遠端約制 / 誘導在老鼠心臟之保護作用
計畫類別: 個別型計畫 計畫編號: NSC91-2314-B-002-232- 執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學醫學院外科 計畫主持人: 陳益祥 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 92 年 10 月 29 日
A Clinically Feasible Protection “Outside the Box” in Acute Myocardial Infarction Model – Skeletal remote preconditioning
Yih-Sharng Chen1, 2, MD; Ming-Chieh Ma1, PhD; Chiang-Ting Chien3, PhD; Tsai-Fu
Chou1,2, MD, PhD; Yung-Zu Tseng1, MD, PhD; Fang-Yue Lin2, MD; Shoei-Shen Wang2, MD; Chau-Fong Chen1, PhD
Department of Physiology1, College of Medicine, National Taiwan University,
Department of Surgery2, and Office for Clinical Research3, National Taiwan University Hospital, Taipei, Taiwan
Authors Yih-Sharng Chen and Chiang-Ting Chien have equally contribution.
Author for correspondence and proofs: Chau-Fong Chen, Department of Physiology, College of Medicine, National Taiwan University, No. 1, Section 1, Jen-Ai Rd., Taipei, Taiwan, ROC.
Tel & fax: 886-2-23922954 Fax: 886-2-23958747
Basic Science Research
Abstract
BackgroundInternal organ remote preconditioning (RPC), such as kidney and mesentery artery, had been proved to have protective effect for myocardial infarction, but it is not feasible in clinical status. This study was performed to evaluate skeletal RPC protection in acute myocardial infarction model.
Methods and Results
RPC was performed by repeated 4-cycle 10-min ischemia-reperfusion of femoral artery. The coronary artery was occluded for 2 hours (time C) to produce infarction 2 hour after RCP (time B). Four groups experiment was designed: I, sham group, without RPC and infarction; II, RPC only; III, infarction only; IV, incorporating both RPC and infarction. The infarct size was significantly reduced for group IV (22.7 ± 7.0%) compared to group III (51.6 ± 8.2%) (p<0.0001). The data pertaining to cardiac enzymes (creatine kinase and troponin I) also revealed significant decrease in the level for group IV compared to group III at time C. Western blotting of heat shock protein (HSP) revealed that consistent elevation of HSP 25 and 70 in group II, III and IV. Antioxidant enzyme in the
myocardium at the area of risk revealed that Mn-superoxidase dismutase and glutathione peroxidase were consistently elevated with HSP data, but not for Cu/Zn-superoxidase dismutase and catalase. This finding suggested that RPC had protective effect via heat shock protein and partial antioxidant enzyme.
Conclusions
The skeletal RPC can produce a protective effect of myocardial infarction that may be applied in clinical setting to limit the myocardial damage when infarction occurs.
Keywords:
Myocardial infarction, muscle, protein, remote preconditioning, infarct size, antioxidant enzyme
Condensed abstract
Remote preconditioning is a feasible cardioprotection “outside of box”. The myocardial protective effect of skeletal muscle remote preconditioning was proved by anatomical infarct size analysis as well as cardiac enzyme reconfirmation. The free radicals scavenger, MPG, can blockade the protective effect of this preconditioning model. We also demonstrated heat shock protein and antioxidant enzyme were involved in the process of remote preconditioning.
Introduction
Ischemic preconditioning (IPC), brief episodes of myocardial ischemia rendering the heart resistant to myocardial injury during subsequent prolonged ischemic episodes, has been widely explored in various experimental models since 1986.1 This form of protection has been also observed in organs other than heart.2-4 The concept of local “remote preconditioning” (RPC) of virgin myocardium has been previously advocated in that a brief period of ischemia in one region of the heart was able to reduce the potential infarct size of the different region of the heart following long duration of coronary occlusion.5 Liauw et al in 1996 also demonstrated that RPC of one skeletal muscle could be practiced to protect the contralateral muscle from an ischemia-reperfusion injury.6
Gho et al extended the intraorgan RPC concept to the interorgan RPC.7 Their results clearly demonstrated RPC applied by way of intestinal repetitive ischemia/reperfusion (I/R) was as protective for myocardial infarction as classic IPC and the protection was abolished by ganglionic blockade with hexamethonium.7 Renal RPC was also later shown to produce the effect of improving myocardial energy metabolism and reducing infarct size in vivo by an adenosine-dependent mechanism.8 Skeletal-muscle ischemic RPC by rapid stimulation of the gastrocnemius muscle combined with partial reduction of blood supply to this muscle has also been shown to elicit a protective effect by way of a reduction to subsequent elicited myocardial infarct size.9 It would appear almost impossible and also unethical, however, to elicit an
intraorgan RPC or the internal organ RPC by such as mesenteric artery I/R or the renal artery I/R or by way of electric stimulation of muscle.
Skeletal RPC seemed to be a clinically feasible way for myocardial protection, but there still were some controversial data exited,10-12 and no suggestive mechanism was clearly
investigated.11 We tested the hypothesis that short periods of limb I/R can induce RPC and reduce the infarct size in an acute infarction model. We also tested the contents of heat-shock protein expression and the antioxidant enzymes in the myocardium in order to delineate the role of free radicals in this RPC condition.
