Urban & Fischer Verlag
http://www.urbanfischer.de/journals/jpp
Short Communication
Cell wall peroxidase against ferulic acid, lignin, and NaCl-reduced root
growth of rice seedlings
Chuan Chi Lin, Ching Huei Kao*
Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China
Received August 16, 2000 · Accepted December 1, 2000
Summary
The changes in cell wall peroxidase activity against ferulic acid (FPOD) and lignin level in roots of NaCl-stressed rice seedlings and their correlation with root growth were investigated. Increasing concentrations of NaCl from 50 to 150 mmol L–1progressively decreases root growth. The reduction of root growth by NaCl is closely correlated with the increase in FPOD activity extracted from the cell wall. In contrast, lignin level was reduced by NaCl. Since proline and ammonium accumulations are associated with root growth inhibition caused by NaCl, we determined the effect of proline or NH4Cl
on root growth and FPOD in roots. Exogenous application of NH4Cl or proline markedly inhibited root
growth and increased FPOD activity in rice seedlings in the absence of NaCl. An increase in FPOD activity in roots preceded inhibition of root growth caused either by NaCl, NH4Cl, or proline. Our
results suggest that cell-wall stiffening catalyzed by FPOD may participate in the regulation of root growth reduction of rice seedlings caused by NaCl.
Key words:ammonium – lignin – NaCl – Oryza sativa – peroxidase – proline
Abbreviations:DW dry weight. – FPOD peroxidase against ferulic acid. – FW fresh weight
Introduction
NaCl is known to inhibit plant growth. However, the mecha-nism underlying this inhibition is not yet clear (Greenway and Munns 1980, Munns and Termaat 1986, Rengel 1992). It has been shown that inhibition of leaf or root growth of cereals is not caused by decreased turgor (Cramer 1992, Munns 1993, Neumann et al. 1994). In contrast, NaCl-induced inhibition in growth has often been related to measured decreases in the
* E-mail corresponding author: kaoch@ccms.ntu.edu.tw
plastic extensibility of the growing cell walls in root and leaf expansion zones (Cramer 1992, Neumann 1993, Prichard et al. 1993, Chazen and Neumann 1994, Neumann et al. 1994, Chazen et al. 1995). Neumann (1993, 1997) suggests that rapid, metabolically regulated changes in the physical prop-erties of growing cells caused by osmotic or other effects, appear to be a factor regulating maize leaf growth response to root salinization. Neumann et al. (1994) also demonstrated that root growth inhibition caused by salinity was associated with cell wall stiffening. A key role of cell-wall peroxidases in the stiffening of the cell wall, and consequently, in the growth
reduction of cell elongation, has been postulated (Fry 1986). Recently, we have reported that ionically bound peroxidase activity was associated with growth inhibition of rice seedling root caused by NaCl (Lin and Kao 1999). This ionically bound fraction of peroxidases, removable from homogenized tissues with high ionic strength buffers, has been equated with the cell wall fraction. However, Mader et al. (1986) argued that at least some ionically bound peroxidase activity might be an artifact of homogenization. Therefore, to test the hypothesis that an increase in cell wall peroxidase activity is associated with growth inhibition of seedling roots of rice, data from per-oxidase activity associated with cell walls is required. Guaia-col is usually used as a substrate to assay peroxidase activ-ity. However, guaiacol is not the natural substrate for the per-oxidase in the cell-wall stiffening process. Ferulic acid has been identified as being ester linked to arabinoxylans in monocotyledonous plants (Kato and Nevins 1985, Hartley and Ford 1989). A key role in the cell-wall stiffening of dimeriza-tion of ferulic acid catalyzed by cell-wall POD has been re-ported (Sanchez et al. 1996). Thus, ferulic acid appears to be the suitable substrate to establish the relationship between cell-wall peroxidase activity and NaCl-inhibited root growth of rice seedlings. The present investigation was therefore de-signed to study the changes in peroxidase activity against fe-rulic acid (FPOD) associated with cell walls during growth re-duction of rice seedling roots caused by NaCl.
