Temperature and Para-Substituent Effects on the Face Selectivity
of 1,3-Dipolar Cycloaddition Reactions of Benzonitrile Oxides with
5-Substituted Adamantane-2-thiones, N-Benzyladamantyl-2-imines,
and 2-Methyleneadamantanes
Tzong-Liang Tsai,
†Wei-Cheng Chen,
‡Chin-Hui Yu,
‡W. J. le Noble,
§and
Wen-Sheng Chung*
,†Department of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan 30050, Republic of China; Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan 300, Republic of
China; and Department of Chemistry, State University of New York, Stony Brook, New York 11794
Received May 19, 1998
The 1,3-dipolar cycloaddition reactions of para-substituted benzonitrile oxides (5-Y) with 5-fluo-roadamantane-2-thione (2-F) and -2-methyleneadamantane (3-F) as well as with variously 5-substituted-N-benzyladamantyl-2-imines (4-X) were examined. They produce two geometrically isomeric∆2-1,4,2-oxathiazolines (7-F,Y), ∆2-isoxazolines (8-F,Y), and∆2-1,2,4-oxadiazolines (11-X,Y), respectively. The face selectivity in the latter reaction was found to be∼1:1 regardless of the variations in 5-substituent and the temperature. For the former two reactions, the para-substituent was varied from electron-withdrawing (Y ) F, Cl, Br, CN, or NO2) to -releasing (Y ) Me, or OMe). The face selectivity was measured in all cases. The differences∆F for the reactions of 2- and 3-F with 5-Y were obtained from linear Hammett plots; they are +0.12 and 0.0, respectively. These low values and information from previous studies imply a concerted one-step mechanism with very little charge distribution differences in the transition states. These effects of temperature on the Z/E product ratios provide us, for the first time, with activation parameter differences between the syn- and anti transition states; their values are discussed. The product bias resulting from the favored attack of nitrile oxide on the zu face is discussed in terms of transition state hyperconjugation based on the experimental results and AM1 calculations.
The study of electronic effects in various sterically unbiased trigonal carbon centers continues to attract considerable theoretical and experimental attention.1,2 Among the many models, transition-state hypercon-jugation1a-c,2and electrostatic field interaction1c-eare the two most popular explanations for the face selectivity results to be found in the literature. 5-Substituted adamantan-2-ones 1-X and their derivatives have proven to be useful probes in research aimed at understanding the electronic factors in face selection.1aThese studies of a variety of reactions indicate that the reagent prefers to attack the face antiperiplanar to the more electron-rich vicinal bonds (zu and en face preference in 1-X when X equals an electron-withdrawing or -donating group, respectively). These results can be reconciled with Cieplak’s transition-state hyperconjugation model.2That interpretation allows but does not demand that the magnitude of the effect may be a function of the electronic nature of the nucleophile or electrophile. There is only
one example of a search for such an effect: in the addition of para-substituted phenyl Grignard reagents to 1-F, the substituent was varied from CF3to NMe2.3However, no effect was found on the face selectivity.
1,3-Dipolar cycloadditions offer a convenient one-step route for the construction of a variety of complex five-membered heterocycles which are synthetically useful compounds.4 Nitrile oxide cycloadditions to terminal alkenes and thiones proceed regioselectively to give 5-substituted∆2-isoxazolines and∆2-1,4,2-oxathiazolines, respectively.5We recently reported6 the 1,3-dipolar cy-†National Chiao Tung University.
‡National Tsing Hua University. §State University of New York.
(1) (a) Bodepudi, V. R.; le Noble, W. J. J. Org. Chem. 1994, 59, 3265; 1991, 56, 2001 and references therein. (b) Halterman, R. L.; McCarthy, B. A.; McEvoy, M. A. J. Org. Chem. 1992, 57, 5585 and references therein. (c) For an excellent article on the electrostatic vs hypercon-jugation effects, see Adcock, W.; Cotton, J.; Trout, N. A. J. Org. Chem. 1994, 59, 1867 and references therein. (d) Coxon, J. M.; Houk, K. N.; Luibrand, R. T. J. Org. Chem. 1995, 60, 418. (e) Paddon-Row: M. N.; Wu, Y.-D.; Houk, K. N. J. Am. Chem. Soc. 1992, 114, 10638 and references therein.
(2) (a) Cieplak, A. S.; Tait, B. D.; Johnson, C. R. J. Am. Chem. Soc. 1989, 111, 8447. (b) Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4540. (c) Johnson, C. R.; Tait, B.; Cieplak, A. S. J. Am. Chem. Soc. 1987, 109, 5875.
(3) Lin, M.-H.; Silver, J. E.; le Noble, W. J. J. Org. Chem. 1988, 53, 5155.
(4) For reviews, see (a) Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 1-176. (b) Caramella, P.; Gru¨ nanger, P. In 1,3-Dipolar Cycloaddition Chem-istry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 291-392. (c) Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis; Torssell, K. B. G., Ed.; VCH: New York, 1988. (d) Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon Press: Oxford, 1990; pp 269-367. (e) Houk, K. N.; Gonzalez, J.; Li, Y. Acc. Chem. Res. 1995, 28, 81. (f) Sustmann, R. Tetrahedron Lett. 1971, 2717, 2721. 10.1021/jo980945n CCC: $18.00 © 1999 American Chemical Society
cloaddition reactions of benzonitrile oxide (5-H) with the sterically unbiased thiones 2-X and terminal alkenes 3-X (where X ) F, Cl, Br, and Ph) and found that the favored products derive from attack of nitrile oxide on the zu-face. Thus, 1,3-dipolar cycloaddition reactions to 2 and 3 provide additional important support for the transition-state hyperconjugation model, which stresses that the newly developingσ* orbital of an incipient bond should attract electron density with the same directional prefer-ence, regardless of the type of reaction.1a,2c
Several important questions remained to be an-swered: (1) Is there any effect of the para-substituent of phenyl nitrile oxide in these reactions, and can one obtain charge distribution information from the Hammett plot studies? (2) Are the reaction rates and the E/Z product ratios of 1,3-dipolar cycloadditions sensitive to the reac-tion temperature, and can one obtain activareac-tion param-eter differences of the two transition states? (3) How do the HOMO and LUMO energies of the dipolarophiles (1-X to 4-X) and dipoles (5-Y) vary with the substituents? With these objectives in mind, we synthesized a series of para-substituted benzonitrile oxides (5-Y) and studied the face selectivity of their reactions with 2-F and 3-F. The results from theoretical calculations (AM1) were compared with experimental results. The activation parameter differ-ences and the difference in ∆F between the two 1,3-dipolar cycloaddition reactions supplement those previ-ously given6and allow us to comment on the mechanism.
Results and Discussion
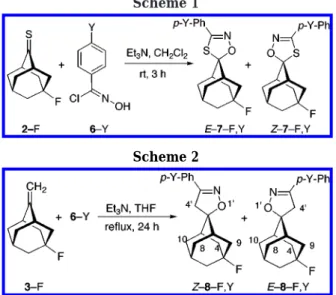
The 1,3-dipolar cycloaddition reactions of 2-X and 3-X with parent nitrile oxide 5-H in methylene chloride at room temperature give mixtures of E- and Z-adducts of 7-X,H and 8-X,H in 75-82% yields (Schemes 1 and 2).6 Compounds 2-F and 3-F were chosen for further study with para-substituted benzonitrile oxides 5-Y (prepared in situ from the reaction of 6-Y with triethylamine); the fluorine-induced chemical shifts and C-F couplings allow
a straightforward assignment of configuration of the two products. Both adducts are stable to the reaction conditions, and they are characterized as 5-fluoro-3′-p-Y-phenyladamantane-2-spiro-5′-(∆2 -1′,4′,2′-oxathiazo-lines) based on their mass and NMR spectra.6 In all instances examined (Y ) NO2, CN, F, Cl, Br, OMe, and Me) the yields of E- and Z-7-F,Y are 78-94%, and the major isomer is E-7-F,Y, which results from syn attack of the nitrile oxide (5-Y) on 2-F (see Table 1).
The configuration assignment of the epimers 7-F,Y was based on the relative shielding power of oxygen vs sulfur directly “above” the flanking methylene groups. Thus, C-4 and C-9 (identified by their19F coupling) are syn to the oxygen since they are shielded vs C-8 and C-10 by a margin of g2.2 ppm. The configurations of Z-7-F,H and Z-7-Br,H were established independently by means of both X-ray diffraction and a13C NMR additivity scheme.6,8 The 13C NMR absorption peaks for the adamantane skeleton in the oxathiazolines E- and Z-7-F,Y are very similar to those observed for parent compounds E- and Z-7-F,H. Thus, the assignment of configuration is straight-forward, based on these13C NMR peaks (see Experimen-tal Section).
The reactions of benzonitrile oxides 5-Y with 3-F were carried out in refluxing THF for 24 h to give two isoxazolines 8-F,Y in 72-88% isolated yields (see Scheme 2 and Table 2). Again, the products were proven to be stable under the reaction conditions; i.e., both products are formed in kinetically controlled processes. In all (5) (a) Huisgen, R.; Fisera, L.; Giera, H.; Sustmann, R. J. Am. Chem.
Soc. 1995, 117, 9671. (b) Sustmann, R.; Sicking, W.; Huisgen, R. ibid. 1995, 117, 9679. (c) Fisera, L.; Huisgen, R.; Kalwinsch, I.; Langhals, E.; Li, X. Mloston, G.; Polborn, K.; Rapp, J.; Sicking, W.; Sustmann, R. Pure. Appl. Chem. 1996, 68, 789. (d) Metzner, P. Pure. Appl. Chem. 1996, 68, 863. (e) Grundmann, C. Synthesis 1970, 344.
(6) Chung, W.-S.; Tsai, T.-L.; Ho, C.-C.; Chiang, M. Y. N.; le Noble, W. J. J. Org. Chem. 1997, 62, 4672.