Material and methods
Wistar rats weighting 200 – 300gm were used for this study. All animal experiments and care were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (published by National Academy Press, Washington DC, 1996). The Laboratory Animal Care Committee of our institution approved all protocols used in this study. General surgical preparation
The rats were anesthetized by intraperitoneal administration of sodium pentobarbital, 35mg/kg. A tracheostomy was performed with the cannula (PE-200), and then was connected to a rodent ventilator. The rats were ventilated with room air at 60breaths/min and a tidal volume of 6ml/kg. The rectal temperature was maintained at 37°C by using a servo-null heating pad.
The right common carotid artery was cannulated with PE-50 catheters and then the arterial pressure and the heart rate was recorded via a Polygraph (Quincy, MA, USA). The internal jugular vein was cannulated for infusing saline at a rate of 2 ml/hr.
Determination of amount of plasma free radicals by chemiluminescence
In order to elicit the role of free radicals involving in RPC, preliminary studies for detecting the free radicals in plasma were conducted by a lucigenin-enchnaced
were measured at three different time points: baseline, five minutes subsequent to RPC, and 120 minutes following RPC.
Whole blood, 0.2ml, was sampled at different time points, and the blood samples were then immediately wrapped in aluminum foil and kept on ice until chemiluminescence (CL)
measurement was undertaken. The measurement was typically conducted within two hours of the sample having been taken.
After a 100-second chemiluminescence background level determination, 1.0ml of 0.1 mM lucigenin (Sigma Chemical Co, St. Louis, MO, USA) in phosphate-buffered saline (pH = 7.4) was injected into the collected blood sample. The plasma free radical thus enhanced by the addition of lucigenin was detected via a Chemiluminescence Analyzing System (CLD-110, Tohoku Electronic Industrial Co., Sendai, Japan) as was reported previously.13 The CL level was monitored continuously for an additional 600 seconds. The total quantity of CL was calculated by integrating the area under the curve and subtracting this value from the background level. The assay was performed in duplicate for each sample and was expressed as CL counts/ten seconds for blood CL.
Remote preconditioning model
The femoral vessels were exposed for performing RPC and an atraumatic arterial vascular clamp was applied to the femoral artery for temporary occlusion. RPC was performed by 4 cycles of 10-minute period of I/R (i.e., 10 minutes of ischemia followed by 10-minutes of reperfusion) of the femoral artery. The occlusion was confirmed by collapse and no pulsation at the distal artery (Fig. 1).
Model of myocardial infarction
Myocardial infarction was conducted 2 hours subsequent to RPC by coronary artery ligation (Fig. 1). Midline sternotomy was performed followed by pericardiotomy. The left anterior
descending artery close to its origin was looped with 7-O Prolene (Ethicon, Inc, Somerville, NJ), about 3 mm away from the left coronary ostium. Ligation of the looped Prolene to produce the coronary occlusion as revealed by the discolorization of the involved myocardium was continued for 2 hours (Fig. 1). After the 2-hour period of myocardial infarction, 5 ml methyl blue was injected via the internal jugular vein catheter and the heart was harvested 2 minutes later. Then the heart was placed in a saline for 10 minutes at 0°C for the subsequent determination of infarct size.
Study protocol in remote preconditioning model
Experimental animals were divided into 4 groups as indicated in Fig. 1. Group I: sham group, neither RPC nor coronary occlusion was performed. Group II: only 4-cycle repeated 10-minute RPC was conducted. Group III: only coronary artery ligation for 2 hours without initial RPC was undertaken. Group IV: coronary artery occlusion was performed 2 hours later after RPC.
For determining the role of free radicals, N-(2-mercaptopropionyl)-glycine (MPG), 20 mg/kg, was infused in group II, III and IV during the period of RCP (initial 80 minutes) to eradicate the free radicals that were produced by RPC, which were abbreviated as MPG + I, MPG + II, MPG + III, and MPG + IV.
Infarct size determination
weighted. Following this, the slices were incubated for 20 minutes at 37°C in a 1% solution of triphnyltertrazolium chloride (TTC) (Sigma, St. Louis, MO) in order to distinguish the necrotic tissue from the viable myocardial tissue.14 The heart slices were then photographed immediately by digital camera (Nikon, Tokyo). Photographic images of heart slices were then projected and traced at 5-fold magnification. The area of necrosis (stained as white) and the vital tissue (stained as pink-red) for each slice was quantified by computerized planimetry. These areas were then multiplied by the weight of each slice, and the results were summed to obtain the volume of area of risk and necrosis. The infarct size (IS) was expressed as a percentage of summated weight of necrosis area in summated weight of area of risk (AR). The definition of AR was based upon the summation of the weight of the area of necrosis and the viable myocardium. The size calculation was performed repetitively by 2 different individuals.
Data collection and timing of collecting
The heart rate and blood pressure were recorded at the beginning of the experiment (time A), prior to the coronary ischemia (time B), and 2 hours after infarction (time C) (Fig. 1). The
plasma creatine kinase MB form (CK-MB) and troponin I (TnI) were collected at these same time points.
Plasma creatine kinase MB form and troponin I assays.
Whole blood (1 m aliquots) was sampled at corresponding experimental times (time A, B and C, Fig. 1) as described above. Subsequent to the sampling, heparinized blood samples were centrifuged at 620g and the plasma was collected and then stored at -70°C for subsequent analysis. The measurement of CK-MB activity (U/L) was performed by a commercial kit (Sigma, St. Louis, MO). The quantity of TnI (ng/ml) in the samples was determined by enzyme-linked immunoassay according to manufacture’s protocol (Diagnostic Automation, CA, USA).
