Varicella Zoster Virus infection among Health Care Workers in Taiwan:
Seroprevalence and Predictive Value of History of Varicella Infection
Mei-Fong Wua, Yu-Wen Yanga, Wen-Yuan Linb,e, Chih-Yen Changc, Maw-Soan Soond,
Chun-Eng Liuc
a
Department of Family Medicine, Changhua Christian Hospital, Changhua, Taiwan
b
Department of Family Medicine, China Medical University Hospital, Taichung,
Taiwan
c
Division of Infectious disease, Department of Internal Medicine, Changhua Christian
Hospital, Changhua, Taiwan
d
Division of Gastroenterology, Department of Internal Medicine, Changhua Christian
Hospital, Changhua, Taiwan
e
School of Medicine and Graduate Institute of Clinical Medical Science, China
Medical University, Taichung, Taiwan
Short running title: Varicella in Health care workers
Co-Authors’ e-mail:
Yu-Wen Yang: 55846@cch.org.tw
Wen-Yuan Lin: wylin@mail.cmu.edu.tw
Chih-Yen Chang: 106018@cch.org.tw
Maw-Soan Soon: 2531@cch.org.tw
Correspondence and reprint request to:
Chun-Eng Liu MD
Division of Infectious disease, Department of internal medicine, Changhua Christian
Hospital
135 Nan-Hsiao street, Changhua City, Changhua 500, Taiwan
Email: 63557@cch.org.tw
Summary
To prevent nosocomial varicella outbreaks, a varicella program was conducted in a
tertiary hospital in Taiwan from 2008 to 2009. This program included antibody testing
against varicella zoster virus (VZV), self-administered questionnaire interview to
obtain previous history of varicella infection or varicella vaccination, and provision of
varicella vaccination to those with seronegativity for VZV. This study analyzed the
results of this program, including seroprevalence of VZV and predictive value of
self-reported varicella infection or vaccination history among health care workers
(HCW) in Taiwan. All HCW (N=3733) in this hospital with a mean age of 34.6 years
participated in this program. The seroprevalence of VZV was 91.1%. Sensitivity,
specificity, positive, and negative predict value of a self-reported history of varicella
infection was 82.3%, 48.6%, 96.3%, and 14.4%, respectively, while that for history of
varicella vaccination was 23.4%, 69.4%, 90.9%, and 6.5%, respectively. The recall
history of younger age, female, medical professionals (doctors, nurses, and
paramedical staff), or HCW at higher risk of exposure to varicella had a higher
sensitivity. However, only those of medical professionals had significantly higher PPV.
This study concludes a positive recall history of varicella infection and vaccination
was not predictive of lack of immunity. To effectively prevent nosocomial infection,
documenting VZV-IgG titers for all HCW and vaccinating of those who are
susceptible is suggested.
Introduction
Varicella (chickenpox), caused by varicella zoster virus (VZV), is a highly
contagious disease that is spread by contact with respiratory droplets and/or vesicle
fluid.(1) It is usually self-limited, but may cause severe complications, such as lower
respiratory tract infection, skin and soft tissue infection, or even death.(1) In Taiwan,
the annual cases of varicella is about 11,000(2) and the estimated varicella-related
hospitalization rate was 60 per 1000 patients. Infants and adults aged from 19 to 38
years or older than 75 years have the highest hospitalization rate.(3)
Varicella is a recognized nosocomial infection among health care workers
(HCW), who, once infected, may transmit infection to susceptible co-workers and
patients under their care.(4) The cost of controlling varicella in the hospital settings can
be substantial because identification of cases, furloughing, and serologic testing of
susceptible HCW are often indicated after each episode of in-hospital exposure to
varicella.(5, 6) Therefore, VZV vaccination has been recommended by the US Centers
for Disease Control and Prevention (CDC) for HCW who are susceptible to
varicella.(7, 8) In Taiwan, the national recommendations regarding varicella vaccination
for susceptible HCW have not been issued. Free VZV vaccination policy is
knowledge, few hospitals in Taiwan follow the guidelines from US CDC because of
the cost related to laboratory testing and providing vaccination. However, several
episodes of nosocomial outbreaks of varicella occurred in hospitals in past years.
