行政院國家科學委員會專題研究計畫 成果報告
Aloe-emodin 誘發肺癌細胞凋亡之蛋白質體及基因層次的探
討
計畫類別: 個別型計畫 計畫編號: NSC93-2320-B-039-025- 執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日 執行單位: 中國醫藥大學藥學系 計畫主持人: 李鳳琴 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 94 年 10 月 27 日
Cancer Letters
已接受
Aloe-emodin induced DNA damage through generation of reactive oxygen
species in human lung carcinoma cells
Hong-Zin Leea,*, Ching-Ju Lin,bWen-Hui Yangc, Wing-Cheung Leungd, Shen-Pen Changa
a
School of Pharmacy, China Medical University, Taichung, Taiwan
b
Department of Physiology, China Medical University, Taichung, Taiwan
c
School of Health Services Management, China Medical University, Taichung, Taiwan
d
Department of Radiation Oncology, Chi Mei Medical Center, Tainan, Taiwan
* Correspondence to: Hong-Zin Lee
School of Pharmacy, China Medical University, 91, Hsueh-Shih Road, Taichung, 40402, Taiwan
Phone: +886-4-22058436 Fax: +886-4-22039203
Abstract
The DNA aggregation was found in aloe-emodin-induced H460 cell apoptosis in this study. Aloe-emodin (40 μM)-induced DNA single strand breaks were observed by comet assay. Aloe-emodin induced decreases in the mRNA of DNA repair enzymes such as
hMTH1, hOGG1 and APE. Although the activity of the radical-scavenging enzyme SOD
was enhanced by aloe-emodin, the effects of aloe-emodin on H460 cell apoptosis were suspected to result from the prooxidant. These results suggest that aloe-emodin induced DNA damage through generation of reactive oxygen species in human lung carcinoma cells.
Keywords: Aloe-emodin; H460 cell, Human lung non-small carcinoma cell line; ROS, reactive oxygen species; Apoptosis; Comet assay; DNA repair enzymes
1. Introduction
Aloe-emodin is a bioactive anthraquinone from the root and rhizome of Rheum
palmatum and is an extremely potent inducer of apoptosis in H460 cells [1]. Many reports
demonstrated that exposure to chemotherapy drug in cancer cells can lead to DNA damage [2,3]. DNA strand breaks and nuclear condensation has been observed on antitumor activity of chemotherapy drug [4,5]. The most abundant lesion produced is DNA single strand breakage, which can be sensitively detected by the alkaline microgel electrophoresis (comet) assay [6,7]. The comet assay has also been previously used to detect DNA strand breaks in anti-tumor study [8,9]. We attempted to examine whether DNA strand breaks and DNA aggregation were involved in aloe-emodin-induced H460 cell apoptosis. One of the principal forms of DNA damage is 8-oxo-2-deoxyguanine (8-oxo dG), which is a result of mis-pairing to adenine during DNA-replication results in the formation of G to T
transversions. The formation of 8-oxo dG may represent an important mechanism during DNA damage [10,11]. As a consequence of the formation of 8-oxo dG, there are four main repair systems that maintain the integrity of the human genome: prevention of
incorporation (human MutT homologue, hMTH1), mismatch repair (human MutY homologue, hMYH), base excision repair (human homologue of the
8-oxoguanine-glycosylase enzyme, hOGG1) and nucleotide excision repair [12,13]. In the current study we investigated mRNA expression of DNA repair enzymes in human lung carcinoma cells exposed to aloe-emodin to determine whether aloe-emodin is able to modulate expression of DNA repair enzymes. Some evidences suggested that
polyphenols-inducing cell toxicity was suspected to result from the prooxidant or the antioxidant action [14,15]. Since the structure of aloe-emodin contains polyphenolic structure, this study would demonstrate the role that prooxidative or antioxidative actions of aloe-emodin play in aloe-emodin-induced H460 cell apoptosis. Free radicals are a family
of molecules, which modulate several important physiological functions including proliferation and apoptosis. Previous studies reported that reactive oxygen species (ROS) participated in cancer and apoptosis through inducing DNA damage [16,17]. Superoxide dismutase (SOD) is a well-known antioxidative enzyme with the activity to convert superoxide to hydrogen peroxide, and at least two types of SOD have been identified, one is mitochondrial Mn SOD, and the other is cytosolic Cu/Zn SOD, which can be inhibited by cyanide. Antioxidant enzymes can antagonize initiation and promotion phases of
carcinogenesis and they are reduced in many malignancies. The most commonly decreased enzyme is the mitochondrial Mn SOD. Mn SOD is reduced in a variety of tumor cells and has been proposed to be a new type of tumor suppressor gene [18,19].