Immunoblotting of heat-shock protein (HSP), superoxidase dismutase, glutathione peroxidase (GPx), and catalase in the heart
For determining the change of the antioxidant enzyme, repeated experiment was performed in different groups (n=3 for each group) to obtain tissue for subsequent analysis. After finishing the protocol and sacrificing the rats, the region of the left ventricle that was considered to be at AR (territory supposed to be supplied by left anterior descending artery) was sampled for the preparation of the cytosolic fraction. From this fraction, protein samples were separated and electrophoretically transferred to nitrocellulose membranes (Amershan-Pharmacia Buckingham, England, UK). Subsequent to the blocking of the membranes, they were then incubated overnight at 4°C with mouse anti-HSP 70 antibody (BD Transduction, San Jose, CA, USA; diluted 1:500), rabbit anti-HSP 25 antibody (Stressgen, Victoria, Canada; diluted 1:1,000), sheep anti-Cu/Zn SOD antibody (Upstate, Lake Placid, NY, USA; diluted 1:1,000), rabbit anti-Mn SOD antibody (Upstate; diluted 1:1,000), sheep anti-GPx antibody (The binding site, Birmingham, England; diluted 1:1,000), or sheep anti-catalase antibody (The binding site, Birmingham; diluted 1:1,000). Following washing, the membranes were incubated for 1 hour at room temperature with either horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Vector, Burlingame, CA, USA), HRP-conjugated goat anti-mouse IgG (Lenico, St. Louis, Missouri, USA), or HRP-conjugated donkey anti-sheep IgG (Jackson, West Grove, PA, USA) as appropriate, and then washed, and the bound antibody visualized using a commercial DAB (3,3’-diaminobenzidine) peroxidase substrate kit (Vector, Burlingame, CA). The densities of the bands with appropriate molecular masses were determined semi-quantitatively by densitometery using an image analytical system (Alpha Innotech, San Leandro, CA, USA) as has been previously described.15
Statistics
Data are reported as mean ± SD. unless otherwise states. In all cases, statistical difference was considered significant when p<0.05. Continuous variables were compared using the Mann-Whiteny U test or by the Kruskal-Wallis test. Hemodynamic variableswere evaluated by two-way repeated-measuresANOVA (group and time) to determine whether there was a principal effectdue to the particular group being investigated, or due to an effect related to the time, or a combined group-time interaction.The infarct size, TnI, and CK-MB were analyzed using a paired Student’s t-test initially, and were then compared by way of correlation using the
Spearman method. The relationship between the data was assessed by linear regression analysis usingthe least-squares method, and the slopes of the resultant regression lineswere compared. All statistical analyses were performed using the SAS softwaresystem.
Results
Preliminary result of plasma free radicals detected by chemiluminescence
Figure 2 depicts the preliminary results of the free radicals counts corresponding to the different time points in different models of I/R (n=8 for each model, Fig. 2A and 2B). Free radicals counts were significantly elevated following 4-cycle I/R of the femoral artery when compared to the baseline, and even more significantly elevated some 2 hours later, subsequent to RPC. Similar change in free radicals count was similar in another RPC model with one-cycle 40-minute I/R at femoral artery. It was the key finding to lead us focusing RPC phenomena on antioxidant enzyme activity and heat shock protein, and determining the I/R course in RPC. Considering the clinical feasibility, we determined the former model for RPC.
Hemodynamic data
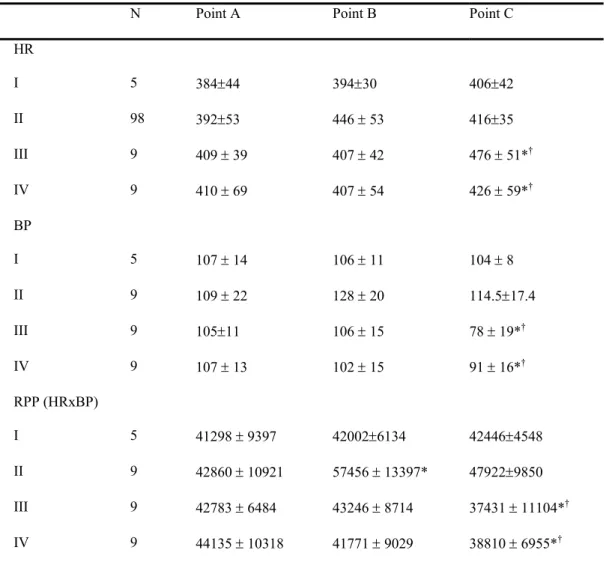
The hemodyanmic data of corresponding groups were comparable in Table 1. Significant changes in heart rate, blood pressure and the rate-pressure product were detected subsequent to the coronary artery having been occluded (time C in group III and IV, Table 1). In the intergroup comparison, the effect of RPC seemed to have the tendency to antagonize the pressure-lowing effect by coronary ligation in spite that the statistic difference did not reach significance (time B and C in group III vs. IV, p>0.05). It may be because the great extent of myocardial infarction that was difficult to detect the difference simply by rate, blood pressure, or the product. Effect of RPC in infarct size
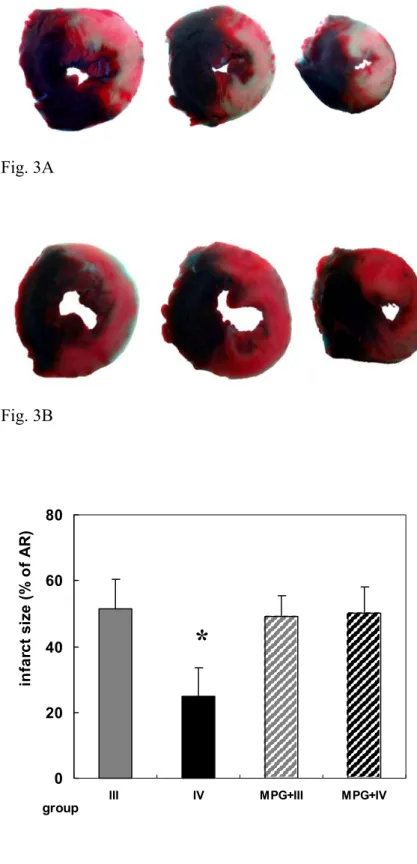
The AR for the groups (III and IV) was similar in size, which indicated uniform location of coronary occlusion (p=0.5006). For group III, the mass of AR was a strong predictor of necrotic area mass (γ=0.881, p=0.0039), but it did not exit in group IV. There was no coronary ischemia for groups I and II, therefore no infarction area was able to be detected (data was not shown in Fig. 3). The IS (necrosis area/AR) was significantly reduced for group IV (Fig. 3B) than for group III (Fig. 3A), 22.7 ± 7.0% for group IV (n=9) versus 51.6 ± 8.2 % for group III (n=9) (p<0.0001, Fig. 3C).