To expedite control of varicella in the hospital setting, some investigators
accepted a past history of varicella infection provided by HCW as a proof of
immunity, and serology is used only in cases of unclear or negative history.(1, 6, 9)
Nevertheless, it remains a debatable issue to serologically screen selected individuals
based on a history of varicella instead of screening all HCW,(10, 11) because the
effectiveness of selective program may depend on the prevalence of the disease in the
population examined and the reliability of recall history of varicella.
To date, the seroprevalence and reliability of a recall history of varicella among
HCW in Taiwan has not been evaluated. The aims of this study were to evaluate the
seroprevalence and the reliability of recall history of varicella among HCW in Taiwan
which may help guide the development of local screening program to control
Methods
Hospital setting
Changhua Christian Hospital (CCH), a 1775-bed tertiary-care hospital providing
primary and tertiary care in middle-Taiwan, with an estimated population of 4.48
million.
Study population
All 3733 HCW in the hospital participated in this varicella control program. The
types of HCW were grouped into physicians (N=537), nurses (N=1580), paramedical
staff (e.g. dietician, pharmacist, rehabilitation staff, laboratory personnel or diagnostic
imaging staff) (N=698), and administrative staff (including maintenance, technical,
and catering et al.) (N=918). "Risk of exposure" to varicella was regarded as "high"
for HCW working in the pediatric department or providing services for varicella (e.g.
department of emergency medicine, dermatology or infectious disease)infectious
diseasesaccording to the definitions of Center for Infection Control at this hospital.
Laboratory investigations and questionnaire interview
occupational medical examination from May 2008 to April 2009. Annual medical
examination is mandatory for all employees in the hospital. VZV antibody was
checked with a commercial enzyme-linked fluorescent immunoassay (ELFA) kit
(VIDAS®, bioMerieux, Marcy l'Etoile, France). ELFA is specific for the detection of
immunoglobulin G (IgG) antibodies to VZV, with declared sensitivity and specificity
of 99.7% and 97.6%, respectively. The patient’s immune serum ratio value was
classified as positive (≥0.9), negative (<0.60), or equivocal (≥0.6 to <0.90). HCW
with either negative or equivocal serum responses were regarded as seronegative, and
would be offered with VZV vaccination (Varilrix®, GlaxoSmithKline, or Varivax
Refrigerated®, CSL-MSD).
A self-administered questionnaire interview to obtain previous history of
varicella infection or vaccination against varicella was performed during health
check-up. The answer was yes, no, or unknown. In this study, we retrospectively
collected the data using a standardized case record form and analyzed the results of
this varicella control program. The study was approved by the Institutional Review
Board of CCH.
Statistical analysis
17.0 ,Chicago17.0, Chicago, IL, USA). Prevalence of VZV antibody and history of
varicella infection or vaccination among different groups (gender, age, type of
occupation, or risk of exposure) were calculated. Difference in proportions were
assessed by the chi-square test, considering a value of P<0.05 as statistically
significant. Sensitivity, specificity, positive predictive value (PPV) and negative
predictive value (NPV) of the recall history for the presence of VZV-IgG were
determined. Relative risk (RR) was calculated by logistic regression model. Gender,
Results
The demographic characteristics of all 3733 HCW are shown in Table I. The
participants were predominantly female (79.2%) and aged ranging from 18 to 68
years (mean age, 34.6 years); 75.2% were younger than 40 years; 42.3% were nursing
staff; and 11.2% of HCW were categorized as the group with high risk of exposure
to varicella infection.
The prevalence of VZV seropositivity was 91.1%. The VZV seronegativity
group included 200 HCW (5.4%) whose serum samples were reported as seronegative
and 133 (3.6%) as sero-equivocal. No significant differences in characteristics were
observed between HCW who were VZV seronegative and those who were
sero-equivocal other than that HCW who were VZV seronegative were older than
those who were sero-equivocal (Table II).
Seropositivity was statistically significantly higher in older HCW than in
younger HCW (Table II & Figure 1).