2. Materials and methods
2.1. Materials
Aloe-emodin (1,8-dihydroxy-3-(hydroxymethyl)-anthraquinone), antipain, aprotinin, dithiothreitol, EDTA, leupeptin, pepstatin, phenylmethylsulfonyl fluoride and Tris were purchased from Sigma Chemical Company (St. Louis, MO, USA). Cu/Zn SOD and Mn SOD antibodies were purchased from Calbiochem (San Diego, CA). Enhanced
chemiluminescent (Renaissance) detection reagents were obtained from NEN Life Science Products (Boston, MA).
2.2. Cell culture
H460 cellsweregrown in monolayerculturein Dulbecco’smodified Eagle’s medium (Life Technologies, Rockville, MD, USA) containing 5% fetal bovine serum
(Hyclone,Logan,UT,USA),100 U/mlpenicillin and 100 μg/mlstreptomycin (Gibco BRL, Rockville, MD, USA) and 2 mM glutamine (Merck, Darmstadt, Germany) at 37℃ in a humidified atmosphere comprised of 95% air and 5% CO2. When H460 cells were treated
with aloe-emodin, the culture medium containing 1% fetal bovine serum was used. All data presented in this report are from at least three independent experiments showing the same pattern of expression.
2.3. DNA extraction and aggregation assay
DNA extraction and precipitation assay were performed as described previously with some modifications [1,4]. Cells were collected and lysed in 400 μlofice-cold lysis buffer (containing 50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 0.3% Triton X-100), incubated on icefor30 min,and then centrifuged.RNase A (100 μg/ml) was added to the supernatant, which was then incubated at 50℃ for 30 min, followed by the addition of200 μg/ml proteinase K and further incubation at 37℃ for 1 h. DNA was extracted with
phenol/chloroform and precipitated at -20℃ with ethanol/sodium acetate. Equal aliquots ofDNA (40 μg)weremixed with aloe-emodin or DMSO and the volume was brought up to 250 μlwith PBS buffer.Thefinalconcentration ofaloe-emodin and DMSO are80 μM and 0.1%, respectively. The samples were extensively vortexed, incubated for 20 min at room temperature and centrifuged for 10 min at 14,000 rpm. The nonprecipitated DNA in solution was determined by measurement of absorbance of the supernatant at 260 nm. The precipitated DNA wasstained with propidium iodide(50 μg/ml) for 30 min at 37℃. After the DNA was stained, it was washed gently and centrifuged. An aliquot of the pellet was recovered and deposited between slide and coverslip. The preparation was photographed with a fluorescence microscope (Olympus IX 70, Germany).