MPG effect on RPC in infarct size
The IS for groups MPG + III (n=9) and MPG + IV (n=9) revealed no significant increase between these 2 groups (Fig. 3C, 48.2 ± 5.3% for MPG + III, 45.1 ± 8.2% for MPG + IV, p>0.05). The IS among groups III, MPG + III, and MPG + IV also did not show the significant difference (p>0.05).
This anatomical finding appeared to demonstrate that the remote preconditioning of distal skeletal muscle did provide a protective effect with about a 50% reduction (51.6% to 22.7%) in infarct size when prolonged coronary artery occlusion occurred.
Effect of RPC in cardiac enzyme (CK-MB and TnI) activity
The CK-MB activity data corresponding to the study groups are depicted in Fig. 4A, and the TnI activity data was are plotted in Fig. 4B. The CK-MB value was found to reveal a significant elevation at time C when compared to the time A and B for groups undergoing coronary ligation (groups III and IV, n=9 for each, Fig. 4A). For group II, there were no significant difference among different time points (Fig. 4A, p>0.05). For group III, CK-MB was at time C was significantly elevated when compared to corresponding values for time A and B (Fig. 4A,
p<0.001). At time C, the CK-MB value was significantly lower for group IV than was the value
of group III (Fig. 4A, p<0.05).
The TnI data also revealed the similar results to the CK-MB data suite (Fig. 4B). For group II, TnI was not different among the different time points as well (p>0.05). For group III, TnI was significant elevated at time C (11.40 ± 1.83 ng/ml), which was different from time A or B, but there was no difference between time B and C (p>0.05). For group IV, TnI data revealed no difference between time A and B (1.05 ± 0.10ng/ml at point A, 1.65 ± 1.21ng/ml at point B, p>0.05), but significant elevation at time C (6.23 ± 3.71ng/ml) when compared to time A (p=0.0068) and time B (p=0.026). Similarly, at time C, TnI was significantly reduced in group IV when compared to group III (p=0.0189, Fig. 4B).
MPG effect on RPC in cardiac enzyme (CK-MB and TnI) activity
In the MPG-treated groups, the result of CK-MB data was similar to that of study groups except the MPG + IV group. There was significant elevation of CK-MB 2 hours after coronary
occlusion (Fig. 5A, MPG+III and MPG+IV), but we could no find the difference between MPG+III and MPG+IV at time C.
For groups MPG + II, MPG + III, and MPG + IV, the result for TnI revealed decreased release of TnI in groups that were pretreated with MPG than those without MPG pretreatment, but there was no significant difference between group MPG + III and MPG + IV at point C (Fig 5B, 5.93 ± 4.71 ng/ml for group MPG + III and 5.01 ± 3.10 ng/ml for group MPG + IV, p>0.05). For corresponding groups MPG + III and MPG + IV, there was significant difference in TnI between point B and C (Fig. 5B).
According to the results above, the skeletal RPC did have the capability of reducing the elevation of then specific myocardial injury markers for the infarction model, which could be blocked by MPG, a free radicals scavenger.
Expressions of HSP 70 and HSP 25
Compared to the results for group I, western blot results revealed the constitutively significantly elevated the levels of expression of HSP 70 in AR of myocardium for groups experiencing coronary ischemia (groups III and IV, n=3 for each group, Fig. 5A). Although no significant difference of HSP 70 content expression was able to be detected between any groups II, III, and IV, the content expression of HSP 70 was also found increased in myocardium for the group II as compared to group I (sham group), which might indicate RPC alone could induce synthesis of the protective HSP in a short time, and the amount was similar to the highly stressed status such as myocardial infarction (Fig. 5A).
The results of western blot study concerning the HSP 25 content expression in the myocardium were similar to those results for HSP 70, such results suggesting that heat shock
proteins, both HSP 70 and 25, were expressed in the myocardium under stress status, either from remote femoral ischemia-reperfusion or myocardial ischemia.
