Ink-jet Printing of Nylon Fabric Using Reactive Dyestuff
全文
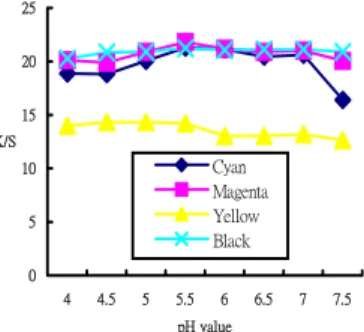
(2) of saturated steam. The steaming time was 15min, 20min, 25min, 30min and 35min.The steamed samples were washed-off in a fresh bath, employing a liquor ratio of 20:1, 2g/l MRT and 2g/l Na2CO3 at 60℃ of hot washing until all unreacted dyes and chemicals were remove from the fabric surface. The washed samples were dried in an oven at 80℃ 2.6 Color yield measurement The printing sample were determined using Datacolor SF-600 PLUS C-T spectrophotometer. The measurement was set under specular excluded with large aperture. The sample was folded twice to ensure opacity and measured three times, the measured results were then averaged. The color yield (K/S value) was calculated for wavelengths of 400-700 nm at 20 nm intervals within the visible spectrum. The K/S values were calculated according to Equation 1: (1) K / S = (1-R)2 / 2R Where , K is the absorption coefficient(depending on the concentration of colorant), S is the scattering coefficient (caused by the dyed substrate ) and R is the reflectance of the colored sample at λmax. The higher the K/S value is, the greater the color yield. 2.7 Color fastness measurement Color fastness to washing was tested by AATCC Test Method 61.2A-2003, color fastness to crocking by AATCC Crockmeter Method 8-2001 and light fastness by AATCC Test Method 16.3-2003. 2.8 Ink limit estimation The ink ejected amount of the printed fabrics was assessed by ErgoSoft Texprint Inklimit Test method, the determination interval of inks amount were from 150% to 400% at 10 % intervals.. can combine with nylon then form the covalent bond[7,8]. This article we selected two different non-volatile organic acid, citric acid and tartaric acid that take as pH adjust agents, probe into the same pH value (pH at 5.5) and different kind acid agent. Figure 2 show that the effect of different acid with K/S value, we can find that adopt citric acid to make pH value adjust agent , except yellow K/S value is almost same with tartaric acid, other colours cyan, magenta and black were all higher than tartaric acid. For this reason we recommended the citric acid for use while printing to nylon fabric with reactive dyestuff. 25 20 15 K/S. Cyan Magenta Yellow Black. 10 5 0 4. 4.5. 5. 5.5. 6. 6.5. 7. 7.5. pH value. Fig. 1. Effect of pH value with K/S value. Citric. Tartaric. 25 20. 3.Result and discussion 3.1 Effect of pH with colour yield Nylon fabric is dyeing with reactive dyestuffs, the pH value acts a quite important role, so it is very important to control pH value in a suitable range, these were effect colour yield. We used sodium bicarbonate and citric acid to adjust pH value from 4.0 to 7.5 at 0.5 intervals, and measure the change of colouration. Figure 1 shows the effect of pH value with colour yield, the colour yield of all colors were increases with pH value raised, but reach highest when pH value is at 5.5, then the colour yield decrease with pH value continuous raised, particularly the colour yield of cyan was seriously down. In general, the functional of reactive dyestuff mainly reacts with amino group of nylon fibre, and form the covalent bond , amino group is in pH value at 5.5 will produce high degree protonation, so can achieve higher color yield, but when pH value increases again, nylon fabric will absence of amino group protonation and lead the color yield to reduce[6]. On the other hand, the colour yield of color will be reduced while the pH value down , because the concentration of neucleophilic amino groups in the substrates were unable to react with reactive dye, so the colour yield of nylon fabric were to reduce. Therefore ink-jet printing of nylon fabrics used reactive dyesyuffs , the pH value of pretreatment paste must adjust at 5.5 which can achieve highest colour yield. 3.2 Effect of different acid agents with colour yield In general , it must be under the alkali condition , the reactive dyestuff will form the covalent bond with the fibres, but printing nylon with reactive dyestuffs, we need to adjust the paste for neutral or weak acid , so the reactive dyestuffs. K/S. 15 10 5 0 Cyan. Magenta. Yellow. Black. Colours. Fig. 2 .Effect of different acid agent with K/S value. 3.3 Effect of fixation steaming condition with colour yield Steam fixation is to make the dyestuff permeate into fibre and form covalent bond, the setting of steaming fixation conditions is very important, while saturated steam achieve better colour yield than overheating steam and heat curing[4]. In this research, 102 ℃ of saturated steam and changes the time of steaming in order to discuss about color yield. Figure 3 show that color yield of yellow was slightly rising when we increased steaming time exceed 30min, color yield was not affected by steaming time. On the other hand, cyan, magenta and black could reach the maximum while steaming time at 30 min, but steaming time increase again, the K/S value has a downward trend . To sum up, the optimum condition analysis result of colour yield on the above various colors, the best performance of colour yield would be under 102℃×30 min of steaming fixation..