2.4. Single cell gel electrophoresis
To examine the DNA damage in these single cell suspensions, we performed an alkaline single-cell gel electrophoresis (comet assay). The comet assay carried out was based on the method of Singh (2002) with some modification [7]. Fully frosted microscope slides werecovered with 85 μlof1% normalmelting agarosein PBS,immediately coverslipped and kept at room temperature for 30 min to allow the agarose to solidify. The removal of the cover glass from the agar layer was followed by the addition of a second layerof75 μlof1% normalmelting agarosecontaining approximately 1 × 105cells at 37℃. Cover glasses were immediately placed and the slides were placed at room temperature for 30 min. After the solidification of the normal melting agarose, the cover glasses were removed and the slides were immersed in the cold lysis solution (pH 11) containing 2.5 M NaCl, 100 mM Na2-EDTA, 10 mM Tris-HCl and 1% Triton X100 for 1 h at room
temperature. Gels are then incubated in an alkaline buffer (0.3 M NaOH, 1 mM Na2-EDTA)
for 20 min at 4℃. The slides were removed from the alkaline buffer and placed on a horizontal electrophoresis tank. The electrophoresis was carried out for 1 h at 25 V. After electrophoresis, the gels were washed gently twice with water and Tris-HCl (0.5 M, pH 7). The slides were immersed in 100% methanol for 30 min at room temperature. The
methanolwasthen removed and thegelswerestained with propidium iodide(40 μg/ml). The gels were washed twice with water and examined by fluorescence microscopy (Olympus IX 70, Germany).
2.5. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNAs were isolated from control or aloe-emodin-treated H460 cells with RNeasy Minikit(QIAGEN,USA)according to themanufacturer’sdescriptions.RNA
concentration was quantified using a spectrophotometer at a wavelength of 260 nm. cDNA was prepared by reversetranscription of1.5 μg oftotalRNA.Gene transcripts were determined by reverse transcriptase-polymerase chain reaction (RT-PCR) with RNA PCR kit (Invitrogen life technologies, USA). The primers of investigated genes are shown in Table 1. The amplification was performed with one denaturing cycle at 95℃ for 5 min, then 30 cycles at 95℃ for 1 min, at 55℃ for 1 min, at 72℃ for 1 min, and one final extension at 72℃ for 10 min. RT-PCR products were separated by electrophoresis on 2% agarose gel and visualized by ethidium bromide staining.
2.6. Fluorescence microscopic measurements of reactive oxygen species (ROS) production
This study used 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, molecular Probes) to detect intracellular generation of ROS. H460 cells
were loaded with 5μM CM-H2DCFDA for 30 min in the dark. After loading, cells were
washed with warm PBS. During loading, the acetate groups on CM-H2DCFDA are
removed by intracellular esterase, trapping the probe inside the H460 cells. Cells loaded with CM-H2DCF were treated with aloe-emodin (40 μM)and analyzed by fluorescence
microscopy. Production of ROS can be measured by changes in fluorescence due to intracellular production of CM-DCF (5-(and-6)-chloromethyl-2’,7’-dichlorofluorescein) caused by oxidation of CM-H2DCF. CM-DCF fluorescence was measured at an excitation
wavelength of 480 nm and an emission wavelength of 520 nm.
2.7. Evaluation of superoxide dismutase (SOD)
Adherent and floating cells were collected at the indicated times and washed twice in ice-cold PBS. Sonicate the cell pellets in cold 20 mM HEPES (Sigma) buffer, pH7.2,
containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose (Sigma). Samples were then centrifuged for 5 min at 1,500 g and 4℃. The supernatant was aspirated and the total SOD and Mn SOD activity was assayed spectrophotometrically at 450 nm with a
commercial kit (Cayman chemical). A standard curve of SOD solution (from 0.025 to 0.25 U/ml) was run for quantification. One unit of SOD is defined as the amount of enzyme needed to exhibit 50 % dismutation of the superoxide radical.
2.8. Protein preparation
Adherent and floating cells were collected at the indicated times and washed twice in ice-cold PBS. Cell pellets were resuspended in modified RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 % Nonidet P-40, 0.25 % sodium deoxycholate, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 5 μg/ml antipain) for 30 min at 4℃. Lysates were clarified by centrifugation at 100,000 × g for 30 min at 4℃ and the resulting supernatant was collected, aliquoted (50 μg/tube) and stored at -80℃ until assay. The protein concentrations were estimated with the Bradford method [20].