(7) (a) Chung, W.-S.; Turro, N. J.; Srivastava, S.; Li, H.; le Noble, W. J. J. Am. Chem. Soc. 1988, 110, 7882. (b) Katada, T.; Eguchi, S.; Sasaki, T. J. Org. Chem. 1986, 51, 314. (c) Li, H.; Silver, J. E.; Watson, W. H.; Kashyap, R. P.; le Noble, W. J. J. Org. Chem. 1991, 56, 5932. (8) Srivastava, S.; Cheung, C.-K.; le Noble, W. J. Magn. Reson. Chem. 1985, 23, 232.
Scheme 1
Scheme 2
Table 1. 1,3-Dipolar Cycloaddition Reaction of Para-Substituted Benzonitrile Oxide (5-Y) with 5-Fluoroadamantane-2-thiones (2-F) in Methylene
Chloride at Room Temperature for 3 h entry σpa Y E-:Z-7-F,Yb yield, %
1 0 H 69:31 82 2 0.78 NO2 c 88 3 0.66 CN 71:29 89 4 0.06 F 68:32 85 5 0.23 Cl 68:32 94 6 0.23 Br 68:32 78 7 -0.17 CH3 66:34 80 8 -0.27 OCH3 65:35 86 aTheσ
pvalues are obtained from Swain, C. G.; Lupton, E. C., Jr. J. Am. Chem. Soc. 1968, 90, 4328.bThe ratios of the adducts were determined by VPC analysis.cRatio could not be determined by this method.
Table 2. 1,3-Dipolar Cycloaddition Reaction of Para-Substituted Benzonitrile Oxide (5-Y) with 5-Fluoro-2-Methyleneadamantanes (3-F) in Refluxing
Tetrahydrofuran for 24 h
entry σp Y Z-:E-8-F,Ya yield, %
1 0.00 H 60:40 72 2 0.78 NO2 61:39 88 3 0.66 CN 60:40 83 4 0.06 F 59:41 85 5 0.23 Cl 61:39 79 6 0.23 Br 60:40 82 7 -0.17 CH3 60:40 77 8 -0.27 OCH3 59:41 81
aThe ratios of the E- and Z-adducts were determined by1 H-NMR.
instances examined, the major isomer (∼60:40 ratio as determined by1H NMR integration) has the Z configu-ration. It should be noted that the major product Z-8-F,Y is derived from syn attack of the nitrile oxide on 3-F. A 5.8% NOE was observed for the flanking pair axial-hydrogens at C-8 and C-10 but not at C-4 and C-9 when the 4′-methylene hydrogens were irradiated, confirming the major isomer to be Z-8-F,Y.
The data in Tables 1 and 2 show the previously noted tendency that all dipoles (5-Y) prefer to approach from the direction antiperiplanar to the electron richest bonds. In the Hammett plots of log(syn/anti) vs σp, linear correlation are found; they give the reaction constant difference∆F as 0.12 for 2-F and 0.0 for 3-F. These small values are accommodated by a one-step concerted mech-anism involving a five-membered dipolar transition state 9. This mechanism is consistent with previous experi-mental data, namely the low sensitivity of the rate constants to solvent polarity.6A charge imbalance in the transition state has been suggested9 to explain the substituent and solvent effects of other concerted 1,3-dipolar cycloadditions. The values of∆F also indicate that the dipole (CNO group) is acting as an electron acceptor and the dipolarophile (CdS or CdC) as an electron donor; the formation of partial charges in the transition states 9 and 10 suggests that bonds a in 9 and 10 have developed further than bonds b. However, this varies somewhat with the polar nature of the dipolarophiles, as indicated by the∆F values.9The fact that the∆F value
in 3-F is smaller than that in 2-F points to a lowering of the polarity of the transition state 10. Recent ab inito calculations by Huisgen and Sustmann5a-cfor the parent nitrone with dipolarophiles CdS vs CdC also support different transition states in these 1,3-dipolar cycload-dition reactions.
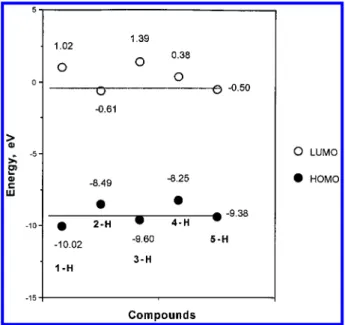
Some 1,3-dipolar cycloaddition reactions are controlled mainly by the HOMO (dipole)-LUMO (dipolarophile) interaction and others by the LUMO (dipole)-HOMO (dipolarophile) interaction. The smaller the energy gap between the controlling orbitals is, the faster is the reaction.4,5The former is accelerated by electron-donating substituents in the dipole and electron-attracting sub-stituents in the dipolarophile, and the latter by electron-donating substituents in the dipolarophile; in each case, the energy gap between the controlling orbitals in the two components is diminished. AM1 calculations of the HOMO-LUMO energy of 1-H to 4-H (Figure 1) reveal that all of the 1,3-dipolar reactions described here fit into the latter category; i.e., the nitrile oxide has a low lying LUMO which interacts with the HOMO of the dipolaro-philes (1-X to 4-X).
The calculated HOMO and LUMO energies of dipole 5-H and dipolarophiles 1-H to 4-H explain why the reaction of nitrile oxide with adamantanone 1-H fails even at high temperature and with a long reaction time. On the other hand, one would expect the 1,3-dipolar cycloaddition reaction of 5-H with N-benzyladamantyl-2-imine 4 to proceed very easily, and indeed, the reactions of imines 4-X with 5-H in methylene chloride at room temperature proceeded smoothly to completion within 0.5 h (Scheme 3). The imines 4-X were prepared from corresponding adamantanones (1-X) with benzylamine in refluxing benzene. In all cases studied, the yields of Z-and E-11-X,H were 83-92%, but no preference in product configuration was found (Table 3). In a highly exothermic reaction like this, according to the Hammond postulate, the transition state is early, and its structure should resemble that of starting reagents. One would also expect that if the reaction temperature is low enough, the face selectivity should be enhanced; however, the Z-/E-11-X,H product ratio stays constant at ca. 50:50 ((2%) even at -60 °C.
The structures of 5-substituted-3′-phenyl-4′-N-benzyl-adamantane-2-spiro-5′-(∆2-1′,2′,4′-oxadiazoline) 11-X,H (9) (a) Dondoni, A.; Barbaro, G. J. Chem. Soc., Perkin Trans. 2, 1973,
1769. (b) Ito, K.; Saito, K.; Takahashi, K. Heterocycles 1993, 36, 21. (c) Ito, K.; Saito, K. Bull. Chem. Soc. Jpn. 1995, 68, 3539. (d) Balsamini, C.; Bedini, A.; Spadoni, G.; Burdisso, M.; Capelli, A. M. Tetrahedron 1994, 50, 3773.
Figure 1. AM1-calculated energies for frontier molecular
orbitals of adamantan-2-ones (1-H), adamantane-2-thiones (2-H), methyleneadamantane (3-(2-H), N-benzyladamantyl-2-imine (4-H), and benzonitrile oxide (5-H), where the two lines show the relative energies of dipolarophiles vs those of dipole 5-H.
were characterized completely by means of1H and13C NMR spectroscopy (Table 4), MS, and HRMS (see Ex-perimental Section). The assignments of configuration were based on the NOE experiments on the 11-F,H where strong signal enhancements (ca. 6%) were observed between protons on C-11 and C-8, C-10 of the Z-11-F,H isomer (identified by their small C-F coupling constants) but not between those on C-11 and C-4, C-9. All other adducts were assigned on the basis of the 13C NMR additivity scheme, which has been shown to be very successful in the assignment of adamantane structures.6-8 It is interesting to note that N-benzyl has a better shielding effect than an oxygen atom directly “above” the flanking methylene carbons in oxadiazolines 11;10thus, C-8 and C-10, which are syn to the N-benzyl in the parent 11-H,H, are shielded by at least 0.67 ppm compared to C-4 and C-9 (Table 4).
Even though the absolute rate equation (eq 1)11readily permits a systematic study to be made of the temperature dependence of the Z/E product,
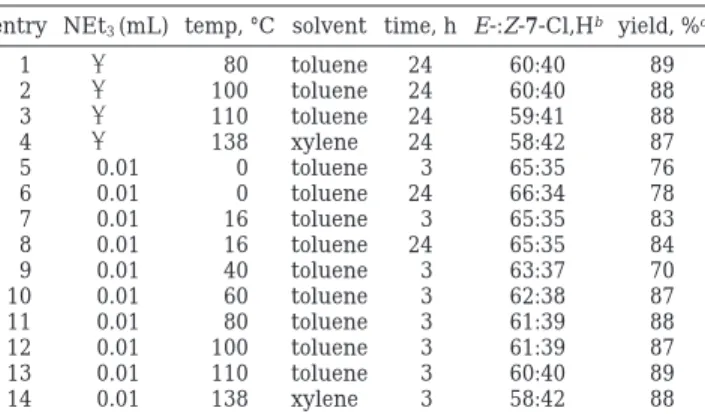
few such studies have been reported in the adamantane system. For example, although various temperatures were used in the reduction and Grignard reactions of adamantanones 1-X, no changes in face selectivity were observed.1,3In the course of our study of the 1,3-dipolar cycloaddition reactions, we noticed that temperature variation did cause a substantial face selectivity change in the 1,3-dipolar cycloaddition reactions. Since 2-Cl and 3-Cl were available in substantial quantity, their reac-tions with 6-H were chosen for a study at various temperatures. The results from temperature effects are summarized in Tables 5 and 6.
