Adjunctive Sarcosine plus Benzoate Improved Cognitive Function in Chronic
Schizophrenia Patients with Constant Clinical Symptoms: A Randomized,
Double-Blind, Placebo-Controlled Trial
Chun-Yuan Lin,a,b,c Sun-Yuan Liang,dYue-Cung Chang,eShuo-Yen Ting,d Ching-Ling Kao,b Yu-Hsin Wu,c,f Guochuan E. Tsai,g and Hsien-Yuan Lane a,h
a Graduate Institute of Clinical Medical Science, College of Medicine, China Medical University, Taichung, Taiwan
b Tsaotun Psychiatric Center, Ministry of Health and Welfare, Nantou, Taiwan c National Changhua University of Education, Changhua, Taiwan
dDepartment of Psychiatry, Changhua Hospital, Ministry of Health and Welfare, Changhua, Taiwan
e Department of Mathematics, Tamkang University, Taipei, Taiwan f Feng-Yuan Hospital, Ministry of Health and Welfare, Taichung, Taiwan g Department of Psychiatry, Harbor-UCLA Medical Center
h Department of Psychiatry, China Medical University Hospital, Taichung , Taiwan Correspondence:
Hsien-Yuan Lane, MD, PhD
Department of Psychiatry, China Medical University Hospital, Taichung, Taiwan No.2, Yuh-Der Road, Taichung 404, Taiwan
E-mail: hylane@gmail.com Tel: +886-921-067260 Fax: 886-4-22361042 Abstract: 199 words; Text: 4405 words; References: 75
Figure: 1; Tables: 3; Supplementary Tables: 2
Objectives
Hypofunction of NMDA receptor is implicated in the pathophysiology, particularly cognitive impairment, of schizophrenia. Sarcosine, a glycine transporter I (GlyT-1) inhibitor, and sodium benzoate, a D-amino acid oxidase (DAAO) inhibitor, can both enhance NMDA receptor-mediated neurotransmission. We proposed simultaneously
inhibiting DAAO and GlyT-1 may be more effective than inhibition of either in improving the cognitive and global functioning of schizophrenia patients. Methods
This study compared add-on sarcosine (2 g/day) plus benzoate (1 g/day) vs. sarcosine (2 g/day) for the clinical symptoms, as well as the cognitive and global functioning, of chronic schizophrenia patients in a 12-week, double-blind,
randomized, placebo-controlled trial. Participants were measured with Positive and Negative Syndrome Scale (PANSS) and Global Assessment of Functioning (GAF) every 3 weeks. Seven cognitive domains, recommended by the Measurement and
Treatment Research to Improve Cognition in Schizophrenia (MATRICS) committee, were measured at weeks 0 and 12.
Results
Adjunctive sarcosine plus benzoate, but not sarcosine alone, improved the cognitive and global functioning of patients with schizophrenia, even when their clinical symptoms had not improved.
Conclusions
This finding suggests NMDA-enhancement therapy can improve the cognitive function of patients with schizophrenia, further indicating this pro-cognitive effect can be primary without improvement in clinical symptoms.
Key words: Schizophrenia, Biological psychiatry, Psychopharmacology, Cognitive function, NMDA receptor
ClinicalTrials.gov Identifier: NCT01047592
Introduction
Schizophrenia is a severe mental disorder, influencing 1% of the global population (Schultz and Andreasen, 1999). Its phenotypes include positive
symptoms, negative symptoms and cognitive impairments. Cognitive dysfunction is thought to be the core manifestation of schizophrenia due to its detrimental impact throughout the lifelong illness (Carrión et al., 2011, Green et al., 2011, Kasper, 2006). There is controversy regarding the effect of current antipsychotic medications
on cognitive dysfunction (Meltzer, 2013, Sergi et al., 2007). Moreover, despite preclinical data, there has been limited positive clinical feedback on putative
pro-cognitive drugs in humans (Millan et al., 2012, Preskorn et al., 2014).