2.9. Western blot analysis
Samples were separated by various appropriate concentrations (12 and 15 %) of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad, Hercules, CA). The SDS-separated proteins were equilibrated in transfer buffer (Tris-HCl 50 mM, pH 9.0-9.4, glycine 40 mM, 0.375 % SDS [Bio-Rad], 20 % methanol [Merck]) and electrotransferred to Immobilon-P Transfer Membranes (Millipore Corporation, Bedford,
MA). The blot was blocked with a solution containing 5 % nonfat dry milk in Tris-buffered saline (Tris-HCl 10 mM, NaCl 150mM [Sigma]) with 0.05 % Tween 20 (TBST; Merck) for 1 h,washed and incubated with antibodiesto β-actin (1:5000 [Sigma], the detection of β-actin was used as an internal control in all of the data of Western blotting analysis), Cu/Zn SOD (1:1000) and Mn SOD (1:1000). Secondary antibody consisted of a 1:20,000 dilution of horseradish peroxidase (HRP)-conjugated rabbit anti-sheep IgG (for Cu/Zn SOD and Mn SOD; Jackson ImmunoResearch). The enhanced chemiluminescent detection system was used for immunoblot protein detection.
2.10. Data analysis and statistics
Values are presented as percentage ± S.D. of control. Statistically significant differenced from thecontrolgroup wereidentified by Student’st test for paired data. A P
value less than 0.05 was considered significant for all tests.
3. Results
3.1. The effect of aloe-emodin on DNA aggregation in H460 cells
Changes in nuclear morphology that involve DNA condensation and nuclear fragmentation are widely considered as an integral criterion of apoptosis. Therefore, the DNA of H460 cells was extracted to investigate the effect of aloe-emodin on DNA aggregation in vitro. DNA aggregation was determined by measurement of absorbance at the 260 nm. As shown in Fig. 1A, the DNA precipitation, which resulted in the decrease in DNA concentration of supernatant, was observed after treatment with 80 μM aloe-emodin for 20 min. DNA aggregates obtained in the presence of DMSO or
aloe-emodin have also been observed by propridiun iodide staining. Aloe-emodin (80 μM) induced very big and fluffy DNA aggregates (Fig. 1C). Fig. 1B showed that 0.1% DMSO had no significant effect on the DNA aggregation.
3.2. The effect of aloe-emodin on DNA damage in H460 cells
To study the DNA damage in the aloe-emodin-treated cells, we performed an alkaline single-cell gel electrophoresis (comet assay). Alkaline comet assay is a sensitive method by which DNA single strand breaks at a single-cell level can be monitored. Fig.2 is a photomicrograph of DNA migration patterns in human lung nonsmall carcinoma H460 cells, processed using comet assay. When cells were treated with 40 μM aloe-emodin for 16 h, DNA damage, as indicated by increased tail migration, is
significantly observed in the majority of the aloe-emodin-treated cells compared to those of control cells. The comet assay results in H460 cells showed that aloe-emodin induced DNA damage in this study.
3.3. The effects of aloe-emodin on the gene expression of DNA repair enzymes in H460 cells
To elucidate whether aloe-emodin affects the gene expression of hMHT1, hOGG1 and apurinic endonuclease, RT-PCR techniques were used in this study. The detection of
β-actin was used as an internal control in the data of PCR. After H460 cells were treated
with 40 μM aloe-emodin for 24 h, there was a significant decrease in the gene expression of hMHT1, hOGG1 and apurinic endonuclease in H460 cells (Fig. 3).
ROS producing and scavenging activities play important roles in the drug-induced apoptosis. In order to demonstrate the role that ROS plays in aloe-emodin-induced
apoptosis, production of ROS was examined by using an oxidant-sensitive fluorescent probe, CM-H2DCFDA. The results showed that treatment with 40 μM aloe-emodin had
significantly increased the intensity of the DCF signal as compared with those in the
control during treatment with aloe-emodin for 1, 4 and 8 h (Fig. 4). The concentration of 40 μM ofaloe-emodin was used to induce H460 cell apoptosis in our serial studies. This result indicates that ROS producing is involved in aloe-emodin-induced cell death in H460 cells.