Entries 1-4 of both Tables 5 and 6 show the results of generating nitrile oxide (5-H) by thermal elimination of HCl from the corresponding hydroximoyl chloride in the absence of triethylamine. At temperatures above 80 °C (but not below), the nitrile oxide can be generated in this way and captured by dipolarophiles 2-Cl or 3-Cl. Basi-cally, the product ratio stays constant when nitrile oxide is prepared by this method. At lower temperatures, 5-H can be generated only in the presence of triethylamine, permitting us to measure the syn/anti product ratios in the wide temperature range of 0-138 °C (entries 5-12 of Tables 5 and 6). The general trend is that as the
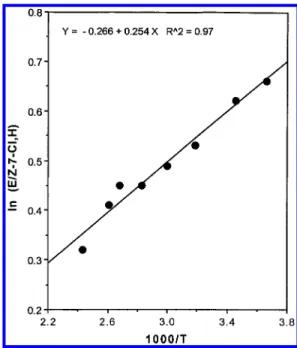
temperature increases, the syn/anti face selectivity de-creases. Plots of eq 2 (Figure 2 and Figure 3) show that the
activation enthalpy difference between the two transition states with 2-Cl is 0.51 kcal/mol and that with 3-Cl is 0.85 kcal/mol. The syn attack has a lower activation enthalpy than anti attack does, in both cases. The difference in∆Sqfor 2-Cl is quite small (∆Sq
syn- ∆Sqanti ≈ -0.53 eu) but larger for 3-Cl (∼ -2.23 eu). In that case, the data clearly suggest a reversal of the major and minor isomers at high temperatures. Solutions of pure isoxazo-(10) For the shielding effect of an oxygen atom on other
adamantane-related structures, see ref 6 and references therein.
(11) For a leading reference see Peterson, R. C. J. Org. Chem. 1964, 29, 3133.
Table 3. 1,3-Dipolar Cycloaddition Reaction of Benzonitrile Oxide (5-H) with
5-Substituted-N-benzyladamantane-2-imine (4-X) in Methylene Chloride at Various Temperature for 0.5 h entry X temp, °C Z-:E-11-X,Ha yield, %
1 F rt 51:49 83 2 F 0 51:49 b 3 F -50 52:48 b 4 F -60 52:48 b 5 Cl rt 50:50 87 6 Br rt 50:50 86 7 Ph rt 50:50 92
aThe ratios of the E- and Z-adducts were determined by 1H NMR.bWhen the ratio was determined, the reaction was not yet
complete.
k ) RT/Nh exp (-
∆G
q/RT)
(1)
Figure 2. The Arrhenius plot of ln(E/Z-7-Cl,H) vs 1000/T from
the 1,3-dipolar cycloaddition reactions of 2-Cl with benzonitrile oxide 5-H. The slope is 254 K-1.
Figure 3. The Arrhenius plot of ln(Z/E-8-Cl,H) vs 1000/T from
the 1,3-dipolar cycloaddition reactions of 3-Cl with benzonitrile oxide 5-H. The slope is 430 K-1.
ln(k
syn/k
anti) ) (∆S
q syn- ∆S
q anti)/R +
(∆H
q anti- ∆H
q syn)/RT (2)
lines E- and Z-8-Cl,H in toluene were heated to reflux for 24 h, but no indication of forming the other isomer through cycloreversion was observed; this indicates that
both products are formed in kinetically controlled pro-cesses. Although the correlation coefficient of our data in Figure 3 is only 0.91, it is clear that the slopes are positive and the intercepts negative in Figures 2 and 3. The kinetic study shows that the syn-attacks of phenyl nitrile oxides (5-Y) to thione 2-X and methyleneadaman-tane 3-X (where X is electron withdrawing) have a smaller activation enthalpy (∆Hq) and a more negative activation entropy (∆Sq) than the anti-attacks. This is consistent with the notion of transition state hypercon-jugation model, in which better interactions exist be-tween the more electron-rich antiperiplanar bonds and theσ* orbital of the incipient bond of dipoles.
Kinetic studies5a-cof 1,3-dipolar additions of nitrones to thiones have revealed that the weakness of the CdS π bond is not responsible for the fast rates; instead, the low HOMO-LUMO energy gap of the CdSπ bond was suggested to be the decisive factor. AM1 calculations15 of the HOMO-LUMO energy of 1-4 (Figure 1) reveal that all the 1,3-dipolar reactions described here are LUMO (dipole)-HOMO (dipolarophile) controlled reac-tions, i.e. a Sustmann type III reaction,4fand that the rates, which diminish in the order k(imine) > k(CdS) > k(CdCH2) . k(CdO) are consistent with the decreased energy gaps. The energy gaps (∆EII) between LUMO (dipole)-HOMO (dipolarophile) are as follows: 7.75 eV (4-H), 7.99 eV (2-H), 9.1 eV (3-H) and 9.52 eV (1-H).
The HOMO-LUMO energies of substituted dipolaro-philes 2-X to 4-X can also be calculated with the AM1 method (see Supporting Information). All of the 5-sub-stituents except phenyl cause lower HOMO and LUMO energies compared to those of the parent compounds 2-H to 4-H. The calculated LUMO energies of para-substi-tuted benzonitrile oxide 5-Y increase with electron-donating substituents (Me and OMe) but decrease with electron-withdrawing groups. The observed small positive values (∆F) in the Hammett plots are consistent with the diminished energy gap for electron-withdrawing para-(12) The author has deposited atomic coordinates for (Z)-7-Br (H) with the Cambridge Crystallographic Data Center. The coordinates can be obtained, on request, from the Director, Cambridge Crystal-lographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK.
(13) (a) Katada, T.; Eguchi, S.; Sasaki, T. J. Chem. Soc., Perkin Trans. 1 1984, 2641. (b) Bonini, B. F.; Maccagnani, G.; Mazzanti, G.; Thijs, L.; Ambrosius, H. P. M. M.; Zwanenburg, B. J. Chem. Soc., Perkin Trans. 1 1977, 1468.
(14) Taguchi, K.; Westheimer, F. H. J. Org. Chem. 1971, 36, 1570. (15) Dewar, M. J. S.; Zoebisch, E. G.; Healy, E. F.; Stewart, J. J. P. J. Am. Chem. Soc. 1985, 107, 3902.
Table 4. Calculatedaand Observedb 13C NMR Chemical Shifts of
5-Substituted-3′-phenyl-4′-N-benzyladamantane-2-spiro-5′-(∆2-1′-2′-4′-oxadiazolines)c
11-H,H Z-11-F,H E-11-F,H Z-11-Cl,H E-11-Cl,H Z-11-Br,H E-11-Br,H Z-11-Ph,H E-11-Ph,H C1, C3 34.43 36.93 (37.53) J ) 10.6 36.94 (37.53)J ) 10.2 37.26 (36.53) 36.91 (36.53) 38.10 (38.43) 38.00 (38.43) 34.86 (35.03) 34.57 (35.03) C2 102.42 100.61 (100.62) 100.64 (100.62) 100.40 (100.12) 100.02 (100.12) 100.31 (100.12) 100.11 (100.12) 101.76 (101.52) 101.58 (101.52) C4, C9 34.73 39.18 (39.73) J ) 19.5 38.67 (39.06)J ) 19.4 44.14 (44.43) 43.40 (43.76) 45.65 (46.23) 45.11 (45.56) 40.29 (40.13) 39.20 (39.46) C5 26.88 90.94 (90.68) J ) 184.3 91.32 (90.47)J ) 184.4 65.95 (66.68) 65.75 (66.47) 62.47 (64.88) 62.69 (64.67) 35.14 (34.58) 35.02 (34.37) C6 37.35 42.36 (42.35) J ) 17.6 42.44 (42.35)J ) 16.8 47.29 (47.05) 47.08 (47.05) 48.81 (48.85) 48.70 (48.85) 42.49 (42.75) 42.42 (42.75) C7 26.67 29.67 (29.77) J ) 9.9 29.61 (29.98)J ) 9.9 29.89 (28.77) 30.21 (28.98) 30.69 (30.67) 30.61 (30.88) 27.43 (27.27) 26.99 (27.48) C8, C10 34.06 32.39 (32.26) J ) 2.0 33.12 (32.94)J ) 1.9 32.12 (31.76) 32.49 (32.43) 32.10 (31.76) 32.67 (32.43) 33.16 (33.16) 33.51 (33.83) C3′ 161.28 161.37 161.36 161.39 161.39 161.38 161.30 161.38 161.09 C11 49.73 49.90 49.79 49.88 49.53 49.83 49.75 49.85 49.37
aCalculated values are in parentheses.bMeasured with a Bruker DRX-300 NMR operated at 75.4 MHz and reported inδ units, CDCl
3 (δ 77.00). J is in hertz. In the parent compound 11-H,H the oxygen is understood to be syn to C4and C9.cThe phenyl groups are not shown here but are reported in Experimental Section.
Table 5. Temperature Effects on the Face Selectivity of 1,3-Dipolar Cycloaddition Reaction of Benzonitrile Oxide
(5-H) with 5-Chloroadamantane-2-thione (2-Cl) in Toluene or Xylenea
entry NEt3(mL) temp, °C solvent time, h E-:Z-7-Cl,Hb yield, %c
1 - 80 toluene 24 60:40 89 2 - 100 toluene 24 60:40 88 3 - 110 toluene 24 59:41 88 4 - 138 xylene 24 58:42 87 5 0.01 0 toluene 3 65:35 76 6 0.01 0 toluene 24 66:34 78 7 0.01 16 toluene 3 65:35 83 8 0.01 16 toluene 24 65:35 84 9 0.01 40 toluene 3 63:37 70 10 0.01 60 toluene 3 62:38 87 11 0.01 80 toluene 3 61:39 88 12 0.01 100 toluene 3 61:39 87 13 0.01 110 toluene 3 60:40 89 14 0.01 138 xylene 3 58:42 88
aFixed concentration of 2-Cl (5 mM) and benzohydroximoyl
chloride 6-H (7.5 mM) in toluene or xylene were studied through-out the series.bThe ratios of the E- and Z-7-Cl,H were determined
by GC.cYields were measured by VPC with an internal standard
thioxanthen-9-one (1 mM).