It is known that peroxidases also influence plant growth through lignin synthesis (Siegel 1953). Lignification of the stems of the halophyte Suaeda maritima has been shown to be negatively correlated with salinity (Hagege et al. 1988). In Phaseolus sp., salinity caused an earlier and stronger lignifi-cation of root vascular tissues (Cachorro et al. 1992). How-ever, it has been reported that salinity had no effect either on lignin content in tomato roots or reduced lignin level in the in-ternodes of Atriplex prostrata grown under salt conditions (Peyrano et al. 1997, Wang et al. 1997). Therefore, effect of NaCl on lignin level in roots of rice seedlings was also stud-ied in the present investigation.
Materials and Methods
Rice (Oryza sativa L., cv. Taichung Native 1) seeds were sterilized with 2.5 % sodium hypochlorite for 15 min and washed extensively with distilled water. These seeds were then germinated in a Petri dish (20 cm) containing distilled water at 37 ˚C under dark condition. After a 1-day incubation, uniformly germinated seeds were selected and transferred to Petri dishes (9.0 cm) containing two sheets of Whatman No. 1 filter paper moistened with 10 mL of distilled water or test solu-tions. Each Petri dish contained 20 germinated seeds. Each treat-ment was replicated 4 times. The germinated seeds were allowed to grow at 27 ˚C in darkness. To avoid the loss by evaporation and taken up by the seeds, a further 3 mL of distilled water or test solution was added to each Petri dish on day 3 of the growth. Fresh weight (FW) and dry weight (DW) of roots were measured at the times indicated.
Cell walls were prepared by homogenizing roots in ice cold phos-phate buffer (50 mmol L–1, pH 5.8) using a pestle and mortar. The
ho-mogenate was centrifuged at 1,000 gn, and washed at least four times
with 50 mmol L–1phosphate buffer (Lee and Lin 1995). The pellet was
collected and used as a cell wall fraction.
POD ionically bound to the cell wall was extracted with 1 mol L–1
NaCl. Cell walls prepared as described above were incubated in 1 mol L–1 NaCl for 2 h with shaking at 30 ˚C, and centrifuged at
1,000 gn. The supernatant was used for FPOD assay. Ferulic acid
POD (FPOD) was assayed according to Sanchez et al. (1996). The oxidation of ferulic acid was measured spectrophotometrically follow-ing the absorance decrease at 310 nm in a reaction mixture contain-ing 1.35 mL Na-phosphate buffer (0.2 mol L–1, pH 5.8), 0.5 mL ferulic
acid (240µmol L–1), 0.5 mL H
2O2(3 mmol L–1) and 0.15 mL enzyme
ex-tract. One unit of FPOD was defined as a decrease of 1 A310per min.
The lignin level in roots was measured by the Sasaki et al. (1996) method, a method originally described by Morrison (1972). Roots were homogenized with a pestle in a mortar in 95 % ethanol. The ho-mogenate was centrifuged at 1,000 gn for 5 min. The pellet was
washed three times with 95 % ethanol and twice with a mixture of eth-anol and hexane (1 : 2, v/v). The material was allowed to air dry and its lignin level measured. The dried sample was washed one time with 2 mL acetyl bromide in acetic acid (1 : 3, v/v). Then 1 mL acetyl bro-mide in acetic acid (1 : 3, v/v) was added to the pellet and incubated at 70 ˚C for 30 min. After cooling of the mixture to room temperature, 0.9 mL of 2 mol L–1NaOH and 0.1 mL 7.5 mol L–1hydroxylamine
hydro-chloride were added, and the volume was made up to 10 mL with acetic acid. After centrifugation at 1,000 gnfor 5 min, the absorbance
of the supernatant was measured at 280 nm (A280).
For all measurements, each treatment was repeated four times. All experiments described here were repeated at least three times. Sim-ilar results and identical trends were obtained each time. The data re-ported here is from a single experiment.
Results and Discussion
Root growth was followed by measuring FW and DW of roots. Figure 1 shows the effect of NaCl on root growth of rice seed-lings. Increasing concentrations of NaCl from 50 to 150mmolL–1 progressively decreased root growth. The reduction of root growth with increasing NaCl concentrations is correlated with an increase in FPOD activity extracted from cell walls (Fig. 1). Sanchez et al. (1996) demonstrated a negative relationship of FPOD and growth capacity in pine hypocotyl, suggesting a role for peroxidase in the cell-wall stiffening. Furthermore, the ability of peroxidases to catalyze wall cross-linking through ferulic acid esterified to polysaccharides has already been shown (Whitemore 1976). It seems that cell-wall stiffening cat-alyzed by FPOD may participate in the regulation of root growth reduction of rice seedlings under NaCl conditions.
