 items and has been named the EORTC QLQ-HCC18. This is hypothesised to
Phase I 47 issues 29 issues - - - Phase II 22 items - - - Phase III 18 items The EORTC QLQ-HCC18 Pre testing Patients from UK, Hong Kong,
Taiwan n=158 22 items
Interviews with patients from the UK, Egypt & Hong Kong n = 32
9 issues removed
Overlapping issues
18 issues removed
Issues in the QLQ-C30
1 new item 5 deleted:
2 low mean scores 3 overlapping 2 new issues
Literature search Interviews with specialists, n = 10
Fig. 1. QLQ-HCC18 – summary of module development. UK, United Kingdom; EORTC, European Organisation for Research and Treat-ment of Cancer.
Table 2
Socio-demographic and clinical details of patients interviewed in Phases 1 and 3
Phase 1, n = 32 Phase 3, n = 158 Mean age (range)/years 60 (29–77) 58 (35–83) Gender (%)
Male 23 (72) 133 (84)
Marital status (%)
Single 3 (9) 9 (6)
Married or living with partner 24 (75) 139 (88) Separated/divorced/widowed 5 (16) 10 (6) Cohabitation status (%)
Living alone 4 (13) 14 (9)
Living with family 27 (84) 138 (87)
Living with other adults 1 (3) 6 (4)
Education (%)
Less than compulsory school 7 (22) 45 (28)
Compulsory school 12 (38) 60 (38)
Post-compulsory school 13 (41) 51 (32)
Unknown 0(0) 2 (1)
Employment (%)
Employed or homemaker (full-time/part-time) 14 (44) 56 (35)
Retired 15 (47) 62 (39)
Unemployed 1 (3) 32 (20)
Other 2 (6) 8 (5)
Centre (%)
Edinburgh Royal Infirmary, UK 17 (53) 3 (2) Bristol Royal Infirmary, UK 0 (0) 15 (10) National Taiwan University, Taiwan 0 (0) 40 (25) Chinese University of Hong Kong 11 (34) 60 (38) Queen Mary Hospital, Hong Kong 0 (0) 40 (25) Medical Research Institute, Alexandria, Egypt 4 (13) 0 (0) Timing of treatment (%)
Before start of treatment 2 (6) 30 (19)
During treatment 7 (22) 33 (21)
Post-treatment 23 (72) 95 (60)
Treatment group (%)
Hepatectomy alone 7 (22) 33 (21)
Hepatectomy and chemoembolisation 3 (9) 14 (9) Hepatectomy and percutaneous ablation 1 (3) 9 (6) Hepatectomy systemic chemotherapy 0 (0) 4 (3) Chemoembolisation alone 14 (44) 27 (17) Chemoembolisation and percutaneous ablation 0 (0) 2 (1) Chemoembolisation and systemic chemotherapy 0 (0) 5 (3) Lipiodol embolisation alone 0 (0) 8 (5) Radiofrequency ablation (RFA) 1 (3) 15 (10)
Transplant 0 (0) 14 (9)
Systemic chemotherapy alone 4 (13) 12 (8)
Ethanol injection alone 0 (0) 5 (3)
Best supportive care alone 2 (6) 10 (6) Co-morbid disease (%)
Hepatitis B 7 (22) 98 (62)
Hepatitis C 3 (9) 16 (10)
Hepatitis B and/or C and/or alcoholic disease 1 (3) 15 (10)
Alcoholic liver disease 1 (3) 9 (6)
Haemochromatosis 5 (16) 1 (1) Other or unknown 15 (47) 19 (12) Child-Pugh grade (%) A 26 (81) 124 (79) B 3 (9) 22 (14) C 3 (9) 9 (6) Unknown 0 (0) 3 (2)
contain scales addressing fatigue (3 items), body image (2 items), jaundice (2 items), nutrition (5 items), pain(2 items) and fevers (2 items) and two single items address-ing sexual interest and abdominal swelladdress-ing. The module development committee of the EORTC QoL group has approved the module and developmental process.
4. Discussion
The EORTC QLQ-HCC18 has been methodologi-cally developed using standard guidelines. It is designed for use with the QLQ-C30 core instrument to assess all major dimensions of health-related QoL in patients with HCC. The content of the questionnaire has been derived not only from the published literature, but also from health professionals dealing with these patients and, most importantly, from the patients themselves. Testing the QLQ-HCC18 in 158 patients from the UK, Taiwan and Hong Kong confirms that the items are understood, relevant and acceptable to patients with different cul-tural backgrounds and with differing HCC aetiology. The hypothesised scale structure and single items, ad-dress aspects of chronic liver disease (nutrition, jaun-dice, fevers, abdominal swelling), as well as QoL issues specific to the primary tumour and its treatment (fati-gue, body image, pain). The fourth phase of question-naire development is currently in preparation and will provide essential information on the psychometric prop-erties of the module.
There are two published questionnaires for HCC with supporting psychometric data, the FACT-Hep and The Liver Cancer QoL Scale [13,21]. The FACT-Hep in-cludes problems related to pancreatic cancer and colo-rectal liver metastases (such as back pain and gastrointestinal symptoms). It may therefore lack specif-icity for patients with HCC. One study in 20 patients with HCC used the FACT Hep[23]. Significant changes in generic aspects of QoL were reported, but HCC symptoms or treatment-related symptoms did not dem-onstrate major changes after treatment. A prospective study after resection of HCC used the generic FACT questionnaire to measure QoL[24]. Significant changes in QoL were reported in several of the core scales, but no disease-specific items were used in this study. The Li-ver Cancer QoL Scale was developed in Chinese patients and has not been widely used elsewhere. Although the EORTC QLQ-HCC18 has only been developed within two language subgroups, it is the first questionnaire to include patients from the East and West in development and therefore has the potential for use in international trials in HCC.