Evidence of the role of NMDA receptors in the cognition and pathophysiology of schizophrenia (Errico et al., 2013, Javitt et al., 2012, Krystal et al., 1994, Sawa, 2009) suggests that enhancement of the NMDA receptor function may improve the cognitive impairment of schizophrenia (Hashimoto et al., 2013, Javitt et al., 2012,
Krystal et al., 1994, Paoletti et al., 2013). Previous research showed that adjuvant NMDA-enhancing agents which directly or indirectly enhance the NMDA function via the NMDA-glycine site, including D-serine (Levy et al., 2015,
Heresco-Levy et al., 2005, Tsai et al., 1998), glycine (Heresco-Heresco-Levy et al., 2004, Javitt et al., 2001), glycine transporter I (GlyT-1) inhibitor (Tsai et al., 2004), and D-amino acid oxidase (DAAO) inhibitor (Lane et al., 2013), reveal beneficial efficacy on clinical symptoms. For example, sarcosine, increasing glycine supply to the synapse by blocking the reuptake of glycine through inhibiting GlyT-1 (McBain et al., 1989),
could decrease positive and negative symptoms of some patients (Lane et al., 2005, Tsai et al., 2004). Whether sarcosine can improve cognitive function is not yet
known. Roche also recently announced two Phase III studies of another GlyT-1 inhibitor, bitopertin, in adults with persistent, predominant negative symptoms,
failed to meet their primary goals (FirstWord Pharma, 2014). In addition, the Cognitive and Negative Symptoms in Schizophrenia Trial (Buchanan et al., 2007) contained a relatively larger sample size along with a design relevant for interpreting
negative symptoms and cognition effects of glycine and D-cycloserine. The results were negative.
D-serine may be more potent than glycine as the neurotransmitter for the coagonist site of the NMDA receptor (Bado et al., 2011, Balu and Coyle, 2014) and may produce pro-cognitive effects in rodents (Bado et al., 2011) and healthy
showed no improvement in negative or cognitive symptoms of schizophrenia and the lower D-serine doses could be a possible factor (Weiser et al., 2012). Although higher-dose D-serine may provide additional opportunities to test its pro-cognitive effects in schizophrenia, the nephrotoxicity is a concern. Another potential approach to simulate NMDA function is to prevent D-serine degradation. DAAO, a
flavoenzyme of peroxisomes that exists in the central nervous system, is responsible for degrading D-serine and D-alanine (Burnet et al., 2011, Verrall et al., 2010). One of the potential agents is sodium benzoate (benzoate), an inhibitor of DAAO, which can slow the metabolism and elevate the synaptic concentration of D-amino acids such as D-serine and therefore enhance NMDA receptor-mediated
neurotransmission (Van den Berghe-Snorek and Stankovich, 1985). In an animal study, a single oral dose of sodium benzoate didn’t change D-serine levels in plasma
or in the brain, however; sodium benzoate induced antipsychotic effects in the phencyclidine model of schizophrenia (Matsuura et al., 2015). Small-scale pilot trials showed that benzoate improve the cognitive function of patients with chronic schizophrenia (Lane et al., 2013) and patients with early-phase Alzheimer disease
(Lin et al., 2014a), supporting the safety and pro-cognitive potential of this DAAO inhibitor.
While D-serine and glycine are endogenous coagonists of NMDA receptors, their availability are different (Papouin et al., 2012). The preferential affinity of D-serine is for synaptic NMDA receptors and glycine is for extrasynaptic NMDA receptors. Moreover, long-term potentiation relies on synaptic NMDA receptors and
long-term depression requires both the activations of synaptic and extrasynaptic receptors (Papouin et al., 2012). Whether modulating D-serine and glycine simultaneously can activate NMDA-related functions, such as cognitive function,
more efficiently than one approach alone remains unclear. The feasibility of combining two NMDA-enhancement approaches by inhibiting both GlyT-1 and DAAO deserves further studies.
To date, few studies have applied comprehensive cognitive measures, such as the domains recommended by the Measurement and Treatment Research to Improve
Cognition in Schizophrenia (MATRICS) committee (Green et al., 2011), to assess the pro-cognitive effects of NMDA enhancing agents (D'Souza et al., 2013,
Kantrowitz et al., 2010, Lane et al., 2013). This study compared the cognitive and clinical efficacy as well as safety of add-on sarcosine plus benzoate vs. sarcosine in patients with chronically stable schizophrenia in a 12-week, placebo-controlled trial.