3.5. Effects of aloe-emodin on superoxide dismutase (SOD) activity in H460 cells
Results described above suggested that aloe-emodin-induced apoptosis is mediated by its prooxidant activity. The antioxidant properties of aloe-emodin, such as the activity of SOD (an antioxidant enzyme), were also detected in aloe-emodin-induced H460 cells apoptosis. This study measured total SOD and Mn SOD activity in H460 cells treated with 40 μM aloe-emodin forvarioustimepoints.40 μM aloe-emodin stimulates a biphasic change in total SOD activity. The initial phase of the aloe-emodin response exhibited a significant increase in total SOD activity compared to those of control cells at 8 h of aloe-emodin treatment. The second phase is followed by a significant decay after treatment with aloe-emodin for 8 h (Fig. 5A). To further investigate whether the activation of Mn SOD by aloe-emodin could be linked to aloe-emodin-induced H460 cell apoptosis, 1 mM potassium cyanide, which will inhibit Cu/Zn SOD, was added to the assay samples. As shown in Fig. 5A, after 4 h treatment with aloe-emodin also induced a biphasic increased in the activity of Mn SOD. These results suggest that the antioxidant properties of
Our study also demonstrated the expression of Cu/Zn SOD and Mn SOD protein levels during aloe-emodin-induced apoptosis by Western blotting techniques. The protein levels ofMn SOD and Cu/Zn SOD werenotchanged during treatmentwith 40 μM aloe-emodin for 24 h (Fig. 5B).
4. Discussion
Ourpreviousstudy demonstrated that24 h ofcontinuousexposureto 40 μM of aloe-emodin induced a typical apoptosis on lung carcinoma cell line H460 [1]. We have also reported that the release of nucleophosmin, a nuclear protein, from the nucleus to cytosol was associated with aloe-emodin-induced H460 cell apoptosis [21]. The nucleus may be an important target area of aloe-emodin during aloe-emodin-induced H460 cell apoptosis. Therefore, we focused attention on the DNA damage in aloe-emodin-induced H460 cell apoptosis. During apoptosis, DNA condensation took place at the early stages of apoptosis and preceded nuclear DNA fragmentation. Therefore, the DNA of H460 cells was extracted to investigate the effect of aloe-emodin on DNA aggregation in vitro. As shown in Fig. 1A, the DNA precipitation, which resulted in the decrease in DNA concentration of supernatant,wasobserved aftertreatmentwith 80 μM aloe-emodin for 20 min. DNA aggregates obtained in the treatment of aloe-emodin have also been observed by propridiun iodide staining.
The alkaline microgel electrophoresis technique (comet) was used for estimation of DNA single strand breaks in this study. Comet assay is a sensitive method by which DNA single strand breaks at a single-cell level can be monitored. When cells were treated with 40 μM aloe-emodin, DNA damage, as indicated by increased tail migration, is significantly observed in the majority of the aloe-emodin-treated cells compared to those of control cells. The results of comet assay showed that aloe-emodin induced DNA damage in H460 cells.
In order to prevent DNA from chemical-mediated damage, there are many specific DNA repair enzymes, which help in the safeguarding and repairing of DNA. To elucidate whether aloe-emodin affects the gene expression of hMHT1, hOGG1 and apurinic
endonuclease, RT-PCR techniques were used in this study. After H460 cells were treated
with 40 μM aloe-emodin, there was a significant decrease in the gene expression of hMHT1,
hOGG1 and apurinic endonuclease in H460 cells.