Based on the self-administered questionnaire interview, a previous history of
varicella infection was reported by 1465 of the HCW (39.2%), while 354 (9.5%)
HCW (aged <50 years), female HCW, medical professionals (doctors, nurses and
paramedical staff), and high-risk exposure group were more likely to report a positive
history of varicella infection. (Table III & Figure1)
Of the 1465 HCW who reported a positive history of previous varicella infection,
1411 (96.3%) were seropositive and 54 (3.7%) were seronegative. Among the 354
HCW who reported a negative history of previous varicella infection, 303 (85.6%)
were seropositive and 51 (14.4%) were seronegative. A positive recall history of
previous varicella infection was significantly associated with a higher prevalence of
VZV seropositivity (96.3% Vs 85.6%, P<0.001). However, there was no statistically
significant difference in terms of seroprevalence between HCW with an uncertain
varicella infection history and those with a negative history (88.1% Vs 85.6%,
P=0.189).In this population, tThe sensitivity of a recall history of previous varicella
infection to detect seropositivity for VZV was 82.3% (1411/(1411+303)), whereas the
specificity was 48.6% (51/(54+51)). In this population, tThe PPV of a recall history of
varicella infection to predict varicella immunity was 96.3% (1411/1465) and the NPV
was 14.4% (51/354). The respective value for sensitivity, specificity, PPV and NPV
will be 41.5%, 83.6%, 96.3%, and 12.3%, when people with unknown history of
varicella infection were included in the group of participants with a negative history
In multivariate logistic regression analysis (Table IV), the recall history of
medical professionals (including doctors, nurses, and paramedical staff) had
statistically higher PPV compared with that of administrative staff. Younger age (<50
year), female, medical professionals and high-risk exposure group had significantly
higher sensitivity. In contrast, neither NPV nor specificity was significantly
influenced by those defined variables. Multivariate logistic regression analysis
showed older HCW had a higher seropositivity rate than younger HCW; doctors or
nurses had higher seropositivity rates than other co-workers.
Receipt of varicella vaccine was reported by 287 HCW (7.7%), 912 (24.4%)
reported a negative vaccination history, and 2534 (67.9%) were unaware of their
vaccination history (Table II). Of those 287 HCW with previous varicella vaccination,
261 (90.9%) were seropositive. Subjects with a previous history of varicella
vaccination didn't have higher seropositivity to VZV than those without such a
vaccination history (90.9% Vs. 91.1%, P=0.932). HCW younger than 40 years and
doctors were more likely to have received varicella vaccination than other HCW
(Figure 1). The sensitivity of VZV vaccination to predict VZV immunity was 23.4%
(261/(261+853)) and the specificity was 69.4% (59/(26+59)). PPV was 90.9%
(261/287) and NPV was 6.5% (59/912). The figure for sensitivity, specificity, PPV
unknown history of vaccination were included in the negative history group.
If recall history of varicella infection and history of vaccination waswere
analyzed together, the sensitivity of positive history to predict VZV immunity was
86.4% (1527/(1527+240)) and the specificity was 36.4% (40/(70+40)). PPV was
95.6% (1527/(1527+70)) and NPV was 14.3% (40/(240+40)). The figure for
sensitivity, specificity, PPV and NPV became 44.9%, 79.0%, 95.6% and 12.3%
respectively, when people with unknown history were included in the negative history
Discussion
To our best knowledge, current study is the first one to document the varicella
susceptibility and the reliability of recall history of varicella infection among HCW in
Taiwan. In this hospital-wide survey, we have demonstrated that the seropositivity for
VZV among HCW was 91.1%. However, only 39.2% of the HCW reported a previous
history of varicella infection. The sensitivity, specificity, PPV and NPV of a recall
history of varicella infection to predict varicella immunity was 82.3%, 48.6%, 96.3%
and 14.4%, respectively. The proportion of HCW who reported havinghadever
received varicella vaccination was low (7.7%), although they are at high risk for
varicella infection and work in the hospital with previous episodes of nosocomial
varicella outbreaks involving the hospital staff.