Methods
Ethical approval was obtained from the Institutional Review Board. Patients with chronic schizophrenia were recruited from the inpatient units of the Department
of Psychiatry, Changhua Hospital, Taiwan. All patients provided written informed consent following a complete description of the study. Patients were enrolled in this study if they (1) were aged from 18 to 60 years, (2) were physically healthy and had
laboratory assessments (including urine/blood routine, biochemical tests, and electrocardiograph) within normal limits, (3) fulfilled the diagnosis of schizophrenia
according to the criteria of the Diagnostic and Statistical Manual of Mental
Disorders-Fourth Edition (DSM-IV) (American Psychiatric Association, 1994 ) (4) remained symptomatic but without clinically significant fluctuation and the
antipsychotic doses were unchanged for at least 2 months prior to this study, (5) had a minimum baseline total score of 60 on the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Exclusion criteria included DSM-IV (American
Psychiatric Association, 1994 ) diagnosis of mental retardation, substance/alcohol abuse or dependence, history of epilepsy, head trauma or CNS diseases, pregnancy or breast-feeding, and an inability to follow the protocol.
Study design
All subjects had been receiving a balanced hospital diet and unchanged institutionalization before and during the trial. After achieving optimal clinical
treatment response, patients’ antipsychotic doses remained constant for at least 2 months prior to enrolment into the study and during the study period. All patients
were randomly assigned to receive the 12-week trial of stable antipsychotic
regimens concomitant with placebo, sarcosine (2 g/day), or sarcosine (2 g/day) plus benzoate (1 g/day) in a 1:1:1 ratio (Figure). These doses were safely used in
previous trials (Lane et al., 2005, Lane et al., 2013, Tsai et al., 2004). Study medications were given twice daily and were provided in coded containers with a
supply of identical in appearance capsules of placebo or either of the active compounds. Patients, caregivers, and investigators (except for the investigational pharmacist) were all masked to the assignment. Patient’s adherence and safety were
closely monitored by the research psychiatrists and the nursing staff.
Measurement of clinical symptoms
Clinical assessments were measured with the PANSS, Global Assessment of Functioning (GAF) (American Psychiatric Association, 1994) , and the Clinical Global Impression-Severity scale (CGI-S) (Guy, 2000) at weeks 0, 3, 6, 9, and 12, and cognitive function was measured at the baseline and at the endpoint. The ratings
scales. Inter-rater reliability was analyzed with the ANOVA test. Only raters reaching the intra-class correlation coefficients of >0.90 during pre-study training
were allowed to rate the study patients.
Primary outcome measures were PANSS total, global composite and
neurocognitive composite of 7 cognitive domains (see below); secondary outcome measures were the 3 PANSS subscales, CGI, GAF, and 7 cognitive domains (see
below).
Measurement of cognitive function
This study was started before a commercial Chinese version of Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) was available. Cognitive function in the
current study was assessed using a battery of tests, which were the same tests or the analogues of tests from MCCB (Green et al., 2004), due to the lack of Chinese
versions of some tests. The tests utilized in this study were reviewed by one of the developers of the MCCB, Dr. Green, in our previous study (Lane et al., 2013). There were seven domains: (1) speed of processing (consisting of 3 tests: Category
Fluency, Trail Marking A, and WAIS-III Digit Symbol-Coding); (2) sustained attention (Continuous Performance Test) (Chen et al., 1998, Sternberg, 1966); (3)
working memory, verbal (backward digit span Silver 2003) and nonverbal (WMS-III, Spatial Span); (4) verbal learning and memory (WMS-(WMS-III, word listing); (5) visual learning and memory (WMS-III, visual reproduction); (6) reasoning and problem solving (WISC-III, Maze), and (7) social cognition (the Mayer–Salovey–
Caruso Emotional Intelligence Test [MSCEIT] Version 2) (Green et al., 2005, Mayer et al., 2003). The Chinese version of the MSCEIT tasks was translated and
back translated from English to Mandarin Chinese with satisfactory reliability, validity (Ma et al., 2012), and applicability (Lin et al., 2013). The first six domains were defined as neurocognition, the processes of linking and appraising information, while the 7th domain, social cognition, was defined as the mental operations
underlying social interactions such as the perception, interpretation, and generation of responses to the intentions and behaviors of others (Mayer et al., 2003).
