Performance Evaluation of Cleaning Solutions Enhanced
with Tetraalkylammonium Hydroxide Substituents
for Post-CMP Cleaning on Poly-Si Film
Tung Ming Pan,a,
*
Tan Fu Lei,cFu Hsiang Ko,b,**
Tien Sheng Chao,b,c,z Ming Chi Liaw,cYing Hao Lee,dand Chih Peng Luda
Department of Electronics Engineering and Institute of Electronics andbDepartment of Electrophysics, National Chiao Tung University, Hsinchu, TaiwancNational Nano Device Laboratories, Hsinchu, Taiwan d
Merck-Kanto, Advanced Chemicals Limited, Taoyuan, Taiwan
The cleaning solutions augmented with tetraalkylammonium hydroxides共TAAHs兲 with various chain-lengths of hydrocarbon substituents were developed for post-poly-Si chemical mechanical polishing共CMP兲 cleaning. The cleaning performance with respect to particle, organic, and metal removal as well as surface roughness was evaluated for a series of 3% NH4OH solutions dosed with 0.26 M of a TAAH and 100 ppm of ethylenediaminetetraacetic acid共EDTA兲. The experimental results demonstrated that the cleaning solutions enhanced with these surfactants共TAAH兲 and a chelating agent 共EDTA兲 achieved significantly better removal efficiencies of particle and metal impurities than the control solution containing 3% NH4OH only. A conceptual model involving surface adsorption and double-layer formation was used to postulate the aqueous-phase surface interactions between the tetraalkylammonium cations and the poly-Si surface, and to explain the removal mechanisms of particle and metal impurities from the surface. The improved electrical properties共current density-electric field and charge-to-breakdown characteristics兲 of the post-CMP capacitor after cleaning further demonstrated the reliability and feasibility of the proposed cleaning recipes. © 2002 The Electrochemical Society. 关DOI: 10.1149/1.1474435兴 All rights reserved.
Manuscript submitted May 7, 2001; revised manuscript received January 3, 2002. Available electronically April 25, 2002.
The increasing complexity and miniaturization of modern inte-grated circuits demand a high device yield, meaning that lower de-fect density in the active region of silicon devices is a necessity.1,2 For instance, the reliability of nonvolatile memory devices such as EEPROM, EPROM 共electrically erasable and erasable program-mable read-only memory, respectively兲, and flash memory is strongly dependent on the polyoxide quality characterized by low leakage current and high breakdown electrical field to prolong their data retention capabilities. Inadequate control over the thickness uniformity and surface roughness of the poly-Si layer directly leads to defect formation on the poly-Si/polyoxide interface, thus severely degrading the electrical properties. In recent years, the chemical mechanical polishing共CMP兲 process has been widely accepted as the mainstream planarization technique in the fabrication of deep submicrometer integrated circuits3in light of its capability to reduce surface roughness. However, as the device’s dimension continues to scale down, the requirement for post-CMP cleaning becomes in-creasingly more stringent to ensure high device yield.
The brush-scrubbing technique in combination with dilute am-monium hydroxide solution has been employed for many years and was considered among the most effective methods for removing particles after the CMP process.4Numerous studies focusing on im-proving oxide layer CMP as well as the post-CMP cleaning effi-ciency have been reported.5-9Jolley7reported that the solution con-taining tetramethylammonium hydroxide 共TMAH兲 showed enhancement of metal removal, and had little effect on the surface roughness for post tungsten-CMP cleaning as compared to ammo-nium hydroxide solution. Cady and Varadarajan8also proposed that the alkaline solution containing TMAH could replace the traditional multisteps cleaning method. The important merits of their proposed cleaning methods are low water consumption, high throughput, and effective particle removal. Our previous study9also indicated that the NH4OH solution spiked with TMAH and a chelating agent was more effective for metal removal. Nevertheless, despite the favor-able results exhibited by the TMAH-containing solution for post-CMP cleaning, the possible surface interactions and cleaning mecha-nisms remain to be elucidated.