It has been suggested that immunity levels of 94% or more are needed to
interrupt virus transmission in the health care settings;(12) therefore, the level of VZV
seropositivity among HCW in this hospital may not be sufficiently high to prevent
further varicella outbreak, and interventions to prevent varicella transmission is
desirable, for which varicella vaccination for HCW without immunity may be the
most cost-effective strategy.(7, 8) Serological testing for all HCW is the most reliable
cost-effectiveness of this approach needs further investigations. The second approach
is to rely on a recall history of varicella infection. Several investigators have adopted
this approach.(4, 11, 13, 14)
In our study, more than half of HCW (51.3%) were unaware of their previous
history of varicella infection; the positive recall history of varicella infection was only
39.2%, which is significantly lower compared with those of other studies, for which
the figure ranges from 49.7% to 85.3%.(4, 6, 9, 10, 12, 13) Consequently, our sensitivity
(41.5%), which is defined as the ability of a positive history to identify all immune
subjects, was lower compared with that of these published studies, which ranges from
50.5% to 59.0%.(4, 10, 12, 13) In our study, younger, female, medical professionals
(doctors, nurses, and paramedical staff), or HCW at higher risk of exposure to VZV
were more likely to report a history of varicella infection, and therefore, their recall
history had a higher sensitivity; however, only the recall history of medical
professionals had significantly higher PPV. The possible explanation might be
because medical professionals have better knowledge about varicella compared with
administrative staff. Our findings suggest that education sessions provided to HCW
before questionnaire survey may increase the reliability of a recall history of varicella.
excellent predictor and advocated testing only those individuals with a negative
history of varicella infection. This recommendation was based on their high
seroprevalence rate (97.7%~98.5%)(4, 11, 13) with PPV might be up to 100%.(4, 11) The
seroprevalence (91.1%) and PPV (96.3%) in our study was lower compared with
those in these studies, which suggests that a reported history of varicella infection
may not ensure the presence of protective VZV-IgG titer. For example, in our study,
still 3.7% (54/1465) HCW who reported a positive varicella history remained
susceptible to varicella. Screening based on a history of varicella may put these
seronegative HCW at risk for nosocomial varicella infection if other measures are not
taken. Considering the severity of nosocomial varicella, a documented VZV-IgG titer
in all HCW should be considered. Whereas a negative history did not predict lack of
immunity (NPV=14.4%), possible reasons might be that most patients got chickenpox
when they were younger than 10 years of age (chickenpox peaked in children of 4-5
years),(3) the recall history might be unreliable and underestimated. Accordingly,
serological testing rather than presumptive vaccination is advisable to those with a
negative or uncertain history of varicella infection.
Studies by Gallagher et al(15) (PPV=95%, NPV=11%) and Almuneef et al(10)
(PPV=89%, NPV=22%) also had similar results to ours and recommended serological
those who are susceptible.
Interestingly, HCW with a positive history of varicella vaccination didn’t have
higher seropositive rate. , the PPV was not improvedhigher when history of varicella
infection and vaccination were analyzed together. These findings suggest that a
history of varicella vaccination may not be a reliable indicator of immunity. The
possible explanation would be that someone of them might not responsed to varicella
vaccination, the immunity for VZV declines with age, people were not familiar with
their vaccination schedules in their childhood or the recall bias.or the recall history of
vaccination was incorrect.
Our study has several limitations and interpretation of our data should be
cautious. First, our study involved HCW of only one hospital and the results might not
be generalized to all HCW of other hospitals around Taiwan. Nevertheless, this
program is the first program to evaluate the varicella susceptibility and reliability of
recall history of varicella among HCW in Taiwan, and the study population was large
and comprised all HCW with different job titles in a tertiary-care hospital. Our
findings may help guide development of a local policy to identify venerable HCW
and define recommendation for immunization. The other limitation is that HCW with
their immunization status before receiving VZV vaccination. Although this strategy
may enhance immunity against varicella for these HCW, some of these HCW who
were seroequivocal might demonstrate seropositivity if the serum tests were repeated.
Thus, our seropositivity rate may have been underestimated. Finally, we didn't provide
education sessions of varicella for HCW before self-administered questionnaire
interview and didn't validate their results after collecting the data, better knowledge
for varicellafor which is likely to improve the reliability of recall history.