Table 6. Temperature Effects on the Face Selectivity of 1,3-Dipolar Cycloaddition Reaction of Benzonitrile Oxide
(5-H) with 5-Chloro-methyleneadamantane (3-Cl) in Toluene or Xylene for 24 ha
entry NEt3(mL) temp, °C solvent Z-:E-8-Cl,Hb yield, %c
1 - 80 toluene 51:49 99 (6) 2 - 100 toluene 51:49 83 (27) 3 - 110 toluene 50:50 93 (99) 4 - 138 xylene 51:49 95 (99) 5 0.01 0 toluene 60:40 89 (88) 6 0.01 16 toluene 60:40 92 (75) 7 0.01 30 toluene 58:42 85 (77) 8 0.01 40 toluene 56:44 87 (82) 9 0.01 50 toluene 54:46 82 (59) 10 0.01 60 toluene 52:48 96 (95) 11 0.01 80 toluene 51:49 83 (82) 12 0.01 100 toluene 52:48 89 (70) 13 0.01 110 toluene 50:50 88 (76) 14 0.01 138 xylene 50:50 91 (89)
aFixed concentration of 3-Cl (5.5 mM) and 6-H (8.2 mM) in
toluene or xylene were studied throughout the series.bThe ratios
of the Z- and E-adducts 8-Cl,H were determined by1H NMR.
cYields were measured by 1H NMR with 1,4-dioxane as an external standard. Values in parentheses are the percentage conversion.
substituents; e.g., 5-NO2 reacts faster and is more selective than 5-Me when treated with 2-F (Table 1). The Hammett plot of the reaction of 5-Y with 3-F leads to a zero reaction constant (∆F ) 0), which indicates that no charge-distribution difference is observed in the two transition states leading to Z- and E-8-F,Y; again, this indicates that electrostatic effects are not important here in these adamantane systems.
Summary
The effects of para-substitution of benzonitrile oxides (5-Y) on the face selectivity of 1,3-dipolar cycloaddition reactions with 2-F and 3-F have been studied. The reaction constant differences∆F for these reactions are obtained from linear Hammett plots, which give a small positive value (+0.12) for thiones (2-F) and a zero slope for methyleneadamantanes (3-F). These small∆F values imply that the 1,3-dipolar cycloaddition is consistent with a concerted-one-step mechanism with a slightly different charge imbalance in the transition states.
Effects of temperature variation on the Z/E product ratios of the 1,3-dipolar cycloaddition reactions of 2-Cl and 3-Cl with parent nitrile oxide 5-H provide us, for the first time, with the activation enthalpy and entropy differences between the two transition states of the syn-and anti-face attacks. The syn-attacks of the dipoles (5-X) on thiones 2-X and methyleneadamantane 3-X have a smaller activation enthalpy (∆Hq) and a more negative activation entropy (∆Sq) than the anti-attacks.
AM1 calculations of the HOMO-LUMO energies of 1-5 reveal that all the 1,3-dipolar reactions described here are LUMO (dipole)-HOMO (dipolarophile) con-trolled reactions; the rates decrease in the order k(imine) > k(CdS) > k(CdCH2) . k(CdO), consistent with decreased energy gaps.
Experimental Section
1H NMR spectra were measured on 300 and 400 MHz spectrometers. The data reported were recorded at 300 MHz. Natural abundance13C NMR spectra were measured using pulsed Fourier transform, on either a Varian Unity-300 or Bruker DRX-300 high-resolution NMR spectrometer operating at 75.4 MHz. Broad-band decoupling, DEPT and 2D H,C-COSY experiments were carried to simplify spectra and aid peak identification. Chemical shifts are given in ppm and J in hertz for both nuclei, with the solvent (usually CDCl3) peak as an internal standard. The reference peak for13C isδ 77.00, which is set at the center peak of CDCl3, and for1H it isδ 7.25 of CHCl3. Gas chromatographic analyses were carried out on an instrument equipped with a flame ionization detector and a reporting integrator. The capillary column employed included HP-1 cross-linked methylsilicone (SE-30, 25 m) and carbowax column (25 m). GC/MS spectral analyses were carried out with EI (at 70 eV).
General Procedure for the Synthesis of 5-Fluoro-3′
-para-substituted-phenyladamantane-2-spiro-5′-(∆2-1′,4′,2′
-oxathiazolines) (E- and Z-7-F,Y). To a well-stirred solution
of 2-F (100 mg, 0.5 mmol) and 6-Y (0.75 mmol) in anhydrous methylene chloride (10 mL) was added an excess of triethy-lamine (0.75 mol equiv). After being stirred for 3 h at room temperature, the mixture was washed with water and dried (MgSO4); after filtration and solvent evaporation, the solid residue was purified on a silica gel column by elution with n-hexane/methylene chloride to give E- and Z-7-F,Y. The isolated yields based on converted 2-F are as follows: 7-F,F 85%, 7-F,Cl 94%, 7-F,Br 78%, 7-F,CN 89%, 7-F,NO288%, 7-F,-Me 80%, 7-F,O7-F,-Me 86%.
E-7-F,F: colorless solid; mp 90-91.5 °C;δH1.70-2.00 (m, 8 H), 2.20-2.30 (m, 1 H), 2.40-2.60 (m, 2 H), 2.70 (bs, 2 H), 7.00-7.15 (m, 2 H), 7.60-7.75 (m, 2 H);δC29.79 (C7, J ) 9.8 Hz), 35.68 (C8,10, J ) 1.8 Hz), 38.31 (C4,9, J ) 19.8 Hz), 42.02 (C1,3, J ) 10.2 Hz), 42.19 (C6, J ) 16.1 Hz), 90.10 (C5, J ) 185.2 Hz), 109.89 (C2, J ) 1.5 Hz), 115.92 (Cm, J ) 22.1 Hz), 124.70 (Ci, J ) 3.5 Hz), 129.69 (Co, J ) 8.6 Hz), 155.22 (C3′), 164.17 (Cp, J ) 252.1 Hz); MS (EI, m/z) 321 (M+, 42), 184 (43), 168 (6), 153 (100), 97 (14); HRMS calcd for C17H17OSF2N 321.1000, found 321.0995. Anal. Calcd for C17H17OSF2N: C, 63.53; H, 5.33; N. 4.36, found C, 63.66; H, 5.40; N. 4.26. Z-7-F,F: colorless solid; mp 129.5-131.5 °C;δH1.50-1.70 (m, 2 H), 1.85-2.18 (m, 6 H), 2.20-2.35 (m, 3 H), 2.66 (bs, 2 H), 7.00-7.15 (m, 2 H), 7.60-7.75 (m, 2 H);δC29.09 (C7, J ) 9.9 Hz), 32.11 (C8,10, J ) 1.8 Hz), 41.47 (C4,9, J ) 19.7 Hz), 41.72 (C1,3, J ) 10.5 Hz), 42.20 (C6, J ) 17.1 Hz), 90.52 (C5, J ) 185.5 Hz), 110.40 (C2, J ) 1.3 Hz), 115.92 (Cm, J ) 22.1 Hz), 124.68 (Ci, J ) 3.5 Hz), 129.70 (Co, J ) 8.6 Hz), 155.48 (C3′), 164.18 (Cp, J ) 252.2 Hz); MS (EI, m/z) 321 (M+, 36), 258 (25), 214 (32), 184 (29), 153 (100), 137 (37), 109 (18); HRMS calcd for C17H17OSF2N 321.1000, found 321.0996.
E-7-F,Cl: colorless solid; mp 157-158.5 °C;δH1.60-2.00 (m, 8 H), 2.20-2.30 (m, 1 H), 2.40-2.60 (m, 2 H), 2.70 (bs, 2 H), 7.30-7.40 (m, 2 H), 7.55-7.65 (m, 2 H);δC29.77 (C7, J ) 9.9 Hz), 35.67 (C8,10, J ) 1.7 Hz), 38.30 (C4,9, J ) 19.8 Hz), 42.06 (C1,3, J ) 10.2 Hz), 42.18 (C6, J ) 17.9 Hz), 90.06 (C5, J ) 185.0 Hz), 111.08 (C2), 126.97 (Ci), 128.85 (Cm), 129.00 (Co), 136.94 (Cp), 155.22 (C3′); MS (EI, m/z) 339 (M++ 2, 18), 337 (M+, 46), 184 (50), 171 (37), 169 (100), 97 (13); HRMS calcd for C17H17OSFN35Cl 337.0705, found 337.0708.
Z-7-F,Cl: colorless solid; mp 154-156 °C;δH1.50-1.60 (m, 2 H), 1.85-2.15 (m, 6 H), 2.15-2.30 (m, 3 H), 2.66 (bs, 2 H), 7.30-7.40 (m, 2 H), 7.50-7.65 (m, 2 H);δC29.10 (C7, J ) 9.7 Hz), 32.12 (C8,10, J ) 2.0 Hz), 41.47 (C4,9, J ) 19.7 Hz), 41.78 (C1,3, J ) 10.5 Hz), 42.20 (C6, J ) 17.2 Hz), 90.47 (C5, J ) 185.6 Hz), 110.58 (C2), 126.97 (Ci), 128.87 (Cm), 129.01 (Co), 136.97 (Cp), 155.47 (C3′); MS (EI, m/z) 339 (M++ 2, 17), 337 (M+, 44), 184 (74), 171 (38), 169 (100), 97 (15); HRMS calcd for C17H17OSFN35Cl 337.0705, found 337.0703.