In comparison to post-oxide-CMP cleaning, information on
poly-Si CMP and the subsequent cleaning methods is scarcely avail-able. This is a particular concern because the CMP process is a proven technology to improve polyoxide quality by reducing the surface roughness of the deposited poly-Si film,10yet the post-CMP cleaning efficiency continues to be a problem. Pietsch et al.11 of-fered an explanation by postulating that the hydrophobic Si-H bond-ing would remain on the poly-Si surface after polishbond-ing with alka-line slurry. As a consequence, both the abrasives and the metallic contamination on the hydrophobic poly-Si surface cannot be effec-tively removed by the alkaline cleaning solutions.
To overcome the difficulty of post-polishing particle removal from the hydrophobic poly-Si surface, it is preferable to operate the cleaning process under a slight etching condition of wafer surface.12 In the present study, several tetraalkylammonium hydroxides 共TAAHs兲 with various chain lengths of hydrocarbon substituents were added to the NH4OH-based cleaning solution. These surfac-tants included TMAH, tetraethylammonium hydroxide共TEAH兲, tet-rapropylammonium hydroxide 共TPAH兲, and tetrabutylammonium hydroxide共TBAH兲. The aim of this study, therefore, is to investigate the influences of the added surfactants on the hydrophilicity and the etching rate of the poly-Si surface, as well as the possible interaction mechanism between TAAH cleaning solution and the poly-Si sur-face. The overall cleaning performance of the solutions can be evaluated by analyzing the particle, organic, and metallic impurities remaining on the surface after cleaning. Furthermore, measurement of the electrical properties of the capacitors can also provide valu-able information to the specific roles of the various tetraalkylammonium-containing solutions during post-CMP clean-ing of poly-Si surfaces.
Experimental
Materials and cleaning solutions.—p-Type silicon wafers共具100典; resistivity 15-25⍀ cm兲 of 15 cm diam were used for device fabri-cation and cleaning experiments in this study. The compositions of the water-based cleaning solutions are specified in Table I. With the exception of the control solution, all other solutions were dosed with a predetermined concentration of TMAH 共Mw 91兲, TEAH 共Mw 147兲, TPAH 共Mw 203兲, and TBAH 共Mw 259兲, and were spiked with ethylenediaminetetraacetic acid共EDTA, Mw 292兲 having four pKa values共i.e., 1.99, 2.67, 6.16, and 10.26兲.13All reagents used were of electronic or higher grades from Merck共Darmstadt, Germany兲. *Electrochemical Society Student Member.
**Electrochemical Society Active Member. zE-mail: tschao@ndl.gov.tw
Capacitor fabrication and cleaning procedures.—Figure 1 de-scribes the fabrication process, including the cleaning procedure, for a capacitor. A 5000 Å buffer oxide was thermally grown on the silicon substrate, and a poly-Si film of 3000 Å thickness共poly-Si-I兲 was subsequently deposited on the oxide in a low-pressure chemical vapor deposition共LPCVD兲 system. The poly-Si film was doped with POCl3at 875°C for 17 min, providing a resistivity of 40-80⍀ cm. The poly-Si film was then polished on a Westech polisher共model 372M兲 with diluted Cabot SC-1 slurry to remove 500 Å poly-Si. After the CMP process, the wafer was treated by spraying with 3% NH4OH solution with 0.95 MHz megasonic, followed by dispensing the various cleaning solutions 共Table I兲 with a PVA 关poly 共vinyl alcohol兲兴 brush. The RCA cleaning was then performed to complete the cleaning operation. Afterward, an inter-polyoxide layer of 120 Å was deposited on the poly-Si-I by growing tetraethylorthosilicate 共TEOS兲 in LPCVD, and the samples were annealed in a rapid ther-mal reactor共950°C, 30 s, in N2ambient兲. Subsequently, a poly-Si-II layer with 3000 Å was deposited and POCl3-doped to 40-80⍀ cm resistivity. After the poly-Si-II layer was defined, an oxide was again thermally grown to 1000 Å by wet oxidation. Contact holes were defined and opened, and Al film was deposited and patterned. Fi-nally, the wafers were sintered at 350°C for 30 min in N2ambient to complete the capacitor fabrication process.