We conclude that seropositive rate of varicella among HCW in this hospital was
not sufficiently high to prevent an outbreak, therefore, documenting VZV-IgG titers
for all HCW and vaccination of those susceptible should be considered to effectively
prevent nosocomial varicella. Though a positive recall history of varicella was
associated with a significantly higher seropositive rate and might be used as a
surrogate of immunity when screening all HCW is not allowed, nevertheless, it will
put the seronegative HCW with a positive varicella history at risk for nosocomial
varicella infection. The balance between
We conclude that seropositive rate of varicella among HCW in this hospital was
associated with a significantly higher seropositive rate, but did not ensure the
presence of protective VZV-IgG titer, whereas a negative history was not predictive of
lack of immunity. A history of varicella vaccination had no value as a predictor of
susceptibility. The recall history of younger, female, medical professionals, or HCW
at higher risk of exposure to varicella had a higher sensitivity; however, only those of
medical professionals had significantly higher PPV. To effectively prevent nosocomial
varicella, documenting VZV-IgG titers for all HCW and vaccination of those
susceptible is suggested. If screening all HCW is impossible and history of varicella
would be used as a proof of immunity, efforts to improvinge PPV and a pilot study to
evaluate the value of PPV are suggestedis indicated. Which level of PPV would tip
the balance in favor of simply testing those with a negative or unknown history of
Acknowledgements
The authors owe our deepest gratitude to Dr. Chien-Ching Hung for his help in the
preparation of the manuscript.
Conflict of interest statement
None declared.
Funding sources
References
1. Holmes CN, Predictive value of a history of varicella infection, Can Fam
Physician 51(2005), pp. 60-5.
2. Center for disease control,TAIWAN, Notifiable infectious disease statistics
system, Taipei (2010).
3. Lin YH, Huang LM, Chang IS, Tsai FY, Chang LY, Disease burden and
epidemiological characteristics of varicella in Taiwan from 2000 to 2005, J Microbiol
Immunol Infect 42(2009), pp. 5-12.
4. Chazan B, Colodner R, Teitler N, Chen Y, Raz R, Varicella zoster virus in health
care workers in northern Israel: seroprevalence and predictive value of history of
varicella infection, Am J Infect Control 36(2008), pp. 436-8.
5. Apisarnthanarak A, Kitphati R, Tawatsupha P, Thongphubeth K, Apisarnthanarak
P, Mundy LM, Outbreak of varicella-zoster virus infection among Thai healthcare
workers, Infect Control Hosp Epidemiol 28(2007), pp. 430-4.
6. Almuneef M, Dillon J, Abbas MF, Memish Z, Varicella zoster virus immunity in
multinational health care workers of a Saudi Arabian hospital, Am J Infect Control
31(2003), pp. 375-81.
7. Lyznicki JM, Bezman RJ, Genel M, Report of the Council on Scientific Affairs,
vaccine, Infect Control Hosp Epidemiol 19(1998), pp. 348-53.
8. Immunization of health-care workers: recommendations of the Advisory
Committee on Immunization Practices (ACIP) and the Hospital Infection Control
Practices Advisory Committee (HICPAC), MMWR Recomm Rep 46(1997), pp. 1-42.
9. Chong CY, Lim SH, Ng WY, Tee N, Lin RV, Varicella screening and vaccination
for healthcare workers at KK Women's and Children's Hospital, Ann Acad Med
Singapore 33(2004), pp. 243-7.
10. Almuneef M, Memish ZA, Abbas ME, Balkhy HH, Screening healthcare
workers for varicella-zoster virus: can we trust the history?, Infect Control Hosp
Epidemiol 25(2004), pp. 595-8.
11. Santos AM, Ono E, Weckx LY, Coutinho AP, de Moraes-Pinto MI, Varicella
zoster antibodies in healthcare workers from two neonatal units in Sao Paulo,
Brazil--assessment of a staff varicella policy, J Hosp Infect 56(2004), pp. 228-31.
12. De Juanes JR, Gil A, San-Martin M, Gonzalez A, Esteban J, Garcia de Codes A,
Seroprevalence of varicella antibodies in healthcare workers and health sciences
students. Reliability of self-reported history of varicella, Vaccine 23(2005), pp.
1434-6.
13. Vandersmissen G, Moens G, Vranckx R, de Schryver A, Jacques P, Occupational
seroprevalence study, Occup Environ Med 57(2000), pp. 621-6.