Recently, many reports indicated that reactive oxygen species participated in cancer and apoptosis through inducing DNA damage [16,22]. ROS producing play an important role in the drug-induced apoptosis. Specifically, oxidative damage to DNA is considered an essential step in cancer development or apoptosis [16,22]. In order to demonstrate the role that ROS play in aloe-emodin-induced apoptosis, production of ROS was examined by using an oxidant-sensitive fluorescent probe, CM-H2DCFDA. The results showed that
treatment with aloe-emodin had significantly increased the intensity of the DCF signal as compared with those in the control. This result indicates that ROS producing is involved in aloe-emodin-induced cell death in H460 cells.
Polyphenolic antioxidants are scavengers of free radicals and modifiers of various enzymatic functions. The structure of aloe-emodin is anthraquinone and contains two phenolic structures. Therefore, this study detected the activity of antioxidant enzymes, such as superoxide dismutase, of H460 cells after treatment with aloe-emodin. Aloe-emodin (40 μM)stimulatesabiphasicchangein totalSOD activity.To furtherinvestigatewhetherthe activation of Mn SOD by aloe-emodin could be linked to aloe-emodin-induced H460 cell apoptosis, 1 mM potassium cyanide, which will inhibit Cu/Zn SOD, was added to the assay samples. As shown in Fig. 5A, after 4 h treatment with aloe-emodin also induced a biphasic increased in the activity of Mn SOD. The present study also demonstrated that the protein levels of Mn SOD and Cu/Zn SOD were not changed during treatment with aloe-emodin.
aloe-emodin-induced H460 cell apoptosis. Aloe-emodin induced ROS production during H460 cell apoptosis. Although the activity of the radical-scavenging enzyme SOD was enhanced by aloe-emodin, H460 cells still died. Therefore, the effects of aloe-emodin on H460 cell apoptosis were suspected to result from the prooxidant rather than the
antioxidant action of aloe-emodin.
Acknowledgements
This work was supported by National Science Council Grant NSC93-2320-B-039-025 of Republic of China.
References
[1] H.Z. Lee, Protein kinase C involvement in aloe-emodin- and emodin-induced apoptosis in lung carcinoma cell. Br. J. Pharmacol. 134 (2001) 1093-1103.
[2] Z. Han, W. Wei, S. Dunaway, J.W. Darnowski, P. Calabresi, J. Sedivy, E.A. Hendrickson, K.V. Balan, P. Pantazis, J.H. Wyche, Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J. Biol. Chem. 277 (2002) 17154-17160.
[3] B. Kowalska-Loth, A. Girstun, A. Piekielko, K. Staron, SF2/ASF protein inhibits camptothecin-induced DNA cleavage by human topoisomerase I. Eur. J. Biochem. 269 (2002) 3504-3510.
[4] A. Sukhanova, J. Devy, M. Pluot, J.C. Bradley, J.P. Vigneron, J.C. Jardillier, J.M. Lehn, I. Nabiev, Human DNA topoisomerase I inhibitory activities of synthetic polyamines: relation to DNA aggregation. Bioorg. Med. Chem. 9 (2001) 1255-1268.
[5] J. Kapuscinski, Z. Darzynkiewicz, Relationship between the pharmacological activity of antitumor drugs Ametantrone and mitoxantrone (Novatrone) and their ability to
condense nucleic acids. Proc. Natl. Acad. Sci. U S A 83 (1986) 6302-6306.
[6] A.J. Lee, N.J. Hodges, J.K. Chipman, Modified comet assay as a biomarker of sodium dichromate-induced oxidative DNA damage: optimization and reproducibility.
Biomarkers 9 (2004) 103-115.
[7] N.P. Singh, P.E. Penn, W.R. Pendergrass, N.S. Wolf, White light-mediated DNA strand breaks in lens epithelial cells. Exp. Eye Res.75 (2002) 555-560.
[8] V. Cardile, C. Scifo, A. Russo, M. Falsaperla, G. Morgia, M. Motta, M. Renis, E. Imbriani, G. Silvestre, Involvement of HSP70 in resveratrol-induced apoptosis of human prostate cancer. Anticancer Res. 23 (2003) 4921-4926.