E-7-F,Br: colorless solid; mp 176-176.5 °C;δH1.70-2.05 (m, 8 H), 2.20-2.35 (m, 1 H), 2.40-2.60 (m, 2 H), 2.70 (bs, 2 H), 7.54 (s, 4 H);δC29.78 (C7, J ) 9.9 Hz), 35.68 (C8,10, J ) 1.9 Hz), 38.31 (C4,9, J ) 19.8 Hz), 42.08 (C1,3, J ) 10.2 Hz), 42.18 (C6, J ) 18.1 Hz), 90.03 (C5, J ) 185.5 Hz), 110.13 (C2), 125.28 (Cp), 127.44 (Ci), 129.03 (Cm), 131.96 (Co), 155.29 (C3′); MS (EI) m/z 383 (M++ 2, 46), 381 (M+, 44), 215 (100), 213 (98), 184 (67), 97 (22); HRMS calcd for C17H17OSFN79Br 381.0154, found 381.0200. Z-7-F,Br: colorless solid; mp 181-181.5 °C;δH1.55-1.65 (m, 2 H), 1.90-2.20 (m, 6 H), 2.27 (bs, 3 H), 2.66 (bs, 2 H), 7.54 (s, 4 H);δC29.12 (C7, J ) 9.9 Hz), 32.14 (C8,10, J ) 1.6 Hz), 41.50 (C4,9, J ) 19.9 Hz), 41.82 (C1,3, J ) 10.4 Hz), 42.23 (C6, J ) 17.0 Hz), 90.46 (C5, J ) 186.0 Hz), 110.66 (C2), 125.32 (Cp), 127.46 (Ci), 129.07 (Cm), 131.99 (Co), 155.57 (C3′); MS (EI, m/z) 383 (M++ 2, 55), 381 (M+, 52), 215 (100), 213 (96), 184 (54), 97 (29); HRMS calcd for C17H17OSFN79Br 381.0154, found 381.0194.
E-7-F,CN: colorless solid; mp 186-187.5 °C;δH1.70-1.92 (m, 6 H), 1.96 (bs, 1 H), 2.40-2.60 (m, 2 H), 2.70 (bs, 2 H), 7.60-7.80 (m, 4 H);δC29.67 (C7, J ) 10.0 Hz), 35.61 (C8,10, J ) 1.9 Hz), 38.24 (C4,9, J ) 19.9 Hz), 42.10 (C6, J ) 19.4 Hz), 42.17 (C1,3, J ) 10.1 Hz), 89.89 (C5, J ) 185.3 Hz), 111.10 (C2), 114.22 (Cp), 118.03 (CCN), 128.06 (Co), 132.44 (Cm), 132.71 (Ci), 155.29 (C3′); MS (EI, m/z) 328 (M+, 100), 184 (88), 168 (65), 160 (71), 128 (16), 97 (38); HRMS calcd for C18H17OSFN2 328.1047, found 328.1051. Anal. Calcd for C18H17OSFN2: C, 65.83; H, 5.22; N. 8.53, found C, 65.43; H, 5.20; N. 8.42. Z-7-F,CN: colorless solid; mp 198-198.5 °C;δH1.55-1.65 (m, 2 H), 1.90-2.15 (m, 6 H), 2.20-2.30 (m, 3 H), 2.55-2.70 (m, 2 H), 7.60-7.80 (m, 4 H);δC29.02 (C7, J ) 9.7 Hz), 32.09 (C8,10, J ) 1.9 Hz), 41.40 (C4,9, J ) 19.9 Hz), 41.90 (C1,3, J ) 10.5 Hz), 42.13 (C6, J ) 17.1 Hz), 90.31 (C5, J ) 185.7 Hz), 110.62 (C2), 114.27 (Cp), 118.04 (CCN), 128.09 (Co), 132.46 (Cm), 132.72 (Ci), 154.82 (C3′); MS (EI, m/z) 328 (M+, 43), 184 (51), 168 (70), 128 (100), 97 (41); HRMS calcd for C18H17OSFN2
328.1047, found 328.1043. Anal. Calcd for C18H17OSFN2: C, 65.83; H, 5.22; N. 8.53, found C, 65.40; H, 5.21; N. 8.49.
E-7-F,NO2: colorless solid; mp 243-243.5 °C;δH1.70-1.95 (m, 6 H), 1.98 (bs, 2 H), 2.25-2.35 (m, 1 H), 2.53 (bs, 2 H), 2.73 (bs, 2 H), 7.80-7.90 (m, 2 H), 8.20-8.30 (m, 2 H);δC29.67 (C7, J ) 10.0 Hz), 35.63 (C8,10, J ) 1.5 Hz), 38.25 (C4,9, J ) 19.9 Hz), 42.10 (C6, J ) 18.0 Hz), 42.22 (C1,3, J ) 10.2 Hz), 89.90 (C5, J ) 185.3 Hz), 111.40 (C2), 123.96 (Co), 128.41 (Cm), 134.43 (Ci), 148.90 (Cp), 155.29 (C3′); MS (EI, m/z) 348 (M+, 77), 184 (100), 168 (63), 150 (30), 97 (59), 79 (30); HRMS calcd for C17H17O3SFN2348.0945, found 348.0952. Anal. Calcd for C17H17O3SFN2: C, 58.61; H, 4.92; N. 8.04, found C, 58.60; H, 5.03; N. 7.96.
Z-7-F,NO2: colorless solid; mp 242-243.5 °C;δH1.61 (bs, 1 H), 1.65 (bs, 1 H), 1.90-2.15 (m, 6 H), 2.20-2.35 (m, 3 H), 2.69 (bs, 2 H), 7.80-7.90 (m, 2 H), 8.20-8.30 (m, 2 H);δC29.03 (C7, J ) 9.9 Hz), 32.10 (C8,10, J ) 1.7 Hz), 41.41 (C4,9, J ) 19.9 Hz), 41.95 (C1,3, J ) 10.6 Hz), 42.14 (C6, J ) 17.4 Hz), 90.31 (C5, J ) 185.7 Hz), 111.92 (C2), 123.97 (Co), 128.44 (Cm), 134.44 (Ci), 148.93 (Cp), 154.48 (C3′); MS (EI, m/z) 348 (M+, 88), 184 (100), 168 (79), 150 (35), 97 (63), 79 (31); HRMS calcd for C17H17O3SFN2348.0945, found 348.0943.
E-7-F,OMe: colorless solid; mp 147-148.5 °C;δH1.75-1.90 (m, 6 H), 1.90-2.00 (m, 2 H), 2.20-2.35 (m, 1 H), 2.50-2.60 (m, 2 H), 2.69 (bs, 2 H), 3.84 (s, 3 H), 6.85-7.00 (m, 2 H), 7.55-7.65 (m, 2 H);δC29.85 (C7, J ) 9.8 Hz), 35.71 (C8,10, J ) 1.7 Hz), 38.35 (C4,9, J ) 19.6 Hz), 41.94 (C1,3, J ) 10.2 Hz), 42.23 (C6, J ) 17.9 Hz), 55.40 (CMeO). 90.21 (C5, J ) 185.0 Hz), 109.03 (C2), 114.09 (Cm), 120.93 (Ci), 129.26 (Co), 155.98 (C3′), 161.68 (Cp); MS (EI, m/z) 333 (M+, 52), 317 (6), 165 (100), 149 (55), 133 (29), 97 (16); HRMS calcd for C18H20O2SFN 333.1200, found 333.1195. Anal. Calcd for C18H20O2SFN: C, 64.84; H, 6.05; N. 4.20, found C, 64.99; H, 6.08; N. 4.17.
Z-7-F,OMe: colorless solid; mp 109-111 °C;δH1.57 (bs, 1 H), 1.61 (bs, 1 H), 1.90-2.20 (m, 6 H), 2.20-2.35 (m, 3 H), 2.67 (bs, 2 H), 3.84 (s, 3 H), 6.85-6.95 (m, 2 H), 7.55-7.70 (m, 2 H);δC29.12 (C7, J ) 9.9 Hz), 32.11 (C8,10, J ) 1.9 Hz), 41.49 (C4,9, J ) 19.7 Hz), 41.58 (C1,3, J ) 10.4 Hz), 42.21 (C6, J ) 17.0 Hz), 55.39 (CMeO). 90.68 (C5, J ) 185.1 Hz), 109.50 (C2), 114.06 (Cm), 120.85 (Ci), 129.27 (Co), 155.28 (C3′), 161.65 (Cp); MS (EI, m/z) 333 (M+, 53), 298 (6), 238 (23), 165 (100), 150 (51), 97 (9); HRMS calcd for C18H20O2SFN 333.1200, found 333.1202. Anal. Calcd for C18H20O2SFN: C, 64.84; H, 6.05; N. 4.20, found C, 64.76; H, 6.05; N. 4.62.
E-7-F,Me: colorless solid; mp 104-106 °C;δH1.70-1.90 (m, 6 H), 1.90-2.00 (m, 2 H), 2.20-2.30 (m, 1 H), 2.38 (s, 3 H), 2.45-2.60 (m, 2 H), 2.70 (bs, 2 H), 7.15-7.25 (m, 2 H), 7.50-7.60 (m, 2 H);δC21.46 (CCH3), 29.84 (C7, J ) 9.9 Hz), 35.69 (C8,10, J ) 1.8 Hz), 38.34 (C4,9, J ) 19.6 Hz), 42.01 (C1,3, J ) 10.2 Hz), 42.23 (C6, J ) 18.0 Hz), 90.16 (C5, J ) 185.1 Hz), 109.11 (C2), 125.66 (Ci), 127.60 (Co), 129.39 (Cm), 141.35 (Cp), 156.28 (C3′); MS (EI, m/z) 317 (M+, 56), 206 (11), 184 (34), 149 (100), 91 (54), 79 (13); HRMS calcd for C18H20OSFN 317.1251, found 317.1243. Anal. Calcd for C18H20OSFN: C, 68.11; H, 6.35; N. 4.41, found C, 68.01; H, 6.33; N. 4.66.