14. Brunell PA, Wood D, Varicella serological status of healthcare workers as a
guide to whom to test or immunize, Infect Control Hosp Epidemiol 20(1999), pp.
355-7.
15. Gallagher J, Quaid B, Cryan B, Susceptibility to varicella zoster virus infection
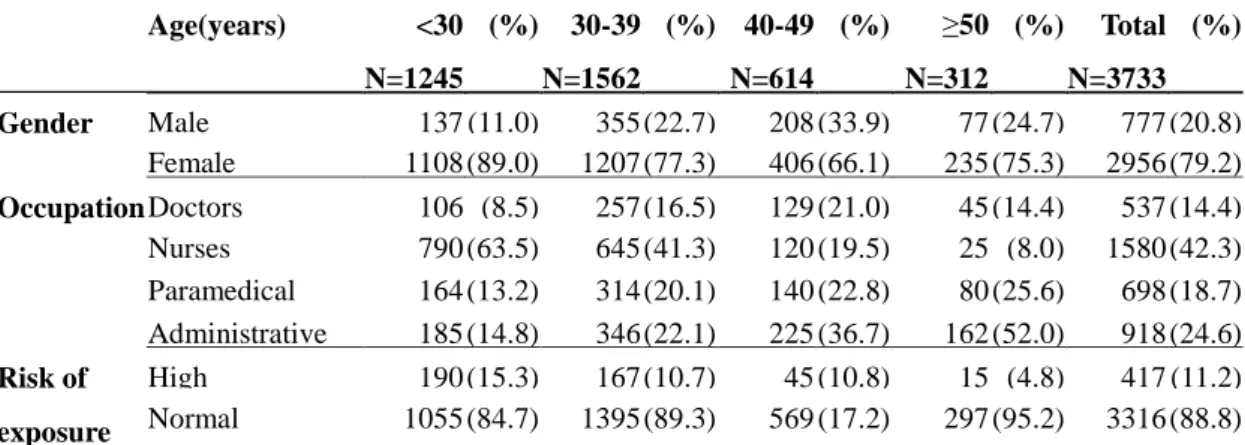
Table I. The baseline characteristics of study population by age groups Age(years) <30 (%) 30-39 (%) 40-49 (%) ≥50 (%) Total (%) N=1245 N=1562 N=614 N=312 N=3733 Gender Male 137 (11.0) 355 (22.7) 208 (33.9) 77 (24.7) 777 (20.8) Female 1108 (89.0) 1207 (77.3) 406 (66.1) 235 (75.3) 2956 (79.2) Occupation Doctors 106 (8.5) 257 (16.5) 129 (21.0) 45 (14.4) 537 (14.4) Nurses 790 (63.5) 645 (41.3) 120 (19.5) 25 (8.0) 1580 (42.3) Paramedical 164 (13.2) 314 (20.1) 140 (22.8) 80 (25.6) 698 (18.7) Administrative 185 (14.8) 346 (22.1) 225 (36.7) 162 (52.0) 918 (24.6) Risk of exposure High 190 (15.3) 167 (10.7) 45 (10.8) 15 (4.8) 417 (11.2) Normal 1055 (84.7) 1395 (89.3) 569 (17.2) 297 (95.2) 3316 (88.8) * Paramedical staff: including dietician, pharmacist, rehabilitation staff, psychologist, laboratory personnel and diagnostic imaging staff.