[9] J.A. Woods, A.J. Young, I.T. Gilmore, A. Morris, R.F. Bilton, Measurement of
menadione-mediated DNA damage in human lymphocytes using the comet assay. Free Radical Res. 26 (1997) 113-124.
[10] M. Murata, S. Kawanishi, Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 316 (2004) 123-128. [11] M. Murata, T. Suzuki, K. Midorikawa, S. Oikawa, S. Kawanishi, Oxidative DNA
damage induced by a hydroperoxide derivative of cyclophosphamide. Free Radical Biol. Med. 37 (2004) 793-802.
[12] M.L. Michaels, J. Tchou, A.P. Grollman, J.H. Miller, A repair system for 8-oxo-7,8-dihydrodeoxyguanine. Biochem. 31 (1992) 10964-10968.
[13] J.L. Parsons, II. Dianova, G.L. Dianov, APE1 is the major 3'-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 32 (2004) 3531-3536.
[14] W.R. Lee, S.C. Shen, H.Y. Lin, W.C. Hou, L.L. Yang, Y.C. Chen, Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem. Pharmacol. 63 (2002) 225-236.
and in vivo inhibitory activities of rutin, wogonin, and quercetin on
lipopolysaccharide-induced nitric oxide and prostaglandin E2 production. Eur. J. Pharmacol. 446 (2002) 187-194.
[16] L.L. Wu, C.C. Chiou, P.Y. Chang, J.T. Wu, Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 339 (2004) 1-9.
[17] G. Varbiro, B. Veres, Jr. F. Gallyas, B. Sumegi, Direct effect of taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med. 31 (2001) 548-558.
[18] K.K. Kiningham, D.K.S. Clair, Overexpresssion of manganese superoxide dismutase selectively modulates the activity of jun-associated transcription factors in fibrosarcoma cells. Cancer Res. 57 (1997) 5265-5271.
[19] J.M. Matés, F.M. Sánchez-Jiménez, Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell. Biol. 32 (2000) 157-170. [20] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram
quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72 (1976) 248-254.
[21] H.Z. Lee, C.H. Wu, S.P. Chang, The release of nucleophosmin from nucleus:
involvement in aloe-emodin-induced human lung nonsmall carcinoma cell apoptosis. Int. J. Cancer 113 (2005) 971-976.
[22] T. Arai, V.P. Kelly, K. Komoro, O. Minowa, T. Noda, S. Nishimura, Cell proliferation in liver of Mmh/Ogg1-deficient mice enhances mutation frequency because of the presence of 8-hydroxyguanine in DNA. Cancer Res. 63 (2003) 4287-4292.
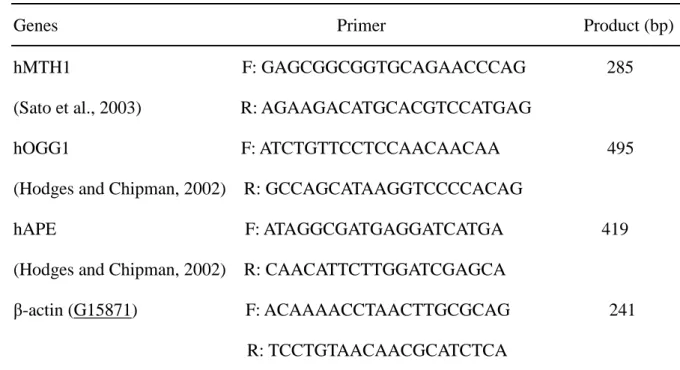
Table 1: Primers for PCR analysis
Genes Primer Product (bp)
hMTH1 F: GAGCGGCGGTGCAGAACCCAG 285
(Sato et al., 2003) R: AGAAGACATGCACGTCCATGAG
hOGG1 F: ATCTGTTCCTCCAACAACAA 495
(Hodges and Chipman, 2002) R: GCCAGCATAAGGTCCCCACAG
hAPE F: ATAGGCGATGAGGATCATGA 419
(Hodges and Chipman, 2002) R: CAACATTCTTGGATCGAGCA
β-actin (G15871) F: ACAAAACCTAACTTGCGCAG 241
Fig. 1. DNA aggregation induced by aloe-emodin. DNA was extracted from H460 cells.