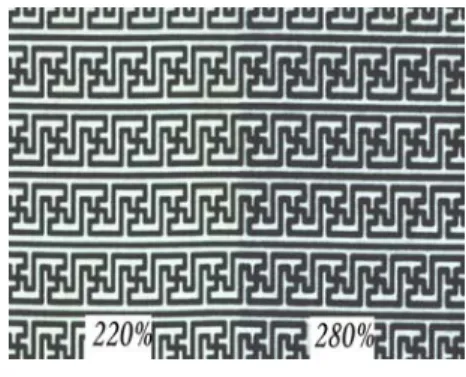
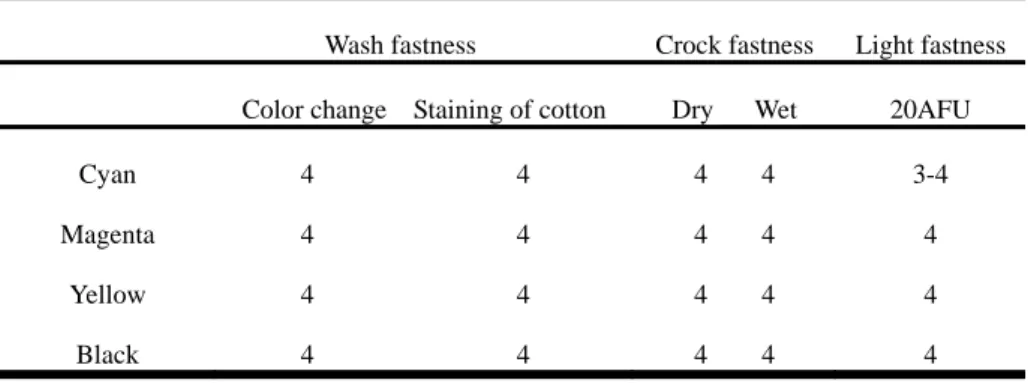
(3) 25 20 15 K/S. Cyan Magenta Yellow Black. 10 5 0 15. 20. 25. 30. 35. Steaming times,min Fig. 3. Effect of steaming times with K/S value. 3.4 Analysis of ink amount limit Even after sizing treatment, every fabrics still contains its maximum absorbability. When the amount of ink exceeds the fabrics absorbed limit, the pattern will spread. Nylon fabric have lower absorbability than cotton, pattern is very easy to migratiom after ink-jet printing. So in order to achieve best quality, it is better to confirm the ink limit before printing. In Figure 4 we find that the fabric in this experiment (100% nylon, 148 ends/inch × 68picks/inch, 70D × 160D) still achieve good pattern sharpness, while the amount of ink reached 240% 3.5 Color fastness of printed fabric Ink-jet printing of nylon fabric used reactive dyestuff to replace acid dyestuff , because reactive. Fig.4.Ink limit measure result. Fig. 5 .The pattern of different ink amount 3.5 Color fastness of printed fabric Ink-jet printing of nylon fabric used reactive dyestuff to replace acid dyestuff , because reactive dyestuff can be form covalent bond with nylon fabric, theoretically, so do not need to use aftertreatment and can achieve good wet fastness. Table 1 show the color fastness result of printed nylon fabrics to light, washing and crocking, we can find four kind of reactive ink , wash fastness and crocking fastness reached grade 4, and light fastness reached more than grade 3-4. 4.Conclusion Nylon fabric is mainly method of ink jet printing were used acid dyestuffs, but because it has bad wash fastness, must through a serious fixing system make aftertreatment with fix agent, this method could prolong the procedure of operation too. This article we find the best colour yield ,while the pH value of pretreatment paste at 5.5, the pH value adjust agent used citric acid better than tartaric acid, the condition of steam fixation was 102℃×30min. To sum up above data, we can believe that ink-jet printing of nylon fabric can use reactive inks. Reference (1) TL Dawson., Spots before the eyes: Can ink jet printers match expectations?, Coloration Tech- nology,vol.117,pp.185(2001) (2) S Erving,Bsiegel and K Siemensmeyer, Text. Chem. Colourist Am. Dyestuff Rep., 1(3) pp.25 (2000) (3) L. W. C. Mile., Textile Printing, Dyers Company Publications