It is known that ammonium strongly inhibits the growth of many plants (Haynes and Goh 1978). Exogenous application of NH4Cl was also found to reduce root growth of rice
seed-lings (Lin and Kao 1996 a). It has also been shown that NaCl was effective in stimulating the accumulation of ammonium in roots of rice seedlings, and that accumulation of ammonium in roots preceded inhibition of root growth and increase in
ion-Figure 1.Effect of NaCl on root growth and FPOD activities in roots of rice seedlings. FW, DW, and FPOD activities were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
ically bound peroxidase activity caused by NaCl (Lin and Kao 1996 a, Lin and Kao 1999). If the increase in FPOD activ-ity is important in regulating growth reduction of rice seedling roots caused by NaCl, then exogenous application of NH4Cl
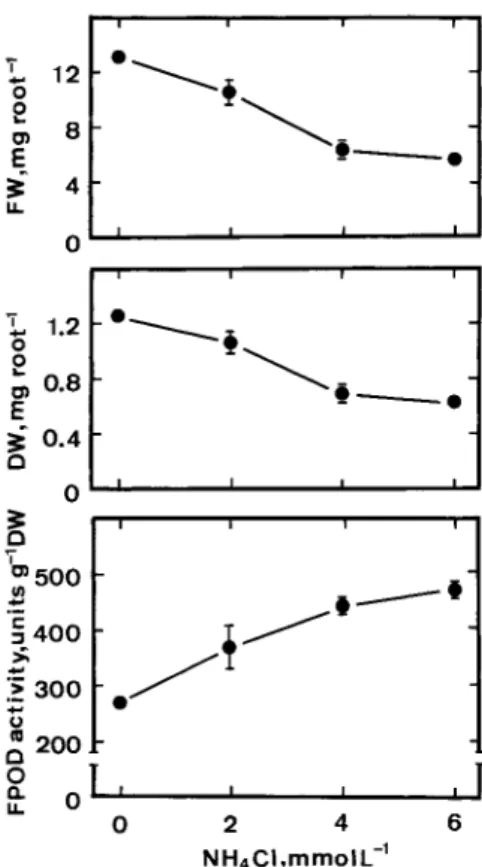
would be expected to increase FPOD activity in roots of rice seedlings. Figure 2 shows that growth of and FPOD activity in roots of seedlings are decreased and increased, respec-tively, with the increasing of NH4Cl concentrations.
We have previously shown that proline accumulation is correlated with root growth inhibition of rice seedlings in-duced by NaCl (Lin and Kao 1996 b), and that exogenous application of proline in the absence of NaCl resulted in a re-duction of root growth (Chen and Kao 1995, Lin and Kao 1996 b). Exogenous application was also found to increase ionically bound peroxidase activity in roots of rice seedlings (Chen and Kao 1995, Lin and Kao 1996 b). In the present in-vestigation, we demonstrated that the reduction of root growth with increasing proline concentrations is correlated with an increase in FPOD activity extracted from cell walls (Fig. 3).
The observations that rice seedlings treated with NH4Cl or
proline, which resulted in an increase in FPOD activity in roots, reduced root growth in the same way that NaCl did, further supports our suggestion that cell-wall stiffening cata-lyzed by FPOD may participate in the regulation of root growth reduction of rice seedlings.