Cognitive performance data of 78 healthy comparison participants (HC), matched with patients in age (p=0.11) and gender (p=0.71), were collected. Inclusion criteria for HC participants included no Axis I or Axis II psychiatric disorder, no neurological illness, no substance dependence or abuse, no family history of major psychiatric disorder, good general physical health, and age between
18 and 60 years. Participants provided written informed consent after the test procedures were fully explained. All cognitive raw scores of schizophrenia patients
were standardized to T scores based on the data of the 78 HC participants. For the cognitive domain that included more than one test, the summary score for the domain was calculated by summing the T scores of the tests included in that domain
and then standardizing the sum to a T score (Kern et al., 2008). A global composite score (for all 7 domains) and a neurocognitive composite score (for the 6
neurocognitive domains without social cognition) were also calculated.
Measurement of safety
Side-effect assessments included the Simpson-Angus Rating Scale (Simpson and Angus, 1970) for extrapyramidal side effects, the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia (Guy, 1976), the Barnes Akathisia Scale
(Barnes, 1989) and Udvalg for Kliniske Undersogelser (UKU) Side-effects Rating Scale (Lingjærde et al., 1987) for systemic side effects. These assessments were conducted at weeks 0, 3, 6, 9, and 12. Routine laboratory tests, including complete blood count (CBC), biochemistry, urine routine, and electrocardiogram were
arranged at the baseline and endpoint.
Statistical analysis
At baseline, the demographic and clinical characteristics, including age, gender, years of education, duration of illness, classification of current
antipsychotics, number of previous hospitalizations, the number of poor responses to antipsychotics, daily antipsychotic dose, severity of clinical symptoms, and
cognitive function were compared by Kruskal-Wallis tests for continuous variables and Pearson’s Chi-square (χ2) tests or Fisher’s exact tests for categorical variables.
The effects of the study drugs on the changes in PANSS, GAF, CGI-S, and MATRIC T scores from the baseline to endpoint were assessed according to the linear mixed effects’ model with treatment, visit and treatment-visit interaction as
fixed effects; baseline value as the covariate (to adjust the effect of baseline
severity); the intercept is the only random effect (to adjust the individual’s effect). The autoregressive of order 1, named AR(1), was used as the covariance type to specify the within-patients’ dependence due to repeated measurements from the same patient. In addition to the placebo group, the sarcosine group was also set as a reference group to compare the differences in clinical and cognitive change between
the sarcosine group and the sarcosine plus benzoate group. All data were analyzed by the SPSS 18.0 statistical package. All p values were based on two-tailed tests
with a significance level of 0.05.
Patients’ characteristics
To enhance drug adherence and minimize environmental factors, we enrolled inpatients in this study. A total of 75 patients were screened. Among them, 63 were eligible and randomized: 21 received placebo; 21, sarcosine; and 21, sarcosine plus benzoate (Figure). The mean age of the 63 patients was 38.4 ± 9.3 (SD) years, the duration of illness was 13.7 ± 6.7 years, the education duration was 11.1 ± 2.7 years,
the number of previous hospitalizations was 3.6 ± 5.6, the number of poor response to antipsychotics, defined as records of failure to achieve clinical improvement after > 8 weeks treatment of an antipsychotic agent, was 4.1 ±1.1, and antipsychotic dose was 13.7 ± 5.6 mg/day (olanzapine-equivalent) (Gardner et al., 2010). Patients’ characteristics at the baseline were similar among the three treatment groups (Table
1).
In total, 49 of them completed the trial: 16 received placebo; 16, sarcosine; and 17, sarcosine plus benzoate (Figure). There was no significant baseline difference between the completers and the patients who dropped out (Supplementary Table
1). Moreover, the characteristics of the three groups of completers were also similar (Supplementary Table 1).