Z-7-F,Me: colorless solid; mp 115-117 °C;δH1.50-1.68 (m, 2 H), 1.90-2.18 (m, 6 H), 2.20-2.35 (m, 3 H), 2.38 (s, 3 H), 2.66 (bs, 2 H), 7.15-7.25 (m, 2 H), 7.50-7.60 (m, 2 H);δC21.49 (CCH3), 29.16 (C7, J ) 9.7 Hz), 32.14 (C8,10, J ) 2.0 Hz), 41.52 (C4,9, J ) 19.7 Hz), 41.72 (C1,3, J ) 10.6 Hz), 42.26 (C6, J ) 17.0 Hz), 90.63 (C5, J ) 185.5 Hz), 109.63 (C2), 125.64 (Ci), 127.63 (Co), 129.41 (Cm), 141.40 (Cp), 156.59 (C3′), MS (EI, m/z) 317 (M+, 31), 266 (17), 206 (100), 184 (16), 149 (55), 91 (28), 79 (6); HRMS calcd for C18H20OSFN 317.1251, found 317.1248.
X-ray Structure Analysis of Z-7-Br,H. A colorless prism
crystal of C17H18ONBrS was crystallized from 30% methylene chloride in hexanes. Its structure was determined by means of single-crystal X-ray analysis on a Rigaku AFC6S diffracto-meter with a graphite monochromated Mo-KR (λ ) 0.71069 Å) radiation at 296 ( 1 K, with anω-2θ type scan at 16°/min (inω). The crystals are monoclinic, with space group P21/c (14) and unit cell dimensions a ) 16.844(5) Å, b ) 24.537(4) Å, c ) 10.048(4) Å,β ) 108.48(3)°, V ) 1600(1) Å3, Z ) 4, F
calcd) 1.512 g cm-3, crystal size (mm) 0.33× 0.41 × 0.48, µ(Mo KR) ) 27.05 cm-1, F(000) ) 744.00, 2688 reflections, 2466 unique
reflections, 772 with I > 3.00σ(I) and with 190 variable parameters. The non-hydrogen atoms were refined anisotro-pically. Hydrogen atoms were included but not refined. The model was finally refined by the full-matrix least-squares methods with weightω ) 1/[σ2(F
0)] to final R values of 0.075 and Rw) 0.0351 (for details, see Supporting Information).12
General Procedure for the Synthesis of 5-Fluoro-3′
-para-substituted-4′-hydrospiro[adamantane-2:5′-∆2
-isox-azolines] (E- and Z-8-F,Y). E- and Z-8-F,Y were synthesized
by the use of a procedure similar to that of Zwanenburg et al.13An excess of triethylamine (1.5 mol equiv) was added to a well-stirred solution of the 3-F1c(50 mg, 0.3 mmol) and 6-Y (1.5 mol equiv) in anhydrous THF (10 mL). The mixture was stirred at reflux for 24 h, diluted with methylene chloride, washed with water, and dried with MgSO4. After filtration and solvent evaporation, the residue was purified on a silica gel column by elution with n-hexane/methylene chloride to give two isomeric adducts E- and Z-8-F,Y. The isolated yields based on converted starting materials 3-F are as follows: 8-F,F 85%,
8-F,Cl 79%, 8-F,Br 82%, 8-F,CN 83%, 8-F,NO288%, 8-F,Me 77%, 8-F,OMe 81%. Z-8-F,F: colorless solid; mp 160.5-161.5 °C;δH1.65-1.85 (m, 6 H), 1.90-2.00 (m, 2 H), 2.20-2.30 (m, 3 H), 2.40-2.60 (m, 2 H), 3.14 (s, 2 H), 7.00-7.15 (m, 2 H), 7.60-7.70 (m, 2 H);δC29.76 (C7, J ) 9.9 Hz), 33.93 (C8,10, J ) 1.6 Hz), 38.23 (C4,9, J ) 19.2 Hz), 39.86 (C1,3, J ) 10.4 Hz), 42.33 (C6, J ) 17.8 Hz), 43.11 (C4′), 89.39 (C2), 90.88 (C5, J ) 184.5 Hz), 115.82 (Cm, J ) 21.9 Hz), 126.22 (Ci, J ) 2.9 Hz), 128.27 (Co, J ) 8.5 Hz), 155.05 (C3′), 163.64 (Cp, J ) 250.6 Hz); MS (EI, m/z) 303 (M+, 100), 286 (35), 135 (24), 79 (9); HRMS calcd for C18H19NOF2303.1438, found 303.1441. Anal. Calcd for C18H19 -NOF2: C, 71.27; H, 6.31; N. 4.62, found C, 71.00; H, 6.33; N. 4.59.
E-8-F,F: colorless solid; mp 157-158 °C;δH1.52 (bs, 1 H), 1.56 (bs, 1 H), 1.95 (bs, 6 H), 2.15-2.30 (m, 5 H), 3.19 (s, 2 H), 7.00-7.15 (m, 2 H), 7.60-7.70 (m, 2 H);δC29.37 (C7, J ) 9.8 Hz), 31.69 (C8,10, J ) 1.9 Hz), 39.31 (C1,3, J ) 10.1 Hz), 40.05 (C4,9, J ) 18.9 Hz), 42.40 (C6, J ) 16.9 Hz), 44.18 (C4′), 89.72 (C2), 90.98 (C5, J ) 185.0 Hz), 115.83 (Cm, J ) 21.9 Hz), 126.20 (Ci, J ) 3.3 Hz), 128.28 (Co, J ) 8.4 Hz), 155.20 (C3′), 163.66 (Cp, J ) 250.6 Hz); MS (EI, m/z) 303 (M+, 100), 286 (33), 135 (26), 79 (9); HRMS calcd for C18H19NOF2 303.1438, found 303.1442. Anal. Calcd for C18H19NOF2: C, 71.27; H, 6.31; N. 4.62, found C, 71.11; H, 6.36; N. 4.62. Z-8-F,Cl: colorless solid; mp 202.5-203 °C;δH1.65-1.85 (m, 6 H), 1.90-2.00 (m, 2 H), 2.20-2.30 (m, 3 H), 2.40-2.55 (m, 2 H), 3.13 (s, 2 H), 7.30-7.40 (m, 2 H), 7.55-7.65 (m, 2 H);δC29.76 (C7, J ) 9.8 Hz), 33.93 (C8,10, J ) 1.7 Hz), 38.22 (C4,9, J ) 19.3 Hz), 39.88 (C1,3, J ) 10.3 Hz), 42.33 (C6, J ) 17.9 Hz), 42.89 (C4′, J ) 2.0 Hz), 89.61 (C2), 90.86 (C5, J ) 184.3 Hz), 127.58 (Co), 128.48 (Ci), 128.96 (Cm), 135.86 (Cp), 155.08 (C3′); MS (EI, m/z) 321 (M++ 2, 38), 319 (M+, 100), 302 (38), 151 (25), 79 (12); HRMS calcd for C18H19NOF35Cl 319.1141, found 319.1138.
E-8-F,Cl: colorless solid; mp 191.5-192 °C;δH1.52 (bs, 1 H), 1.56 (bs, 1 H), 1.85-2.00 (m, 6 H), 2.10-2.35 (m, 5 H), 3.18 (s, 2 H), 7.30-7.40 (m, 2 H), 7.55-7.65 (m, 2 H);δC29.33 (C7, J ) 9.9 Hz), 31.65 (C8,10, J ) 2.0 Hz), 39.29 (C1,3, J ) 10.0 Hz), 40.00 (C4.9, J ) 19.0 Hz), 42.36 (C6, J ) 16.8 Hz), 43.93 (C4′), 89.94 (C2), 90.96 (C5, J ) 184.8 Hz), 127.57 (Co), 128.40 (Ci), 128.94 (Cm), 135.85 (Cp), 155.24 (C3′); MS (EI, m/z) 321 (M++ 2, 35), 319 (M+, 100), 302 (39), 151 (22), 79 (11); HRMS calcd for C18H19NOF35Cl 319.1141, found 319.1136. Anal. Calcd for C18H19NOFCl: C, 67.60; H, 5.99; N. 4.38, found C, 67.25; H, 6.05; N. 4.34. Z-8-F,Br: colorless solid; mp 206-207 °C;δH1.60-1.85 (m, 6 H), 1.90-2.00 (m, 2 H), 2.23 (bs, 3 H), 2.40-2.55 (m, 2 H), 3.13 (s, 2 H), 7.45-7.60 (m, 4 H);δC29.76 (C7, J ) 9.9 Hz), 33.92 (C8,10, J ) 2.1 Hz), 38.22 (C4,9, J ) 19.0 Hz), 39.87 (C1,3, J ) 10.4 Hz), 42.33 (C6, J ) 17.6 Hz), 42.84 (C4′), 87.82 (C2), 90.87 (C5, J ) 183.4 Hz), 124.16 (Cp), 127.80 (Co), 128.91 (Ci), 131.91 (Cm), 155.18 (C3′); MS (EI, m/z) 365 (M++ 2, 100), 363 (M+, 100), 348 (27), 346 (27), 197 (18), 79 (10); HRMS calcd for C18H19NOF79Br 363.0635, found 363.0635. Anal. Calcd for
C18H19NOFBr: C, 59.35; H, 5.26; N. 3.84, found C, 59.61; H, 5.28; N. 3.71.
E-8-F,Br: colorless solid; mp 177-178.5 °C;δH1.52 (bs, 1 H), 1.56 (bs, 1 H), 1.90-2.00 (m, 6 H), 2.15-2.30 (m, 5 H), 3.18 (s, 2 H), 7.50-7.55 (m, 4 H);δC29.35 (C7, J ) 9.7 Hz), 31.67 (C8,10, J ) 1.9 Hz), 39.31 (C1,3, J ) 10.1 Hz), 40.02 (C4.9, J ) 18.9 Hz), 42.38 (C6, J ) 16.7 Hz), 43.89 (C4′), 89.72 (C2), 90.95 (C5, J ) 184.8 Hz), 124.18 (Cp), 127.81 (Co), 128.88 (Ci), 131.92 (Cm), 155.33 (C3′); MS (EI, m/z) 365 (M++ 2, 100), 363 (M+, 100), 348 (25), 346 (26), 197 (19), 79 (11); HRMS calcd for C18H19NOF79Br 363.0635, found 363.0640. Anal. Calcd for C18H19NOFBr: C, 59.35; H, 5.26; N. 3.84, found C, 59.65; H, 5.27; N. 3.72.