Trust,PP.251-264(1981) (4) S.M.Burkinshaw, S.N. Chevli, D.J.Marfell., Print- ing of nylon 6,6 with reactive dyes Part 1: preliminary studies, Dyes and Pigments,vol, 45, pp. 235- 242 (2000) (5) Young-A.Son,Jin-Pyo Hong, Hyeong-Tae Lim, Tae-Kyung Kim., A study of heterobifunctional reactive dyes on nylon fibers: dyeing properties, dye moiety analysis and wash fastness, Dyes and Pigments, vol, 66, pp.231-239(2005) (6) Young-A.Son, Jin-pyo Hong, Hyeong-Tae Lim, Tae-Kyung Kim., A study of heterobifunctional reactive dyes on nylon fiber: dyeing properties, dye moiety analysis and wash fastness, Dyes and Pigments,vol,66, pp.231-239(2005) (7) A.A.Haroun.H.F.Mansour., Effect of cationisation on reactive printing of leather and wool, Dyes and Pigments, vol.72, pp.80-87 (2007) (8) A A Mousa,Y A Youssef,R Farouk and E A El-Kharadly., Dyeing of nylon 6 fabric with a new bifunctional vinyl sulphone reactive disperse dye, Coloration Technology, vol, 122, pp.338-344 (2006).
(4) Table 1. Color fastness result of printed cotton fabrics to light, washing and crocking Wash fastness. Crock fastness. Color change Staining of cotton. Dry. Light fastness. Wet. 20AFU. Cyan. 4. 4. 4. 4. 3-4. Magenta. 4. 4. 4. 4. 4. Yellow. 4. 4. 4. 4. 4. Black. 4. 4. 4. 4. 4.
(5)
數據
相關文件
The purpose of using information technology is to facilitate language learning, not to replace teachers. Does e-learning replace
好了既然 Z[x] 中的 ideal 不一定是 principle ideal 那麼我們就不能學 Proposition 7.2.11 的方法得到 Z[x] 中的 irreducible element 就是 prime element 了..
For pedagogical purposes, let us start consideration from a simple one-dimensional (1D) system, where electrons are confined to a chain parallel to the x axis. As it is well known
The observed small neutrino masses strongly suggest the presence of super heavy Majorana neutrinos N. Out-of-thermal equilibrium processes may be easily realized around the
incapable to extract any quantities from QCD, nor to tackle the most interesting physics, namely, the spontaneously chiral symmetry breaking and the color confinement..
(1) Determine a hypersurface on which matching condition is given.. (2) Determine a
(b) An Assistant Master/Mistress (Student Guidance Teacher) under school-based entitlement with a local first degree or equivalent qualification will be eligible for
DVDs, Podcasts, language teaching software, video games, and even foreign- language music and music videos can provide positive and fun associations with the language for