Table II. The serum results of varicella antibody (anti-VZV IgG) by different
characteristics
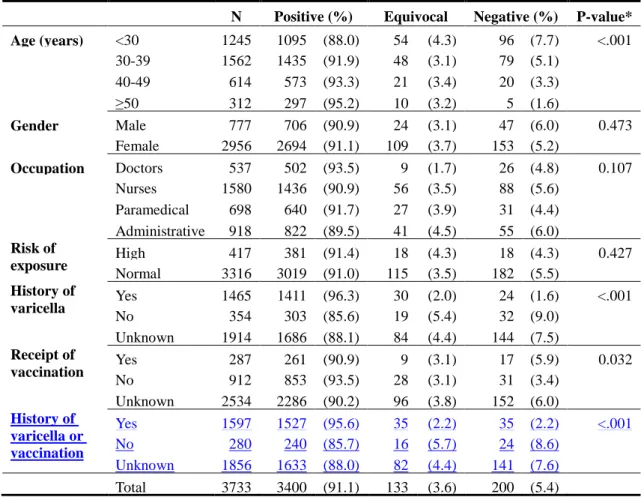
N Positive (%) Equivocal Negative (%) P-value* Age (years) <30 1245 1095 (88.0) 54 (4.3) 96 (7.7) <.001 30-39 1562 1435 (91.9) 48 (3.1) 79 (5.1) 40-49 614 573 (93.3) 21 (3.4) 20 (3.3) ≥50 312 297 (95.2) 10 (3.2) 5 (1.6) Gender Male 777 706 (90.9) 24 (3.1) 47 (6.0) 0.473 Female 2956 2694 (91.1) 109 (3.7) 153 (5.2) Occupation Doctors 537 502 (93.5) 9 (1.7) 26 (4.8) 0.107 Nurses 1580 1436 (90.9) 56 (3.5) 88 (5.6) Paramedical 698 640 (91.7) 27 (3.9) 31 (4.4) Administrative 918 822 (89.5) 41 (4.5) 55 (6.0) Risk of exposure High 417 381 (91.4) 18 (4.3) 18 (4.3) 0.427 Normal 3316 3019 (91.0) 115 (3.5) 182 (5.5) History of varicella Yes 1465 1411 (96.3) 30 (2.0) 24 (1.6) <.001 No 354 303 (85.6) 19 (5.4) 32 (9.0) Unknown 1914 1686 (88.1) 84 (4.4) 144 (7.5) Receipt of vaccination Yes 287 261 (90.9) 9 (3.1) 17 (5.9) 0.032 No 912 853 (93.5) 28 (3.1) 31 (3.4) Unknown 2534 2286 (90.2) 96 (3.8) 152 (6.0) History of varicella or vaccination Yes 1597 1527 (95.6) 35 (2.2) 35 (2.2) <.001 No 280 240 (85.7) 16 (5.7) 24 (8.6) Unknown 1856 1633 (88.0) 82 (4.4) 141 (7.6) Total 3733 3400 (91.1) 133 (3.6) 200 (5.4) * chi-square test
Table III. The distribution of selective variables in relation to history of varicella infection
or receipt of vaccination
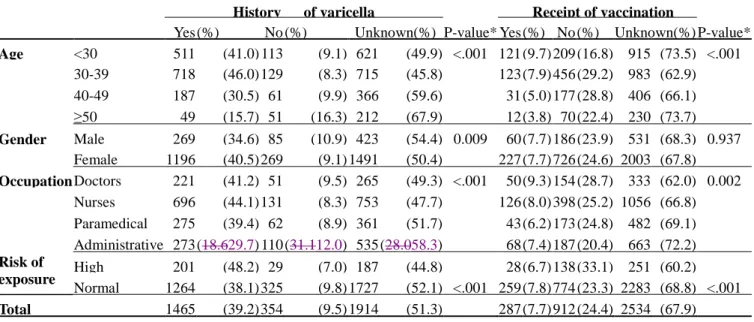
History of varicella Receipt of vaccination Yes (%) No (%) Unknown(%) P-value* Yes (%) No (%) Unknown(%) P-value* Age <30 511 (41.0) 113 (9.1) 621 (49.9) <.001 121 (9.7) 209 (16.8) 915 (73.5) <.001 30-39 718 (46.0) 129 (8.3) 715 (45.8) 123 (7.9) 456 (29.2) 983 (62.9) 40-49 187 (30.5) 61 (9.9) 366 (59.6) 31 (5.0) 177 (28.8) 406 (66.1) ≥50 49 (15.7) 51 (16.3) 212 (67.9) 12 (3.8) 70 (22.4) 230 (73.7) Gender Male 269 (34.6) 85 (10.9) 423 (54.4) 0.009 60 (7.7) 186 (23.9) 531 (68.3) 0.937 Female 1196 (40.5) 269 (9.1) 1491 (50.4) 227 (7.7) 726 (24.6) 2003 (67.8) Occupation Doctors 221 (41.2) 51 (9.5) 265 (49.3) <.001 50 (9.3) 154 (28.7) 333 (62.0) 0.002 Nurses 696 (44.1) 131 (8.3) 753 (47.7) 126 (8.0) 398 (25.2) 