Z-8-F,CN: could not obtained in pure form.δH1.65-1.90 (m, 6 H), 1.96 (bs, 2 H), 2.27 (bs, 3 H), 2.45-2.60 (m, 2 H), 3.21 (s, 2 H), the aromatic region overlapped with impurities; δC29.72 (C7, J ) 10.2 Hz), 33.90 (C8,10), 38.19 (C4,9, J ) 19.2 Hz), 39.90 (C1.3, J ) 10.2 Hz), 42.28 (C6, J ) 17.8 Hz), 42.58 (C4′), 90.38 (C2), 90.75 (C5, J ) 184.7 Hz), the aromatic region overlapped with impurities.
E-8-F,CN: colorless solid; mp 242-243 °C;δH1.50-165 (m, 2 H), 1.90-2.00 (m, 6 H), 2.15-2.35 (m, 5 H), 3.19 (s, 2 H), 7.60-7.70 (m, 2 H), 7.70-7.80 (m, 2 H);δC29.29 (C7, J ) 9.8 Hz), 31.63 (C8,10, J ) 1.7 Hz), 39.34 (C1,3, J ) 10.1 Hz), 39.96 (C4,9, J ) 19.0 Hz), 42.32 (C6, J ) 16.7 Hz), 43.47 (C4′), 90.82 (C5, J ) 185.2 Hz), 90.93 (C2), 113.23 (Cp), 118.36 (CN), 126.79 (Co), 132.48 (Cm), 134.22 (Ci), 154.87 (C3′); MS (FAB+, m/z) 311 (M+ + 1, 12), 307 (12), 154 (100), 136 (83), 77 (35); HRMS (FAB+) calcd for C
19H19N2OF 311.1569, found 311.1557.
Z-8-F,NO2: mp 298-299 °C but color turned from none to brown at 253 °C;δH1.65-1.85 (m, 6 H), 1.96 (bs, 2 H), 2.26 (bs, 3 H), 2.50 (bs, 2 H), 3.18 (s, 2 H), 7.78-7.88 (m, 2 H), 8.20-8.30 (m, 2 H);δC29.69 (C7, J ) 9.7 Hz), 33.89 (C8,10, J ) 2.0 Hz), 38.16 (C4,9, J ) 19.4 Hz), 39.00 (C1,3, J ) 10.4 Hz), 42.27 (C6, J ) 18.0 Hz), 42.48 (C4′, J ) 1.9 Hz), 90.66 (C5, J ) 184.9 Hz), 90.88 (C2), 124.00 (Cm), 127.03 (Co), 136.06 (Ci), 148.35 (Cp), 154.45 (C3′); MS (EI, m/z) 330 (M+, 100), 313 (37), 300 (18), 162 (18), 97 (22), 79 (25); HRMS calcd for C18H19N2O3F 330.1381, found 330.1378.
E-8-F,NO2: colorless solid; mp 290.291 °C;δH1.50-1.65 (m, 2 H), 1.97 (bs, 6 H), 2.15-2.35 (m, 5 H), 3.23 (s, 2 H), 7.75-7.88 (m, 2 H), 8.20-8.30 (m, 2 H);δC29.29 (C7, J ) 9.9 Hz), 31.63 (C8,10, J ) 2.0 Hz), 39.36 (C1,3, J ) 10.0 Hz), 39.96 (C4,9, J ) 19.2 Hz), 42.32 (C6, J ) 16.6 Hz), 43.53 (C4′), 90.77 (C5, J ) 185.0 Hz), 91.19 (C2), 124.00 (Cm), 127.04 (Co), 136.02 (Ci), 148.35 (Cp), 154.63 (C3′); MS (EI, m/z) 330 (M+, 100), 313 (19), 300 (13), 162 (25), 97 (16), 79 (16); HRMS calcd for C18H19N2O3F 330.1381, found 330.1372.
Z-8-F,OMe: colorless solid; mp 161.5-163 °C;δH1.65-1.85 (m, 6 H), 1.94 (bs, 2 H), 2.22 (bs, 3 H), 2.50 (bs, 2 H), 3.14 (s, 2 H), 3.83 (s, 3 H), 6.85-6.95 (m, 2 H), 7.55-7.65 (m, 2 H);δC 29.77 (C7, J ) 10.0 Hz), 33.92 (C8,10, J ) 1.6 Hz), 38.24 (C4,9, J ) 19.0 Hz), 39.82 (C1,3, J ) 10.4 Hz), 42.33 (C6, J ) 17.8 Hz), 43.22 (C4′), 55.32 (COMe), 88.73 (C2), 91.01 (C5, J ) 184.5 Hz), 114.04 (Cm), 122.48 (Ci), 127.84 (Co), 155.60 (C3′), 160.87 (Cp); MS (EI, m/z) 315 (M+, 100), 298 (25), 285 (5), 174 (10), 147 (15), 97 (8), 77 (11); HRMS calcd for C19H22NO2F 315.1636, found 315.1625.
E-8-F,OMe: colorless solid; mp 170-172 °C;δH1.50 (bs, 1 H), 1.55 (bs, 1 H), 1.96 (bs, 6 H), 2.10-2.30 (m, 5 H), 3.18 (s, 2 H), 3.84 (s, 3 H), 6.90-6.95 (m, 2 H), 7.55-7.65 (m, 2 H);δC 29.41 (C7, J ) 9.9 Hz), 31.72 (C8,10, J ) 1.8 Hz), 39.29 (C1,3, J ) 10.1 Hz), 40.08 (C4,9, J ) 18.9 Hz), 42.43 (C6, J ) 16.7 Hz), 44.35 (C4′), 55.35 (COMe). 89.11 (C2), 91.16 (C5, J ) 184.6 Hz), 114.08 (Cm), 122.49 (Ci), 127.88 (Co), 155.78 (C3′), 160.92 (Cp); MS (EI, m/z) 315 (M+, 100), 298 (6), 285 (4), 174 (12), 147 (18), 97 (10), 77(11) HRMS calcd for C19H22NO2F 315.1636, found 315.1628.
Z-8-F,Me: colorless solid; mp 127-128 °C;δH1.65-1.85 (m, 6 H), 1.94 (bs, 2 H), 2.23 (bs, 3 H), 2.38 (s, 3 H), 2.45-2.55 (m, 2 H), 3.15 (s, 2 H), 7.15-7.25 (m, 2 H), 7.50-7.60 (m, 2 H);δC 21.42 (CCH3), 29.82 (C7, J ) 9.7 Hz), 33.96 (C8,10, J ) 1.6 Hz), 38.27 (C4,9, J ) 19.0 Hz), 39.88 (C1,3, J ) 10.3 Hz), 42.38 (C6, J ) 17.7 Hz), 43.17 (C4′, J ) 1.6 Hz), 88.93 (C2, J ) 1.6 Hz), 91.00 (C5, J ) 184.5 Hz), 126.31 (Co), 127.15 (Ci), 129.37 (Cm), 140.15 (Cp), 155.99 (C3′); MS (EI, m/z) 299 (M+, 100), 282 (34), 158 (12), 131 (23), 91 (29) HRMS calcd for C19H22NOF 299.1687, found 299.1678. Anal. Calcd for C19H22NOF: C, 76.22; H, 7.41; N. 4.68, found C, 76.06; H, 7.40; N. 4.68.
E-8-F,Me: colorless solid; mp 165-166.5 °C;δH1.52 (bs, 1 H), 1.56 (bs, 1 H), 1.96 (bs, 6 H), 2.15-2.35 (m, 5 H), 2.38 (s, 3 H), 3.20 (s, 2 H), 7.15-7.25 (m, 2 H), 7.50-7.60 (m, 2 H);δC 21.43 (CCH3), 29.41 (C7, J ) 9.9 Hz), 31.71 (C8,10, J ) 2.0 Hz), 39.30 (C1,3, J ) 10.1 Hz), 40.06 (C4,9, J ) 18.9 Hz), 42.43 (C6, J ) 16.8 Hz), 44.24 (C4′), 89.27 (C2), 91.12 (C5, J ) 184.6 Hz), 126.31 (Co), 127.64 (Ci), 129.38 (Cm), 140.18 (Cp), 156.16 (C3′), MS (EI, m/z) 299 (M+, 100), 282 (25), 269 (9), 158 (11), 131 (20), 91 (26); HRMS calcd for C19H22NOF 299.1687, found 299.1685. Anal. Calcd for C19H22NOF: C, 76.22; H, 7.41; N. 4.68, found C, 76.10; H, 7.46; N. 4.70.