Studies of Mn-SOD, Cu/Zn-SOD, GPx, and Catalase expressions
With regard to the antioxidant enzyme content in the myocardium, there were no difference in Cu/Zn-SOD and catalase content in the AR of myocardium was noted between any of the groups (n=3 for each enzyme detection; Fig. 6A). The content of Mn-SOD and GPx were significantly higher for groups II, III, and IV when compared with group I (Fig. 6B), which reveals a similar trend to the induction of HSP 70 and 25 expression. This finding suggests that enhanced mitochondrial antioxidants activity during RPC may be associated with the HSP protection on the myocardial ischemia, and is another possible mechanism to explain the RPC phenomenon.
Discussion
This study model was designed to simulate the clinical situation of myocardial infarction and to test the hypothesis that skeletal muscle remote preconditioning can reduce the extent of the subsequent infarcted area. We also firstly tried to use cardiac enzyme in vivo as well as IS as end-point for validation. Our model was different from the previous ischemia-reperfusion model for preconditioning. Interorgan RPC induced by mesentery artery7 or kidney ischemia-reperfusion8 or by repetitive muscle stimulation and graded artery stenosis,9 was not practical and not really feasible clinically. The present model, however, would appear to be more simple and clinically feasible. The results have clearly demonstrated the protective effect of RPC in acute myocardial infarction model, and the elevation of HSPs and antioxidant enzymes suggested the mechanism may be related to free radicals.
Prolonged coronary occlusion model (ranged from 90 minutes to 2 hours)16,17 had been applied to delineate the intraorgan preconditioning effect with or without hypothermia, and local hypothermia seemed to have additive protective effect.17 However, it is difficult to perform intraorgan preconditioning and local hypothermia for the acute occlusion model. Compared our result in the infarction model of 2-hour coronary occlusion with the similar prolonged occlusion model (2 hours),16,17 RPC with 2 hours interval can produce similar protective infarct-reducing effect to the previous intraorgan PC.16,17
The infarction model in our experimental study, however, was different from the model used by others as revealed by the literature. Previous models tended to use “index” (prolonged) I/R18 as an endpoint to evaluate the extent of myocardial infarction was not a good example for simulating the clinical myocardial infarction, but the “index” I/R did not necessarily represent a good example for simulating a “clinical” myocardial infarction. In the reperfusion phase for “index” I/R, the temporarily occluded coronary artery might or might not be recannulized after prolonged period of ischemia. If it was recannulized, it seems to be a model more similar to myocardial infarction with emergent revascularization, and the damage was mainly derived from reperfusion, not simple infarction. Our model using infarction model by ligation of coronary artery as an endpoint of myocardial damage was a scenario quite compatible with the clinical condition of acute myocardial infarction. The model described above was both reproducible and reliable if the location of coronary ligation was kept consistent, since the AR was similar for the different study groups.
Reduce Myocardial Infarct Size and Cardiac Enzyme by Skeletal Muscle RPC – correlation between cardiac enzyme and infarct size
We applied both CK-MB and TnI corresponding data to re-validate the data obtained from the anatomical analysis which may have operator-related bias during area calculation. By applying the Spearman correlation to the data suites for cardiac enzyme levels and infarct size, we were able to demonstrate a good correlation (Fig. 7), therefore we suggest that the degree of elevation of TnI and CK-MB levels may act as a reliable marker to determine the effect of preconditioning. This alternative method may act not only as a revalidation tool, but also its application may save time, and reduce/prevent the inaccuracy and the possible operator bias when performing the infarct size determination. Such a procedure may be considered as one of the standard validation technique, because it is a method that can easily be applied and can be rechecked serially in different time periods as the experiment is underway. This may be the first study that clearly correlates the relationship between detected myocardial enzyme markers and anatomical infarct area.
Kharbanda et al19 had conducted a similar experimental study in a swine model with a
40-minute ischemia/reperfusion of the coronary artery following skeletal RPC, although their “index” I/R model is not really similar to a clinical setting as we mentioned previously. These workers demonstrated that the RPC group in their study did result in a better preservation of myocardial function and a smaller infarct size, but they did not work further for the possible mechanism for skeletal RPC. The results of Kharbanda et al combined with ours both contribute to an exploration of the future potential for applying this new feasible protection to clinical myocardial infarction in a clinical setting.
There was difference in the level of CK-MB between groups III and MPG+III at time C (p=0.017, see Fig. 4A and 5A), that whether was due to the release of CK-MB affected by MPG remained further investigation. The release of TnI level was found lower in MPG-treated groups,
especially for III and MPG+III (p=0.036, Fig. 4B and 5B), which whether was due to MPG effect remained further investigation as well.
Induction of HSP Expression and Antioxidant Enzymes by RPC: Role of Free Radicals
In spite that HSP 70 was found to accumulate to maximal tissue level within 6 hours in the previous observation,20 Cornelussen’s data21 as well as ours demonstrate that a significant increase in HSP expression in cardiac tissue was able to be detected within 2 to 4 hours subsequent to stress or RPC. We expected that it might have the potential for increase to be even more pronounced in experiments that were extended to 12 or 24 hours post stress or RPC as the past experiment.
Another striking finding of the present study is that we noted about a 1.9-fold up-regulation of HSP 70 level in a location of the heart similar to where the infarct was located following RPC on a lower limb (Group II; Fig. 5), which is similar to the group III (2.3-fold) and group IV (2.2-fold). This might be indicated that HSP 70 may act as one of the important proteins initially involved in preconditioning even though it is typically induced by hyperthermia,22 classic preconditioning,20 or remote skeletal I/R.