PANSS, GAF and CGI-S
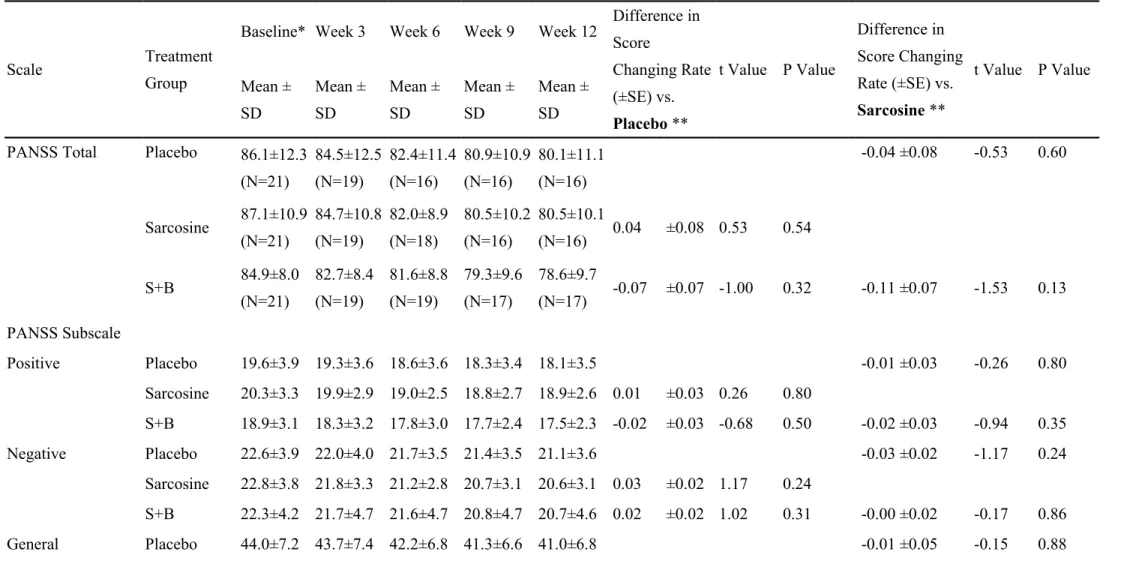
Of all the 63 patients, their PANSS, GAF, and CGI-S at the baseline were similar among the three treatment groups (Table 2). The sarcosine plus benzoate group was better than the placebo group in improving the GAF (mean difference in score changing rate [±SE] = 0.16 ± 0.06, P = 0.005) after 12 weeks of treatment; however, there was no significant group difference in improvement in PANSS, and CGI-S scores (Table 2).
Of the 49 completers, their PANSS, GAF, and CGI-S at the baseline were also similar among the three treatment groups (Supplementary Table S2). Likewise, the sarcosine plus benzoate group was also better than the placebo group in improving
GAF after 12 weeks of treatment; however, there was no significant group difference in improvement in PANSS, and CGI-S scores (Supplementary Table S2).
Cognitive battery
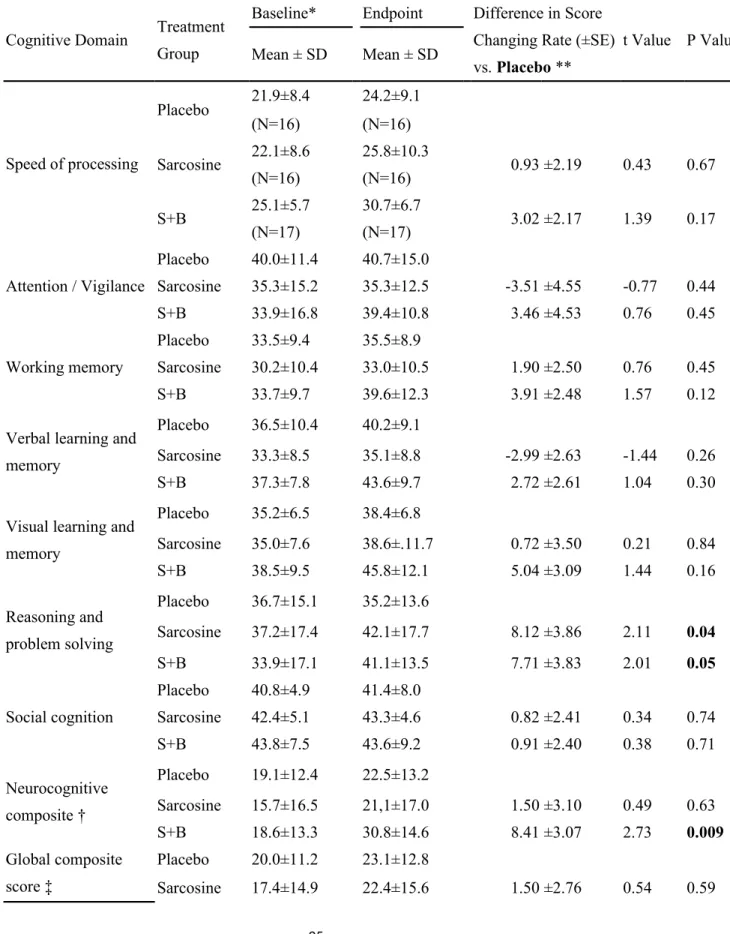
Table 3 presents the cognitive functions among three treatment groups over the 12-week treatment. The cognitive function of the patients who did not complete the 12-week study was not measured at the endpoint. Therefore, the improvement in cognitive function was compared among the 49 completers. In line with previous research (Corbera et al., 2013, McCleery et al., 2014), schizophrenia patients had
lower baseline cognitive performance than HC participants (not shown). Similarly to previous report on the psychometric properties of multiple cognitive domains like the MATRICS Consensus Cognitive Battery (Kern et al., 2011), standardized scores
from domains measured by more than one test tended to be lower than domains measured by a single test (Table 3). Moreover, the patients in this study exhibited severe levels of cognitive impairment in the neurocognitive composite and global
composite score (Table 3). These results were consistent with those reported by McCleery et al. (McCleery et al., 2014). After 12 weeks, the sarcosine plus benzoate group was superior to the placebo group in improving global composite cognition
(mean difference in score changing rate [±SE] = 7.77 ± 2.74, P = 0.007) and neurocognitive composite (mean difference in score changing rate [±SE] = 8.41 ±
3.07, P = 0.009). The sarcosine plus benzoate group was modestly superior to the sarcosine group in improving global composite cognition (mean difference in score changing rate [±SE] =6.37 ± 2.86, P = 0.03), neurocognitive composite score (mean
difference in score changing rate [±SE] =7.02 ± 2.42, P=0.03), and verbal learning and memory (mean difference in score changing rate [±SE] =5.67 ± 2.72, P = 0.04).