General Procedure for the Synthesis of
5-Substituted-3′-phenyladamantane-2-spiro-5′-(∆2-1′,2′,4′
-oxadiazo-lines) E- and Z-11-X,H. E- and Z-11-X,H were synthesized
by a procedure similar to that of Ito9b,c and Westheimer et al.13,14About 0.5 g of molecular sieves are added to a solution of 0.2 g of ketone 1-X and benzylamine (1.5 mol equiv of ketone) in 10 mL of benzene. The mixture was stirred at reflux for 15 h, and the molecular sieve was removed and washed with solvent. After solvent evaporation, the solid residue 4-X was added to benzohydroximoyl chloride 6-H (1.5 mol equiv of ketone) in 10 mL of dry dichloromethane. Excess of triethy-lamine (1.5 mol equiv) was added to the mixture. The mixture was stirred at room temperature for 0.5 h, washed with water, and then dried with MgSO4. After filtration and solvent evaporation, the residue was purified on a silica gel column by n-hexane/methylene chloride to give two isomeric adducts E- and Z-11-X,H. Recrystallization from n-hexane/methylene chloride at room temperature gave white needle crystalline Z-11-H,H. All the E-11-X,H adduct except E-11-F,H could not be isolated by liquid chromatography and recrystallization. The 13C NMR spectra can be obtained, however, from E- and Z-11-X,H mixtures. The isolated yields based on converted starting materials 1-X are as follows: F,H 83%, Cl,H 87%, 11-Br,H 86%, 11-Ph,H 92%. 11-H,H: colorless solid, mp 205-207 °C;δH1.55-1.80 (m, 6 H), 1.80-1.95 (m, 2 H), 2.00-2.15 (m, 2 H), 2.15-2.35 (m, 4 H), 4.48 (s, 2 H), 7.10-7.45 (m, 8 H), 7.55-7.68 (m, 2 H);δC 26.67 (C7), 26.88 (C5), 34.56 (C8,10), 34.43 (C1,3), 34.73 (C4,9), 37.35 (C6), 49.73 (C11), 102.42 (C2), 126.80 (d), 126.96 (s), 127.01 (d), 128.34 (d), 128.41 (d), 128.56 (d), 130.45 (d), 139.67(s), 161.28 (C3′); MS (EI) m/z 359 (M++ 1, 6), 358 (M+, 22), 238 (4), 237 (4), 193 (21), 150 (16), 121 (54), 91 (100), 79 (80); HRMS calcd for C24H26ON2358.2047, Found 358.2037; Anal. Calcd for C24H26ON2: C, 80.41; H, 7.31, found: C, 80.35; H, 7.30. Z-11-F,H: colorless solid; mp 153.5-155 °C;δH1.55-1.80 (m, 4 H), 1.85-2.05 (m, 4 H), 2.10-2.20 (m, 1 H), 2.35-2.55 (m, 4 H), 4.47 (s, 2 H), 7.10-7.27 (m, 5 H), 7.27-7.45 (m, 3 H), 7.55-7.70 (m, 2 H);δC29.67 (C7, J ) 9.9 Hz), 32.39 (C8,10, J ) 2.0 Hz), 36.93 (C1,3, J ) 10.6 Hz), 39.18 (C4,9, J ) 19.5 Hz), 42.36 (C6, J ) 17.6 Hz), 49.90 (C11), 90.94 (C5, J ) 184.3 Hz), 100.61 (C2), 126.36 (s), 126.64 (d), 127.27 (d), 128.32 (d), 128.57 (d), 128.71 (d), 130.74 (d), 139.14 (s), 161.37 (C3′); MS (EI) m/z 376 (M+, 48), 255 (10), 235 (7), 193 (4), 168 (5), 105 (58), 91 (100), 77 (9); HRMS calcd for C24H25ON2F 376.1952, found 376.1955. Anal. Calcd for C24H25ON2F: C, 76.57; H, 6.69; N. 7.44, found C, 76.62; H, 6.63; N. 7.49.
E-11-F,H: colorless solid; mp 132-132.5 °C;δH1.47 (bs, 1 H), 1.51 (bs, 1 H), 1.77 (bs, 1 H), 1.78 (bs, 1 H), 1.79-1.95 (m, 2 H), 2.10-2.35 (m, 5 H), 2.39 (bs, 2 H), 4.50 (s, 2 H), 7.15-7.30 (m, 5 H), 7.15-7.30-7.45 (m, 3 H), 7.60-7.68 (m, 2 H);δC29.61 (C7, J ) 9.9 Hz), 33.12 (C8,10, J ) 1.9 Hz), 36.94 (C1,3, J ) 10.2 Hz), 38.67 (C4,9, J ) 19.4 Hz), 42.44 (C6, J ) 16.8 Hz), 49.79 (C11), 91.32 (C5, J ) 184.4 Hz), 100.64 (C2), 126.40 (s), 126.66 (d), 127.31 (d), 128.33 (d), 128.60 (d), 128.73 (d), 130.76 (d), 139.16 (s), 161.36 (C3′); MS (EI) m/z 376 (M+, 57), 255 (13), 235 (11), 193 (5), 168 (7), 105 (68), 91 (100), 77 (10); HRMS calcd for C24H25ON2F 376.1952, found 376.1944.
Z-11-Cl,H: colorless solid; mp: 182-183.5 °C;δH1.67 (bd,
J ) 12.8 Hz, 2 H), 1.90-2.10 (m, 4 H), 2.10-2.25 (m, 3 H), 2.35 (bs, 2 H), 2.71 (bd, J ) 12.3 Hz, 2 H), 4.46 (s, 2 H),
7.15-7.30 (m, 5 H), 7.15-7.30-7.50 (m, 3 H), 7.60-7.70 (m, 2 H);δC29.89 (C7), 32.13 (C8, 10), 37.26 (C1,3), 44.14 (C4,9), 47.29 (C6), 49.88 (C11), 65.95 (C5), 100.40 (C2), 126.38 (s), 126.65 (d), 127.30 (d), 128.36 (d), 128.60 (d), 128.73 (d), 130.78 (d), 139.17 (s), 161.39 (C3′); MS (EI) m/z 394 (M++ 2, 8), 392 (M+, 22), 357 (M+ -35Cl, 1), 266 (6), 105 (48), 91 (100), 77(13); HRMS calcd for C24H25ON35Cl 392.1658, found 392.1663. Anal. Calcd for C24H25ONCl: C, 73.36; H, 6.41; N. 7.13, found C, 73.23; H, 6.42; N. 7.25. E-11-Cl,H: δC30.21 (C7), 32.49 (C8, 10), 36.91 (C1,3), 43.40 (C4,9), 47.08 (C6), 49.53 (C11), 65.95 (C5), 100.02 (C2), 161.05 (C3′). Z-11-Br,H: colorless solid; mp: 186-187 °C;δH1.65-1.80 (m, 2 H), 2.00-2.25 (m, 5 H), 2.25-2.40 (m, 4 H), 2.85-3.00 (m, 2 H), 4.45 (s, 2 H), 7.15-7.30 (m, 5 H), 7.30-7.45 (m, 3 H), 7.60-7.70 (m, 2 H);δC30.69 (C7), 32.10 (C8, 10), 38.10 (C1,3), 45.65 (C4,9), 48.81 (C6), 49.83 (C11), 62.47 (C5), 100.30 (C2), 126.38 (s), 126.65 (d), 127.31 (d), 128.35 (d), 128.60 (d), 128.73 (d), 130.77 (d), 139.14 (s), 161.38 (C3′); MS (EI) m/z 438 (M++ 2, 14), 436 (M+, 14), 357 (M+-79Br, 4), 266 (16), 236 (15), 149 (16), 105 (49), 91 (100), 77(17); HRMS calcd for C24H25ON79Br 436.1152, found 436.1155. Anal. Calcd for C24H25ONBr: C, 65.91; H, 5.76; N. 6.40, found C, 65.94; H, 5.77; N. 6.58. E-11-Br,H: δC30.61 (C7), 32.67 (C8, 10), 38.00 (C1,3), 45.11 (C4,9), 48.70 (C6), 49.75 (C11), 62.68 (C5), 100.11 (C2), 161.30 (C3′). Z-11-Ph,H: colorless solid; mp: 114.5-116 °C;δH 1.65-1.80 (m, 4 H), 1.94 (bs, 2 H), 2.05-2.20 (m, 3 H), 2.30-2.40 (m, 2 H), 2.45-2.60 (m, 2 H), 4.53 (s, 2 H), 7.10-7.45 (13 H), 7.60-7.70 (m, 2 H);δC27.43 (C7), 32.16 (C8, 10), 34.86 (C1,3), 35.14 (C5), 40.29 (C4,9), 42.49 (C6), 49.85 (C11), 101.76 (C2), 124.93 (d), 125.72 (s), 126.76 (d), 127.10 (d), 128.11 (d), 128.34 (d), 128.47 (d), 128.62 (d), 130.56 (d), 139.52 (s), 149.60 (s), 161.38 (C3′); MS (EI) m/z 434 (M+, 57), 314 (11), 266 (19), 155 (28), 105 (48), 91 (100), 77 (14);HRMS calcd for C30H30ON2 434.2360, found, 434.2368. Anal. Calcd for C30H30ON2: C, 82.91; H, 6.96; N. 6.45, found C, 83.03; H, 6.99; N. 6.56.
E-11-Ph,H:δC26.99 (C7), 33.51 (C8, 10), 34.57 (C1,3), 35.02 (C5), 39.20 (C4,9), 42.42 (C6), 49.37 (C11), 101.58 (C2), 161.09 (C3′).
Computational Methods. The AM115 method was
em-ployed to calculate all the reactants of the 1,3-dipolar cycload-dition reactions including dipolarophiles (1-X to 4-X) and benzonitrile oxide 5-Y. The reactants were fully optimized without any constraint. Frequency calculations were carried out for every optimized structures. All the reactants are located in the local minimum on the potential energy surface con-firmed by all real-frequencies. The calculations were performed with the SPARTAN 5.0 program.16
Acknowledgment. This work is supported at NCTU
by the National Science Council of the Republic of China
(Grant NSC-87-2113-M-009-002).
Supporting Information Available: AM1-calculated
en-ergies for frontier molecular orbitals of 2- to 5-X.1H and13C NMR spectra for compounds E-7-F,Y (Y ) Cl, Br), Z-7-F,Y (Y ) F, Cl, Br, NO2and Me), Z-8-F,Cl and Z- and E-8-F,X (X ) CN, NO2and OMe), and E-11-F,H; X-ray crystallography data for Z-7-Br,H. This material is available free of charge via the Internet at http://pubs.acs.org.
JO980945N
(16) Spartan version 5.0, USA: Wavefunction, Inc., 1998. We thank the National Center for High-performance Computing for support of the calculation.