When facing cardiac stress, cardiac HSP 70 expression has been suggested to provide a protective effect upon cardiac tissue making it somewhat resistant to myocardial ischemia and dysfunction by its chaperoning properties.23,24 Further, in vivo studies have also demonstrated for rat heart-derived myocytes that showed overexpression of HSP 70 renders myocytes more tolerant to oxidative challenge.25 Several studies pertaining to rat heart preparations have shown that enhanced productions of either endogenously or exogenously reactive oxygen species by coronary artery occlusion or hyperthermia can up-regulate cardiac HSP 70 expression.20,26 This
study of HSP expression tended to focus upon the hyperthermia induction effect for HSPs, or upon late classical preconditioning.20,27 To the best of our knowledge, there was no previous
publications describing any investigation exploring the relationship between the RPC and HSP. Previous study has shown that extracellular free radicals may, through an activation of unknown stressor, act to increase the level of membrane phospholipid hydrolysis by activating phospholipase C, and result thereby in increases in intracellular concentrations of inositol 1,4,5-triohosphate and diacylglycerol, these increases being associated with subsequent activation of protein kinase C to provoke HSP 70 synthesis.20,28
Abundant expression of HSP 70 by transgenic animals or gene transfection models has suggested the role of HSP 70 as an agent involved in preserving myocardial function subsequent to heart injury by ischemic reperfusion insult.23 The question arises, however, what is the consequent target for HSP? Besides HSP reported properties of protein refolding, assembly, transport, and degradation, Suzuki et al29 have reported that transfection of human HSP 72 into
rat heart can enhance mitochondrial Mn-SOD activity. Our Western blot results from the analysis of the expression of antioxidant enzymes also revealed an up-regulation of Mn-SOD as well as GPx levels by skeletal RPC. As revealed by this present study, the enhancement of mitochondrial Mn-SOD content and activity as elicited by previous induction by skeletal RPC on the expression of Mn-SOD, could be rationally expected to exert an extra protecting effect upon subsequent heart damage, in accordance with previous suggestion that conserved Mn-SOD could maintain mitochondrial function and reduce this organelle’s participation in cardiomyocyte-involved apoptosis.29
Although the function and effect of low molecule-weight HSPs remain largely unknown, 30 the content of the HSPs were mainly related to subject age,31 and located abundantly in lung,
adrenal and heart.20 Our study has highlighted the effect of the skeletal RPC process was able to enhance both the low- and high-molecule-weight HSP in heart tissue, although the effect of skeletal RPC still remained unclear for organ other than heart, which warrants further investigation.
Combined with the results of this study, we speculate that free radicals play an important role in the RPC process, such a scenario being similar to the late ischemic preconditioning.
Perspectives: Clinical implication
Exercise could offer some level of resistance to myocardial infarct extension, and the study model also highlighted the feasibility of RPC to protect myocardium from further damage when ischemia developed into infarction. In the emergency room or intensive care unit, it is relatively easy to perform temporary leg ischemia-reperfusion by means of a pressure tourniquet as commonly applied for temporary hemostasis during orthopedic surgery for a patient if such patient’s condition of unstable angina is confirmed.
From clinical experience, ventricular dilatation usually developed following myocardial infarction, even though emergent angioplasty is performed to reopen the occluded coronary artery.32-34 There are several trials including medical and surgical treatment studies undertaken to attempt to effectively manage the chronic remodeling process, but to date, no perfect therapeutic treatment has been established to prevent ventricular dilatation in the acute stage. It would appear that the only factor able to prevent dilatation is limiting the infarct size.33,34
This animal-based experimental model has revealed an encouraging result, namely that application of RPC is able to reduce the dimension of subsequent myocardial infarct size as also infarct-elicited cardiac damage. The skeletal RPC is simple, drug-free, non-invasive and capable
and may be extended to the infarct stage in order to limit the potential ongoing damage. Therefore, it may decrease the incidence of chronic heart failure.
Conclusion
The application of the skeletal remote preconditioning technique using a 4-cycle 10-minute ischemia-reperfusion model prior to an elicited myocardial infarction could result in reduction of the elicited infarct size and also a reduction in the level of myocardial enzyme leakage The relationship between the effect of RPC, heat shock protein and antioxidant enzyme present in the myocardium at risk would likely suggest that the free radicals may act an important trigger in remote preconditioning-induced myocardial protection.
References
1. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136.
2. Peralta C, Closa D, Xaus C, et al. Hepatic preconditioning in rats is defined by a balance of adenosine and xanthine. Hepatology. 1998;28:768-773.
3. Lee H, Schroeder C, Shah P, et al. Preconditioning with ischemia or adenosine protects skeletal muscle from ischemic tissue reperfusion injury. J Surg Res. 1996;63:29-34.
4. Kitagawa K, Matsumoto M, Tagaya M, et al. 'Ischemic tolerance ' phenomenon found in the brain. Brain Res. 1990;528:21-24.
5. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic "preconditioning" protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893-899.
6. Liauw SK, Rubin BB, Lindsay TF, Romschin AD, Walker PM. Sequential ischemia/reperfusion results in contralateral skeletal muscle salvage. Am J Physiol. 1996;270:H1407-H1413.
7. Gho BCG, Shoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial
protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193-2200.
8. Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, Kinoshita M. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effect of "remote preconditioning". J Am Coll Cardiol. 1999;33:556-564.