Instrumental analysis and electrical characterization.—The cleaning performance of the wet cleaning recipes specified in Table I was evaluated by various surface analytical methods, including measurements of the contact angle and the trace impurities. The contact angles were measured by injecting one drop共0.01 mL兲 of cleaning solution onto the poly-Si surface, which had been previ-ously treated with dilute HF solution to remove native oxide. Then the image of the liquid drop on the wafer surface was recorded and analyzed. The residual particle (⬎0.2 m) after cleaning was counted by the Tencor Surfscan model 4500 system. The residual organics was determined with the Hitachi thermal desorption system 共TDS兲 model UG-21 in conjunction with the atmospheric pressure ionization mass spectrometer 共APIMS兲 model UG-400P. The de-sorption temperature of the TDS-APIMS was ramped from room temperature to 600°C at 10°C/min. The surface outgassing was ana-lyzed at m/z 30, 44, and 58. The metallic impurities共Ca, Fe, Cu, Zn, and K兲 were determined using the Rigaku total reflection X-ray fluorescence spectrometer 共TXRF, model 3700兲 with an incident angle of 0.07°. The detection limits are 5⫻ 1010, 5⫻ 109, 5 ⫻ 109, 3⫻ 109, and 1011for Ca, Fe, Cu, Zn, and K, respectively. The polyoxide thickness of the capacitor was obtained using a Kei-thley C-V共capacitance-voltage兲 system. The electrical properties of the polyoxide, including the current density-electric field共J-E兲 and the time-dependent dielectric breakdown 共TDDB兲 characteristics, were also measured by using a Hewlett-Packard HP-4145B semi-conductor parameter analyzer.
Results and Discussion
Effects of cleaning solutions on poly-Si surface character-istics.—The physical and chemical interactions between the poly-Si surface and the TAAH-containing solutions strongly dictate the post-CMP cleaning performance of the solutions. One of the most useful parameters characterizing the surface modification is the contact angle共兲 measurement. As shown in Fig. 2, the decrease in contact angle as a function of time is due to the共slow兲 reoxidation of the poly-Si surface by O2present in the different cleaning solutions. In addition, the control solution 共solution A, containing 3% NH4OH only兲 exhibited the largest contact angle. This result reflects the hydrophilic nature of solution A because the surface structure of the poly-Si layer is predominantly hydrophobic in Si-H bonding. The extent of hydrophilicity for solution B共with TMAH兲 laid between those of solution A and solutions C共with TEAH兲, D 共with TPAH兲, Figure 1. Capacitor structure and the fabrication steps for polyoxide
capaci-tors using the CMP process.
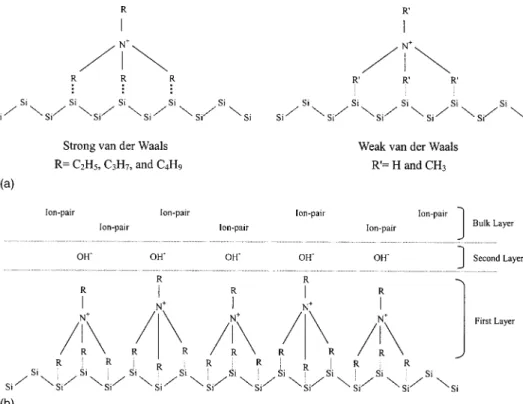
and E 共with TBAH兲, whose values significantly decreased and clustered closer to each other as time progressed. This phenomenon can be explained by the surface adsorption model schemed in Fig. 3a. This figure illustrates that the longer chain lengths of alkyl groups in solutions C, D, and E共corresponding to carbon numbers of 2, 3, and 4, respectively兲 tend to form stronger van der Waals attraction force with the poly-Si surface, whereas solutions A and B 共carbon numbers 0 and 1, respectively兲 exhibit weaker interaction with the surface. By further adapting the Stern-Gouy-Chapman model14for surface adsorption and double-layer formation, we pos-tulated that the tetraalkylammonium cations of TEAH, TPAH, and TBAH were first adsorbed onto the poly-Si surface. This positively charged layer is referred to as the ‘‘primary layer,’’ as shown in Fig. 3b. The second layer, in opposition, was negatively charged with the anions 共e.g., hydroxide兲. Consequently, the ion pairs from TEAH, TPAH, and TBAH were formed in the bulk layer. This model is also useful to vindicate the origin of surface roughness for each cleaning solution, as is discussed later in the paper.