The development of the EORTC QLQ-HCC18 has primarily involved patients from the UK, Taiwan and China and is currently available in Arabic, Chinese (Cantonese), English and Taiwanese (Manderin).
Although most EORTC questionnaire modules are developed in more European countries, half of the worldÕs total patients with HCC are Chinese because of the chronic hepatitis B carrier rate (in excess of 10%). This questionnaire was therefore deliberately developed in a relevant population in which it will mainly be used in the future. The Phase 4 part of ques-tionnaire development (reliability and validity testing) will partly incorporate a UK randomised study, centres from Hong Kong and Taiwan and other centres from across Europe will be invited to participate. This mul-ti-lingual approach is essential to examine the cross-cul-tural validity of the module. The EORTC QLQ-HCC18 is therefore suitable for use in clinical trials of HCC. Further international testing will be performed to con-firm the clinical and psychometric validity of the questionnaire.
Conflict of Interest Statement None declared.
Acknowledgements
The authors acknowledge D. Alderson, M.D. Finch-Jones, R. Jones and F. Gordon (Bristol Royal Infirmary) for allowing us to study patients under their care. We are grateful to Mr. N. Dowidar (Egypt) for his contribution to the early Phase 1 part of the study. The authors are grateful to G. Velikova, T. Conroy, M. Sprangers, D. Osoba and E. Greimel, members of the EORTC Quality of Life Group who reviewed the development of this module. This work was sponsored by the EORTC Quality of Life Group, The National Science Council, Taiwan and the UK Medical Research Council (J.M.B. Clinician Scientist award).
References
1. Parkin DM, Laara E, Muir CS. Estimates of the world-wide frequency of sixteen major cancers in 1980. Int J Cancer 1988, 41, 184–197.
2. El-Sarag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology 2001, 33, 62–65.
3. Faivre J, Formann D, Esteve J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe. Eur J Cancer 1998, 34, 2184–2190. 4. Di Maio M, De Maio E, Perrone F, Pignata S, Daniele B.
Hepatocellular carcinoma: systemic treatments. J Clin Gasteroen-terol 2002, 35, 109–114.
5. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. The Lancet 2003, 362, 1907–1917.
6. Lau WY, Leung TWT, Ho SKW, Chan M, Machin D, Lau J, et al. Adjuvant intra-arterial lipiodol-iodine-131 for resectable
hepatocellular carcinoma: a prospective randomised trial. The Lancet 1999, 353, 797–801.
7. Yuen MF, Poon RTP, Lai CL, Fan ST, Lo CM, Wong KW, et al. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepa-tology 2002, 36, 687–691.
8. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002, 35, 519–524. 9. Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson
NK. Health-related quality-of-life assessments and patient-physi-cian communication: a randomized controlled trial. JAMA 2002, 288, 3027–3034.
10. Idler EL, Russell LB, Davis D. Survival, functional limitations and self-rated health in the NHANES I epidemiological follow-up study, 1992. Am J Epidemiol 2000, 152, 874–883.
11. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993, 85, 365–376.
12. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: devel-opment and validation of the general measure. J Clin Oncol 1993, 11, 570–579.
13. Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer thera-py-hepatobiliary questionnaire. J Clin Oncol 2002, 20, 2229–2239. 14. Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, et al. Development of a disease specific quality of life questionnaire module to supplement the EORTC core cancer quality of life questionnaire, the QLQ-C30 in patients with pancreatic cancer. Eur J Cancer 1999, 35, 939–941.
15. Kavadas V, Blazeby JM, Conroy T, Sezer O, Holzner B, Koller M, et al. Development of an EORTC disease-specific quality of life questionnaire for use in patients with liver metastases from colorectal cancer. Eur J Cancer 2003, 39, 1259–1263.
16. Sprangers MAG, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organisation for Research and Treatment of Cancer approach to quality of life assessment: guidelines for developing questionnaire modules. Qual Life Res 1993, 2, 287–295.
17. Sprangers MAG, Cull A, Groenvold M, Bjordal K, Blazeby JM, Aaronson NK, et al. The European Organisation for Research and Treatment of Cancer approach to developing questionnaire modules: an update and overview. Qual Life Res 1998, 7, 291–300. 18. Bottomley A, Vachalec S, Bjordal K, Blazeby JM, Flechtner H, Ruyskart P. The development and utilisation of the European Organisation for Research and Treatment of Cancer quality of life group item bank. Eur J Cancer 2002, 38, 1611–1614.
19. Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et aland on behalf of the EORTC Quality of Life Group. Development of an EORTC disease specific quality of life module for use in patients with gastric cancer. Eur J Cancer 2001, 37, 966–971.
20. Blazeby JM, Alderson D, Winstone K, Steyn R, Hammerlid E, Arraras J, et al. Development of a EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. Eur J Cancer 1996, 32, 1912–1917.
21. Wan C-H, Fang J-Q, Zhang C-Z, Lin Y, Luo Y-M. Development and evaluation of a quality of life scale for patients with liver cancer. MAPI Research Institute Quality of Life Newsletter 2000, 24, 14.
22. Cull A, Sprangers MAG, Bjordal K, Aaronson NK. on behalf of the EORTC Quality of Life Study Group. EORTC quality of life study group translation procedure. Internal report of the EORTC Study Group on Quality of Life; 1998. ISBN-2-930064-15-3. 23. Steel J, Baum A, Carr B. Quality of life in patients diagnosed with
primary hepatocellular carcinoma: hepatic arterial infusion of cisplatin versus 90-YTTRIUM microspheres (therasphere). Psy-cho-Oncology 2004, 13, 73–79.
24. Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg 2001, 136, 693–699.