9. Birnbaum Y, Sharon LH, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641-1646.
10. Gunaydin B, Cakici I, Soncul H, et al. Does remote organ ischemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493-496.
11. Przyklenk K, Darling C, Dickson E, Whittaker P. Cardioprotection 'outside the box'. Basic Res Cardiol. 2003;98:149-157.
12. Oxman Y, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707-H1712.
13. Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free radicals production and apotosis formation in rat kidney subjected to
ischemia/reperfusion. J Am Soc Nephrol. 2001;12:973-982.
14. Lasley RD, Noble MA, Konyn PJ, Mentzer RMJ. Different effects of an adenosine A1 analogue and ischemic preconditioning in isolated rabbit hearts. Ann Thorac Surg.
1995;60:1698-1703.
15. Ma MC, Huang HS, Chen CF. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol. 2002;13:1008-1016.
16. Hale SL, Kloner RA. Combination therapy for maximal myocardial infarct size reduction. Heart Dis. 2001;3:351-6.
17. Hale SL, Kloner RA. Ischemic preconditioning and myocardial hypothermia in rabbit with prolonged coronary artery occlusion. Am J Physiol (Heart Circ Physiol). 1999;276:H2029-H2034.
18. Kevin LG, Camara AKS, Riess ML, et al. Ischemic preconditioning alters real-time measure of O2 radicals in intact heart with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566-574.
19. Kharbanda RK, Mortensen UM, White P, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881-2883.
20. Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, et al. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461-97.
21. Cornelusesen RN, Garnier AV, van der Vusse GJ, et al. Biphasic effect of heat stress pretreatment on ischemic tolerance of isolated rat hearts. J Mol Cell Cardiol. 1998;30:365-72. 22. Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. Whole-body hyperthermia provides biphasic cardioprotection against ischemia/reperfusion injury in the rat. Circulation. 1998;98:1414-21.
23. Marber MS, Mestril R, Chi SH, et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446-56.
24. Trost SU, Omens JH, Karlon WJ, et al. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest. 1998;101:855-862.
25. Chong KY, Lai CC, Lille S, Chang C, Su CY. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol. 1998;30:599-608. 26. Arnaud C, Joyeux M, Garrel C, et al. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br J Pharmacol.
27. Chiu JH, Tsou MT, Tung HH, Tai CH, Tsai SK, et al. Preconditioned somatothermal stimulation on median nerve territory increases myocardial heat shock protein 70 and protects rat hearts against ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;125:678-85.
28. Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001;69:1-15.
29. Suzuki K, Murtuza B, Sammut IA, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106(suppl I):I-270-76. 30. Wakayama T, Iseki S. Expression and cellular localization of the mRNA for the 25-kDa heat-shock protein in the mouse. Cell Biol Int. 1998;22:295-304.
31. Lutsch G, Vatter R, Offhauss U, Wieske M, Grone HJ, Klemenz R, Schminke I, Stahl J, Benndorff R. Abundance and location of the small heat hock proteins HSP25 and alphaB-crystallin in rat and human heart. Circulation. 1997;96:3466-3476.
32. McKay RG, Pfeffer MA, Pasternak RC, et al. Left ventricular remodeling following myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693-702. 33. Gaudron P, Eilles C, Kugler I, et al. Progressive left ventricular dysfunction and remodeling after acute myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755-63.
34. Meneveau N, Bassand JP, Bauters C, et al. Influence of late reopening of the infarct-related artery on left ventricular remodeling after myocardial infarction. IRIS study group. Eur Heart J. 1997;18:1261-8.
Figure legend
Figure 1: Protocol for remote preconditioning (RPC). Time A, B, & C indicate the timing of detection of plasma cardiac enzymes (CK-MB and troponin I). RPC is produced by four cycles of repetitive 10-minute ischemia and 10-minute reperfusion of the femoral artery. Group I: sham group, n=5. Group II: only RPC, n=9. Group III: coronary ligation for two hours to create infarction, n=9. Group IV: RPC followed by infarction. PC:
preconditioning, n=9. MPG + groups: the study groups (I to IV) were pretreated with N-(2-mercaptopropionyl)-glycine) infusion at the initial 80 minutes, n = 9 each groups except MGP+I (n=5).
Fig.2: Preliminary data of free radicals (FR) count by chemiluminescence (CL). FR was detected at 3 time points: baseline (A), 5 minutes after 4 repetitive 10-min remote skeletal
ischemia/reperfusion(R I/R) or one 40-min remote skeletal ischemia/reperfusion (40 min ischemia followed by 40 min reperfusion, R I/R), and 2 hours after remote
preconditioning (R I/R) (B). Fig. 2A showed elevation in FR count 5 minutes after repetitive remote preconditioning (4 cycles 10-min ischemia/reperfusion at femoral artery), and more significant elevation 2 hours later when comparing to the baseline FR. Fig. 2B showed FR change in one cycle 40-minute remote preconditioning model.
Similar elevation of FR was noted in this model 2 hours after remote preconditioning, but no significant FR change 5 minutes after remote preconditioning with this model. *: P<0.05 when comparing to baseline. The result may hint that free radicals are involved in the process of remote preconditioning.