To test the causal relationship between FPOD activity and root growth reduction caused by NaCl, NH4Cl or proline,
2-day-old seedlings were transferred to distilled water, NaCl, NH4Cl,
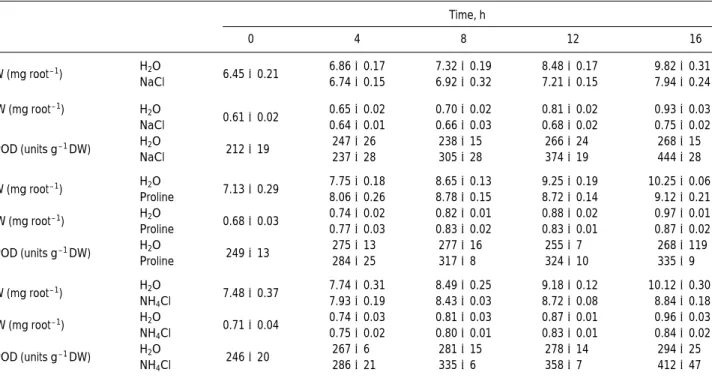
and proline, respectively, for 4, 8, 12, and 16 h. Changes in FPOD and root growth were then monitored. As indicated in Table 1, an increase in FPOD activity was observed at 8 h af-ter treatment, whereas root growth reduction was observed at 12 h after treatment, indicating that an increase in FPOD ac-tivity in roots preceded inhibition of root growth caused by NaCl, NH4Cl, or proline. In previous studies, we observed
that proline or ammonium accumulation occurred at 4 h after NaCl treatment (Lin and Kao 1996 a, b). Clearly, the links be-tween NaCl treatment, proline, ammonium, FPOD, and root growth are well established.
Peroxidases were found to be related to lignin synthesis (van Huystee 1987, Sato et al. 1993, Polle et al. 1994). Lignifi-cation is part of cell wall differentiation and irreversibly inhib-its cell elongation (Sauter and Kende 1992). It has been
Figure 2.Effect of NH4Cl on root growth and FPOD activities in roots
of rice seedlings. FW, DW, and FPOD activities were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
Table 1.Changes in root growth and FPOD activities in roots of NaCl-, proline-, or NH4Cl-treated rice seedlings. Rice seeds were germinated in
distilled water for 2 days, and then were transferred to distilled water, NaCl (150 mmol/L) and NH4Cl (4 mmol/L), respectively.
Time, h 0 4 8 12 16 FW (mg root– 1) H2O 6.45±0.21 6.86±0.17 7.32±0.19 8.48±0.17 9.82±0.31 NaCl 6.74±0.15 6.92±0.32 7.21±0.15 7.94±0.24 DW (mg root– 1) H 2O 0.61±0.02 0.65±0.02 0.70±0.02 0.81±0.02 0.93±0.03 NaCl 0.64±0.01 0.66±0.03 0.68±0.02 0.75±0.02 FPOD (units g– 1DW) H2O 212±19 247±26 238±15 266±24 268±15 NaCl 237±28 305±28 374±19 444±28 FW (mg root– 1) H2O 7.13±0.29 7.75±0.18 8.65±0.13 9.25±0.19 10.25±0.06 Proline 8.06±0.26 8.78±0.15 8.72±0.14 9.12±0.21 DW (mg root– 1) H2O 0.68±0.03 0.74±0.02 0.82±0.01 0.88±0.02 0.97±0.01 Proline 0.77±0.03 0.83±0.02 0.83±0.01 0.87±0.02 FPOD (units g– 1DW) H2O 249±13 275±13 277±16 255±7 268±119 Proline 284±25 317±8 324±10 335±9 FW (mg root– 1) H2O 7.48±0.37 7.74±0.31 8.49±0.25 9.18±0.12 10.12±0.30 NH4Cl 7.93±0.19 8.43±0.03 8.72±0.08 8.84±0.18 DW (mg root– 1) H2O 0.71±0.04 0.74±0.03 0.81±0.03 0.87±0.01 0.96±0.03 NH4Cl 0.75±0.02 0.80±0.01 0.83±0.01 0.84±0.02 FPOD (units g– 1DW) H2O 246±20 267±6 281±15 278±14 294±25 NH4Cl 286±21 335±6 358±7 412±47
Figure 3.Effect of proline on root growth and FPOD activities in roots of rice seedlings. FW, DW, and FPOD activities were determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
shown that salinity caused lignification of plants (Hagege et al. 1988, Cachorro et al. 1992). Thus, it is of great intreast to know whether NaCl has an effect on lignin level in roots of rice seedlings. Contrary to our expectation, increasing con-centration of NaCl progressively decreased lignin level in roots of rice seedlings (Fig. 4). It has been shown that syrin-galdazine, a hydrogen donor, has a particularly high affinity for peroxidases associated with lignification (Goldberg et al. 1983). Recently, we used syringaldazine as the substrate to establish whether ionically bound peroxidase activity is re-lated to the reduction of root growth caused by NaCl. It was shown that the increase in ionically bound peroxidase
Figure 4.Effect of NaCl on the levels of lignin in roots of rice seed-lings. Lignin was determined after 5 days of treatment. Vertical bars represent standard errors (n=4).
against syringaldazine was only observed at a concentration of 150 mmol L–1(Lin and Kao 1999). It is clear that lignification
plays no role in regulating root growth reduction of rice seed-lings caused by NaCl.