EqualaliquotsofDNA (40 μg)weretreated with 0.1% DMSO or80 μM aloe-emodin. The samples were votexed, incubated and centrifuged. (A) The nonprecipitated DNA in solution was determined by measurement of absorbance of the supernatant at 260 nm. Aloe-emodin (C)- or DMSO (B)-induced the precipitated DNA was stained with propidium iodide and examined by fluorescence microscopy. Results are representative of three independent experiments. Bar(1 cm)= 100 μm.
Fig. 2. Aloe-emodin induced DNA single strand breaks in H460 cells. Cells were incubated
with (B) or without (A) 40 μM aloe-emodin in the presence of 1% serum for 16 h. Single cell gel electrophoresis was determined as described in Materials and Methods. The
symbols of + and - indicated the anode and cathode, respectively, during electrophoresis of negatively charged DNA. Results are representative of three independent experiments. Bar (1 cm)= 33 μm.
Fig. 3. Effects of aloe-emodin on the mRNA expression of hMHT1, hOGG1 and apurinic endonuclease in H460 cells. Aloe-emodin-induced the gene expression of hMHT1, hOGG1
and apurinic endonuclease was detected by RT-PCR. H460 cells were incubated with 0.1% DMSO or40 μM aloe-emodin in the presence of 1% serum for 24 h. RNA samples were prepared from control or aloe-emodin-treated cells. PCR products run on a 2% agarose gel. Lane M, molecular weight marker; lane1 (hOGG1), control cells; lane 2 (hOGG1),
aloe-emodin-treated cells; lane 4 (hMHT1), control cells; lane 5 (hMHT1),
aloe-emodin-treated cells; lane 7 (apurinic endonuclease), control cells; lane 8 (apurinic
endonuclease), aloe-emodin-treated cells; lane 10 (β-actin), control cells; lane 11 (β-actin),
aloe-emodin-treated cells. Lanes 3, 6, 9 and 12 were no template control in PCR reaction. Results are representative of three independent experiments.
Fig. 4. Effects of aloe-emodin and chelerythrine on ROS production in H460 cells. Cells
were cultured for 48 h before drug treatment in 12-well plates. Intracellular levels of ROS were examined by 5-H2DCFDA (5 μM)for 30 min in the dark, washed and then treated
with 0.1 % DMSO (A, B and C) or 40 μM aloe-emodin (D, E and F) in the presence of 1 % serum at 37℃ for 1, 4 and 8 h and cells were washed and observed by using fluorescence microscopy. Results are representative of three independent experiments. All photographic exposures were for 10 s.
Fig. 5. Effects of aloe-emodin on SOD activity in H460 cells. A: Cells were cultured for 48
h before drug treatment in 10 cm dish. Cellswereincubated with orwithout40 μM aloe-emodin in the presence of 1 % serum for 1, 2, 4, 8, 16 and 24 h. Cell lysates were analyzed by commercial kit. The total SOD activity was assayed by monitoring the
absorbance at 450 nm using a plate reader. The addition of 2 mM potassium cyanide to the assay will inhibit Cu/Zu SOD, resulting in the detection of only Mn SOD activity. Data are the mean percent control ± S.D. for 3 separate experiments. * P < 0.001 compared to the control values. B: The effects of aloe-emodin on the protein levels of Cu/Zn SOD and Mn SOD were detected by Western blot analysis in H460 cells. Cells were incubated with or without40 μM aloe-emodin in the presence of 1 % serum for 1, 2, 4, 8, 16 and 24 h. Cell lysates were analyzed by 12 % (Mn SOD) and 15 % (Cu/Zn SOD) SDS-PAGE, and then probed with primary antibodies as described in Materials and Methods. Results are representative of three independent experiments.