1056 (66.8) Paramedical 275 (39.4) 62 (8.9) 361 (51.7) 43 (6.2) 173 (24.8) 482 (69.1) Administrative 273 (18.629.7) 110 (31.112.0) 535 (28.058.3) 68 (7.4) 187 (20.4) 663 (72.2) Risk of exposure High 201 (48.2) 29 (7.0) 187 (44.8) 28 (6.7) 138 (33.1) 251 (60.2) Normal 1264 (38.1) 325 (9.8) 1727 (52.1) <.001 259 (7.8) 774 (23.3) 2283 (68.8) <.001 Total 1465 (39.2) 354 (9.5) 1914 (51.3) 287 (7.7) 912 (24.4) 2534 (67.9) * chi-square test
27
Table IV. The relative risk (RR) of positive predict value (PPV), negative predict value
1
(NPV), sensitivity and specificity for HCW with positive history of previous Varicella 2
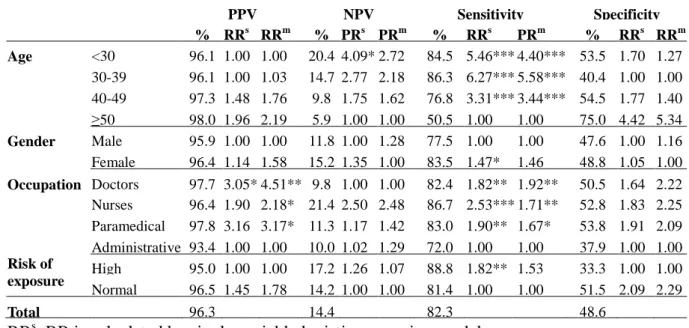
infection by different characteristics 3 PPV NPV Sensitivity Specificity % RRs RRm % PRs PRm % RRs PRm % RRs RRm Age <30 96.1 1.00 1.00 20.4 4.09* 2.72 84.5 5.46*** 4.40*** 53.5 1.70 1.27 30-39 96.1 1.00 1.03 14.7 2.77 2.18 86.3 6.27*** 5.58*** 40.4 1.00 1.00 40-49 97.3 1.48 1.76 9.8 1.75 1.62 76.8 3.31*** 3.44*** 54.5 1.77 1.40 ≥50 98.0 1.96 2.19 5.9 1.00 1.00 50.5 1.00 1.00 75.0 4.42 5.34 Gender Male 95.9 1.00 1.00 11.8 1.00 1.28 77.5 1.00 1.00 47.6 1.00 1.16 Female 96.4 1.14 1.58 15.2 1.35 1.00 83.5 1.47* 1.46 48.8 1.05 1.00 Occupation Doctors 97.7 3.05* 4.51** 9.8 1.00 1.00 82.4 1.82** 1.92** 50.5 1.64 2.22 Nurses 96.4 1.90 2.18* 21.4 2.50 2.48 86.7 2.53*** 1.71** 52.8 1.83 2.25 Paramedical 97.8 3.16 3.17* 11.3 1.17 1.42 83.0 1.90** 1.67* 53.8 1.91 2.09 Administrative 93.4 1.00 1.00 10.0 1.02 1.29 72.0 1.00 1.00 37.9 1.00 1.00 Risk of exposure High 95.0 1.00 1.00 17.2 1.26 1.07 88.8 1.82** 1.53 33.3 1.00 1.00 Normal 96.5 1.45 1.78 14.2 1.00 1.00 81.4 1.00 1.00 51.5 2.09 2.29 Total 96.3 14.4 82.3 48.6 RRs: RR is calculated by single-variable logistic regression model.
4
RRm: RR is calculated by multi-variable logistic regression model, which includes age, 5
gender, occupation and risk of exposure) 6
* P<0.05, **P<0.01, ***P<0.001 7
28
Legend to Figure. The results of seropositivity and positive history of varicella and
1
vaccination history according age group (Figure 1A) and type of work (Figure 1B). 2
29 Figure 1(A) 1 2 3 4
30 Figure 1(B)
1 2