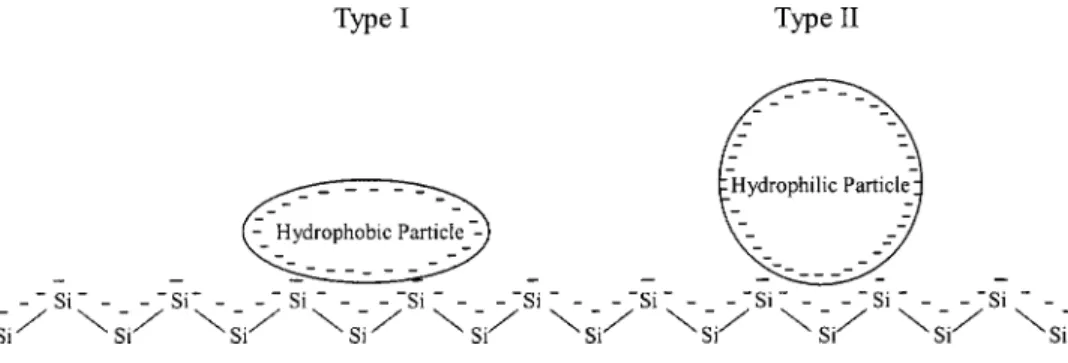
The physical and chemical properties of poly-Si after CMP and cleaning.—The particle number and surface roughness were charac-terized for the poly-Si surface after the post-CMP cleaning process. Figure 4 presents the results of residual particle numbers after clean-ing with each type of solution. Evidently, the TAAH-containclean-ing so-lutions manifested much improved particle removal capability as compared to the solution without TAAH 共i.e., solution A兲. In an attempt to justify these results, we hypothesized the possible surface interaction with two types of particles having either hydrophilic or hydrophobic nature, as illustrated in Fig. 5. It has been previously documented15that the zeta potentials of particle and poly-Si sur-faces are both negative at pH⭓12, a condition normally attained by solutions containing TMAH. As a result, the hydrophobic particle shows stronger interaction with the hydrophobicity of the poly-Si surface than the hydrophilic particle, and is more difficult to remove from the surface. From this perspective, the TAAH components in solutions B, C, D, and E become critical to the removal of hydro-phobic particles by penetrating into the particle-surface interfacial region. As mentioned earlier in Fig. 3, the tetraalkylammonium ions are effectively adsorbed onto the poly-Si surface as well as the par-ticle surface. This surface adsorption phenomenon facilitates parpar-ticle
removal from the poly-Si surface due to the reduction of interfacial attraction. On the contrary, the NH4OH in solution A hardly pen-etrates into the particle-surface interfacial region because of its hy-drophilic characteristics, and hence could not remove particles 共par-ticularly of hydrophobic nature兲 as effectively.
In order to satisfy the total cleaning criteria of the TAAH-containing solutions, the residual organic and metallic impurities were also analyzed with TDS-APIMS and TXRF techniques, respec-tively. The results indicated that the peak intensities of residual C2H6, C3H8, and C4H10after cleaning with solutions B, C, D, and E were in the range of one- to twofold higher than those cleaned with solution A. However, the differences of these peak intensities became insignificant when RCA cleaning was performed following Figure 3. Surface adsorption model for
共a兲 cleaning solutions and 共b兲
double-layer formation.