Fig. 3: The demonstration of stained myocardium and infarct size (infarction are/area at risk, %). Fig. 3A: Transverse section of heart from group III (coronary ischemia without remote preconditioning). The white portion represents necrosis area, and the pink portion represents viable myocardium of the area at risk. Fig. 3B: Transverse section of heart from group IV (coronary ischemia with remote preconditioning). A significant decrease in the necrosis area of the area at risk, and similar perfused area (blue portion) can be noted for group III and IV. Fig. 3C: There was significant difference of infarct size between group III and IV, which meant remote preconditioning possessed the protective effect for myocardial ischemia. In group MPG+III and MPG+IV, it indicated that MPG can block the protective effect for myocardial infarction caused by skeletal RPC. AR: area at risk. Bar indicates S.D. *: P<0.05 compared with group III, MPG + III or MPG + IV.
Fig. 4: Cardiac enzyme activity for the different groups. Fig. 4A: CK-MB content in different groups (n=9 for group II, III, and IV, n=5 for group I). Remote preconditioning could reduce the elevation of CK-MB when myocardial infarction occurred. Bar represents mean, error bar shows S.D. CK-MB: creatine kinase MB form, U/L. Fig. 4B: TnI data for different groups. It revealed a significant elevation in plasma level for the infarction group (Group III and IV) at time C when compared to time A or B, and a significant difference in TnI level between group III and IV at time C. a: P<0.05, compared with point A, B; b: P<0.05, compared with group III.
Fig. 5: The Ck-MB (Fig. 5A) and TnI (Fig. 5B) in groups pretreated with
N-(2-mercaptopropionyl)-glycine (MPG + individual group). CK-MB and TnI were noted significant increase after infarction (MPG+III and MPG+IV at time C). There was no significant difference in the cardiac enzyme level at time C between the MPG+III and
MPG+IV, which indicates the skeletal remote preconditioning is abolished by the MPG. For MPG+I, n=5; for MPG+II, MPG+III, and MPG+IV, n=9 for each group. a: P<0.05,
compared with point A, B.
Fig. 6: Heat shock protein (HSP) content for members of different groups as indicated by western blotting. The HSP content was obtained from the area at risk of the myocardium (territory of left anterior coronary artery). Fig 6A: Heat shock protein 70 (HSP 70) content for the different groups. The result shows an increased HSP 70 level for either the remote-preconditioning group (group II) or coronary-infarction group (III) or both, which suggests that HSP 70 acts as an initial protein involved in the process of remote preconditioning. *: P<0.05, compared with group I. Fig. 6B: Note that the result for HSP 25 content, is similar to that for HSP 70 content.
Fig. 7: Western blotting results for antioxidant enzymes for the four different groups. Fig. 6A: Immunoblot and densitometric units for Cu/Zn SOD and catalase. Regarding these results, there appears to be no significant difference between any of the different groups. Fig. 6B: Elevated expression of Mn-SOD and glutathione peroxidase (GPx) enzymes for the three stress groups (Groups II, III and IV). This finding may hint that remote preconditioning is involved the mitochondrial antioxidant process. *: P<0.05 when compared to group I. Fig. 8: Correlation among the infarct size (IS), troponin I (TnI), and CK-MB. Spearman
correlation was performed to reveal significant correlation between IS and TnI
(p=0.0408), IS and CK-MB (p=0.0039), but not high significant correlation between TnI and CK-MB (p=0.0795). After Log-transformed linear regression of markers (CKMB or TnI) on IS was done to create a univariate linear regression model. Fractional polynomial
correlation formula between IS and CKMB was displayed, IS = 0.187999 + 0.08268 × ln(CKMB-940.84) (Fig. 7A, coefficient 0.08268, p=0.009); good correlation between IS and TnI, IS = -0.353593 + 0.260017 × ln(TnI+8.43) (Fig. 7B, coefficient 0.260017, p=0.027); but fair correlation between TnI and CKMB (Fig. 7C, coefficient 0.119059, p=0.074).
Table 1: Hemodynamic data in different groups
N Point A Point B Point C HR I 5 384±44 394±30 406±42 II 98 392±53 446 ± 53 416±35 III 9 409 ± 39 407 ± 42 476 ± 51*† IV 9 410 ± 69 407 ± 54 426 ± 59*† BP I 5 107 ± 14 106 ± 11 104 ± 8 II 9 109 ± 22 128 ± 20 114.5±17.4 III 9 105±11 106 ± 15 78 ± 19*† IV 9 107 ± 13 102 ± 15 91 ± 16*† RPP (HRxBP) I 5 41298 ± 9397 42002±6134 42446±4548 II 9 42860 ± 10921 57456 ± 13397* 47922±9850 III 9 42783 ± 6484 43246 ± 8714 37431 ± 11104*† IV 9 44135 ± 10318 41771 ± 9029 38810 ± 6955*†
BP: mean blood pressure, HR: heart rate, RPP: heart rate-pressure product; *: p<0.05 when compared to point A; †:
Figures
Fig. 3A Fig. 3B 0 20 40 60 80
III IV MPG+III MPG+IV
group in fa rc t siz e ( % o f A R ) Fig. 3C
*
0 600 1200 1800 2400 3000 3600 4200 4800
I II III IV I II III IV I II III IV
Groups CK-MB Acti vi ty A B C a a b Group II (n=9) Group I (n=5) Group III (n=9) Group IV (n=9) Fig. 4A
0 2 4 6 8 10 12 14 16
I II III IV I II III IV I II III IV
Groups Trop on in I Act ivit y A B C a a b Group I (n=5) Group II (n=9) Group III (n=9) Group IV (n=9) Fig. 4B
HSP 70 0 2000 4000 6000 8000 10000 12000 I II III IV Group De ns it om et ri c U n it s Fig. 6A