Acknowledgements.This study has been financially supported by the National Science Council of the Republic of China (NSC 90-2313-B-002-002).
References
Chazen O, Neumann PM (1994) Plant Physiol 104: 1385 –1392 Cachorro P, Ortiz A, Ros Barcelo A, Cerda A (1993) Phyton 33: 33 – 40 Chazen O, Hartung W, Neumann PM (1995) Plant Cell Environ 18:
727–735
Chen SL, Kao CH (1995) Plant Growth Regul 17: 67–71 Cramer GR (1992) J Exp Bot 43: 857– 864
Fry SC (1986) Annu Rev Plant Physiol 37: 165 –186
Goldberg R, Catesson AM, Czaninski Y (1983) Z Pflanzenphysiol 110: 267– 279
Greenway H, Munns R (1980) Annu Rev Plant Physiol 31: 149 –190 Hagege D, Kevers C, Boucau J, Gaspar T (1988) Plant Physiol
Bio-chem 26: 609 – 614
Hartly RD, Ford CW (1989) In: Lewis NG, Paice MG (eds) Plant Cell Wall Polymers, American Chemical Society Symposium Series 399, American Chemical Society, Washington, DC, pp 137–145 Haynes RI, Goh KM (1978) Biol Rev 53: 465 – 510
Kato Y, Nevins DJ (1985) Carbohydr Res 154: 177–187
Lee TM, Lin YH (1995) Plant Sci 106: 1–7
Lin CC, Kao CH (1996 a) Plant Growth Regul 18: 233 – 238 Lin CC, Kao CH (1996 b) Plant Sci 114: 121–128
Lin CC, Kao CH (1999) Plant Soil 216: 147–153
Mader M, Nessel A, Schloss P (1986) In: Greppin H, Penel C, Gaspar T (eds) Molecular and Physiological Aspects of Plant Peroxidases, Univ. of Geneva Press, Geneva, pp 247– 260
Morrison IM (1972) J Sci Food Agric 23: 455 – 463 Munns R (1993) Plant Cell Environ 16: 15 – 24
Munns R, Termaat A (1986) Aust J Plant Physiol 13: 143 –160 Neumann PM (1993) Plant Cell Environ 16: 1107–1114 Neumann PM (1997) Plant Cell Environ 20: 1193 –1198
Neumann PM, Azaize H, Leon D (1994) Plant Cell Environ 17: 303 – 309
Peyrano G, Taleisnik E, Quiroga M, de Forchetti SM, Tigier H (1997) Plant Physiol Biochem 35: 387– 393
Polle A, Otter T, Seifert F (1994) Plant Physiol 106: 53 – 60
Prichard J, Hetherington R, Fry C, Tomos D (1993) J Exp Bot 44: 1281–1289
Rengel Z (1992) Plant Cell Environ 15: 625 – 632
Sanchez M, Pena MJ, Revilla G, Zarra I (1996) Plant Physiol 111: 941– 946
Sasaki M, Yamamoto Y, Matsumoto H (1996) Physiol Plant 96: 193 – 198
Sato YM, Sugiyama M, Gorecki RI, Fukuda H, Kokmamine A (1993) Planta 189: 584 – 589
Sauter M, Kende H (1992) Plant Cell Physiol 33: 1089 –1097 Siegel SM (1953) Physiol Plant 6: 134 –139
van Huystee RB (1987) Annu Rev Plant Physiol 38: 205 – 219 Wang L-W, Showalter AM, Ungar IA (1997) Am J Bot 84: 1247–1255 Whitmore FW (1976) Phytochemistry 15: 375 – 378