Figure 4. Number of particles remaining on the polished poly-Si surface after cleaning with various solutions.
the post-CMP cleaning with the various TAAH solutions. Further-more, no deterioration in the electrical properties was observed for the capacitors at the level of residual organics. With regard to the control of metallic contamination, the 1999 International Technol-ogy Roadmap for Semiconductors共ITRS兲 specifically recommended that the control limit for surface metal should be at least four orders of magnitude more critical than that for organic contamination. In this study, the metallic impurity on the poly-Si surface was below the TXRF detection limit for all cleaning solutions except solution A. The metal levels detected on poly-Si using cleaning solution A are approximately 1013atoms/cm2. It was found that the metal cat-ion and the adsorbed tetraalkylammonium catcat-ion were of the same charge type; hence, the electrostatic repulsive force helped the re-moval of metal ions. However, the electrostatic interaction was not sufficient to explain the removal of metal oxides 共e.g., chromium oxide, iron oxide兲. The more plausible removal mechanism can be ascribed to the presence of chelating agent in the cleaning solution, namely the EDTA 共100 ppm兲. Under alkaline conditions (pH ⬎ 12), the hydrogen ion was fully dissociated from EDTA due to its pKavalue (pK4⫽ 10.26). As a result, the EDTA in the solutions formed a hexadentate coordination with the surface metal, thereby efficiently removing them from the poly-Si surface. This result was consistent with our previous examination9 that spiking with low dosage of EDTA in cleaning solutions was beneficial for metal removal.
Yet another important factor influencing the device yield is the surface roughness. Tardif and co-workers16have reported that the charge-to-breakdown of their capacitor increased initially with the dipping time in hydrofluoric acid 共HF兲 solution due to improved surface suitability; however, it gradually degraded because of the increase in surface roughness caused by excessively long dipping
time. Therefore, it is imperative to evaluate the potential effect of the TAAH solutions on surface roughness. Figure 6 presents the results of the poly-Si surface roughness measurement as well as the
Figure 6. The poly-Si surface roughness and etching rate after post-CMP cleaning with various solutions.
Figure 7. The J-E characteristics for the top gate applied with共a兲 positive and共b兲 negative bias using different solutions for post-CMP cleaning.
wet etching rate for the cleaning solutions. It was found that the surface roughness exhibited strong correlation with the etch rate, suggesting that the degree of roughness formation was primarily dictated by etching rate. This figure also indicates that the TMAH-containing solution共solution B兲 had appreciably higher etching rate than the others. This result can be attributed to the strong adsorption of tetraalkylammonium cations of the long-chain TAAHs 共e.g., TEAH, TPAH, and TBAH兲 in the ‘‘primary’’ layer, as illustrated in Fig. 3, preventing direct etching of the surface by the hydroxides existing in the second and bulk layers. In contrast, the cations in the TMAH-containing solution only weakly interacted with the poly-Si surface, so the wet etching by the hydroxide occurred much more readily. Furthermore, the lower etching rate of solution A was due to the highly hydrophilic nature of NH4OH relative to the hydrophobic poly-Si surface. Consequently, the NH4OH共or hydroxide兲 could not effectively approach the surface, resulting in a lower etching rate than solution B.
Electrical properties of capacitors after cleaning.—Figure 7a and b shows the typical J-E characteristics under positive and nega-tive biases, respecnega-tively, for the capacitors cleaned with the various TAAH solutions. The electric field (E) was obtained by E⫽ V/T, where V is the applied voltage and T represents the effective oxide thickness determined by C-V measurement. The results show that the capacitors cleaned with solutions C and D generally demon-strated higher electric breakdown field and lower leakage current than those cleaned by other solutions for either positive or negative bias. Further investigation of the breakdown field distribution共Fig. 8兲 indicates that the polished poly-Si-I film cleaned with solution D showed the highest positive and negative electric breakdown fields than the others. This result was an apparent consequence of the favorable performance by solution D with respect to low degree of poly-Si surface roughness and effective particle removal.
Figure 8. The breakdown field distribution for the top gate applied with共a兲 positive and共b兲 negative bias using different solutions for post-CMP
clean-ing. Figure 9. The charge-to-breakdown (Qbd) characteristics under共a兲 positive
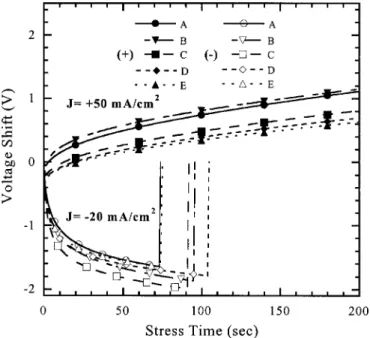
To investigate the polyoxide reliability, charge-to-breakdown (Qbd) measurements were performed on the capacitors. In Fig. 9a and b the Weibull distributions are shown under positive and nega-tive constant current density at ⫹50 and ⫺20 mA/cm2 stress, re-spectively. The distribution for the post-CMP capacitor cleaned with solution D showed a slightly higher Qbd due to higher particle re-moval efficiency and a smoother poly-Si surface in the polyoxide. It is clear that the capacitor with polished poly-Si-I had a higher Qbd than unpolished poly-Si-II. This observation indicates that polished poly-Si-I was a smoother poly-Si-I/polyoxide interface and that the unpolished poly-Si-II was a rougher polyoxide/poly-Si-II interface. Figure 10 depicts the charge-trapping characteristics of the polished poly-Si films cleaned with various solutions. Obviously, the gate voltage shift of these solutions increased with time at⫹Vgand⫺Vg constant current stress. These figures also reveal that capacitors with solution D cleaned after the CMP process exhibited a smaller volt-age shift than those cleaned with the other solutions. This improve-ment implies that the capacitor traps fewer electrons and has a lower electron-trapping rate than others, and that unpolished poly-Si-II tends to have a higher electron trapping rate than polished poly-Si-I. Furthermore, the rougher polyoxide/poly-Si-II interface leads to a smaller conduction area and a higher local current density, subse-quently leading to a higher electron trapping rate.
A previous work has mentioned the centroids of trapped charge (Xt) in the polyoxides.17By the bidirectional I-V measurement and by the shifts of Fowler-Nordheim共F-N兲 I-V characteristics before and after stress for both polarities, the centroids of the trapped charges are calculated from the following relationship
Xt⫽ Tox关⌬Vg⫹/共⌬Vg⫹⫹ ⌬Vg⫺兲兴 关1兴 where Xtis measured from the polyoxide/poly-Si-II interface,⌬Vg⫹ denotes the voltage shift when poly-Si-II is positively biased,⌬Vg⫺ represents the voltage shift when poly-Si-II is negatively biased, and Toxis the polyoxide thickness.
Figure 11 presents the centroid of trapped charges (Xt) at various ⫹Vgand⫺Vginjection times for these solutions. The Xtof capaci-tors using cleaning solutions C, D, and E for polished poly-Si film appeared closer to polyoxide/poly-Si-II interface than those using cleaning solutions A and B. This phenomenon is due to the fact that the surface morphology of using cleaning solution B for poly-Si film
after the CMP process was much rougher than that of other solu-tions; therefore, centroids moved away from the polyoxide/poly-Si-II interface.
Conclusion
The cleaning performance of the various TAAH-containing solu-tions and the effects of hydrocarbon chainlength on the tetraalkyl-ammonium cations were investigated in this study. It was found that overall cleaning efficiency with respect to particle, organic, metal, and surface roughness could be significantly improved over the use of conventional NH4OH solution. These improvements were mainly due to surface modification by adsorption of tetraalkylammonium ions that facilitated removal of microcontaminants. The chelating agent共EDTA兲 in the solution was also instrumental in the removal of surface metals. Electrical characterization of the post-CMP ca-pacitors cleaned with the TAAH-containing solutions further con-firmed their improved cleaning performance. The results indicated that the solution containing TPAH and EDTA was the foremost choice among the TAAH solutions based on the overall cleaning efficiency as well as the electrical properties of the capacitor.
Acknowledgment
The authors thank Dr. Ming-Shih Tsai of National Nano Device Laboratories for very helpful discussions, and Dr. Walter Den for his technical and editorial assistance with this paper. Financial support was provided by Merck-Kanto Advanced Chemicals, Ltd,共contract no. C87140兲, and by the National Science Council of Taiwan 共con-tract no. NSC88-2215-E009-045兲.
The National Chiao Tung University assisted in meeting the publication costs of this article.
References
1. S. M. Sze, VLSI Technology, Chap. 14, 2nd ed., McGraw-Hill, New York共1988兲. 2. A. V. Ferris-Prabhu, Introduction to Semiconductor Device Yield Modeling, Chap.
1, Artech House, Boston, MA共1992兲.
3. G. Bai, C. Chiang, J. N. Cox, S. Fang, and D. S. Gardner, in Digest of Technical papers of the Symposium on VLSI Technology, 48共1996兲.
4. D. Hymes, I. Malik, J. Zhang, and R. Emami, Solid State Technol., 209共July 1997兲. 5. Y. Z. Hu, R. J. Gutmann, T. P. Chow, and B. Witcraft, Thin Solid Films, 332, 391
共1998兲.
6. Y. L. Wang, C. Liu, M. S. Feng, and W. T. Tseng, Mater. Chem. Phys., 52, 23
共1998兲.
7. M. Jolley, Solid State Phenom., 65, 105共1999兲.
Figure 10. The gate voltage shifts vs. time for post-CMP capacitors cleaned with different solutions.
Figure 11. The centroids of trapped charges (Xt) of different solutions for
8. W. A. Cady and M. Varadarajan, J. Electrochem. Soc., 143, 2064共1996兲. 9. T. M. Pan, T. F. Lei, C. C. Chen, T. S. Chao, M. C. Liaw, W. L. Yang, M. S. Tsai,
C. P. Lu, and W. H. Chang, IEEE Electron Device Lett., EDL-21, 338共2000兲. 10. T. F. Lei, J. Y. Cheng, S. Y. Shiau, T. S. Chao, and C. S. Lai, IEEE Trans. Electron
Devices, ED-45, 912共1998兲.
11. G. J. Pietsch, G. S. Higashi, and Y. H. Chabal, Appl. Phys. Lett., 64, 3115共1994兲. 12. T. S. Chao, T. M. Pan, M. C. Liaw, C. C. Chen, W. L. Yang, T. F. Lei, M. S. Tsai, B. T. Dai, H. C. Lin, T. Y. Huang, C. P. Lu, and W. H. Chang, in The International Symposium on Semiconductor Manufacturing, The Ultra Clean Society of Japan and IEEE, p. 125共1998兲.
13. J. Bjerrum, G. Schwarzenbach, and L. G. Sillen, Stability Constants, p. 76, The Chemical Society, Burlington House, London共1957兲.
14. P. C. Hiemenz, Principles of Colloid and Surface Chemistry, 2nd ed., Chap 12, Marcel Dekker, New York共1986兲.
15. F. Tardif, J. Palleau, T. Lardin, O. Demolliens, A. Vincent, and J. Torres, Micro-electron. Eng., 33, 195共1997兲.
16. F. Tardif, T. Lardin, C. Paillet, B. Beneyton, P. Patruno, D. Levy, K. Barla, and W. Sievert, Microelectron. Eng., 28, 121共1995兲.
17. E. Avni, O. Abramson, Y. Sonnenblick, and J. Shappir, J. Electrochem. Soc., 135, 182共1988兲.