The sarcosine group was modestly superior to the placebo group in improving reasoning and problem solving (mean difference in score changing rate [±SE] =8.12
± 3.86, P = 0.04) (Table 3).
Side effects
The three treatment groups had minimal extrapyramidal syndrome at the beginning of the study. The baseline score on the Simpson-Angus Rating Scale was
0.7 ± 1.8 in the placebo group, 0.2 ± 0.7 in the sarcosine group and 1.0 ± 1.0 in the sarcosine plus benzoate group. The baseline AIMS was 0.2 ± 1.1 in the placebo group, 0.2 ± 0.7 in the sarcosine group, and 0.1 ± 0.4 in the sarcosine plus benzoate group. The baseline Barnes Akathesia Scale was 0.5 ± 1.5 in the placebo group, 0.2
± 0.6 in the sarcosine group, and 0.1 ± 0.4 in the sarcosine plus benzoate group. There were no significant differences among the three groups in the Simpson-Angus
Rating Scale (P = 0.11), AIMS (P = 0.79), and Barnes Akathesia score (P = 0.56). At the endpoint, the severity of extrapyramidal syndrome remained minimal and did not reveal significant differences among the three groups. The mean of the Simpson-Angus Rating Scale at the endpoint was 0.4 ± 1.3 in the placebo group, 0 ±
0 in the sarcosine group, and 0.4 ± 0.7 in the sarcosine plus benzoate group (P = 0.14). The endpoint AIMS was 0 ± 0 in the placebo group, 0.1 ± 0.5 in the sarcosine
group, and 0 ± 0 in the sarcosine plus benzoate group (P = 0.36). The endpoint Barnes Akathesia score was 0.6 ±1.8 in the placebo group, 0.1 ± 0.5 in the sarcosine
Treatment-emergent adverse events other than extrapyramidal syndrome in the placebo group included increased dream activity (N = 2), micturition disturbance (N = 1), increased tendency to sweating (N = 1), pruritus (N = 1), weight gain (N = 2),
weight loss (N = 1), and increased sexual desire (N = 1); in the sarcosine group, increased tendency to sweating (N = 1), increased dream activity (N = 1), weight
gain (N = 1), and weight loss (N = 2); in the sarcosine plus benzoate group,
increased sleepiness (N = 1), increased duration of sleep (N = 2), weight gain (N = 1), and weight loss (N = 1). These systemic side effects were all mild and brief, not warranting medical treatment. They were likely coincidental.
The routine blood cell count, biochemistry, urine routine, and
electrocardiogram after treatment remained unchanged (data not shown). No dropout occurred due to adverse effects.
Discussion
Cognitive impairment, a core feature throughout the course of schizophrenia, is related to functional outcome. However, it is poorly managed with currently
available antipsychotics (Allott et al., 2011, Green et al., 2000, Mohamed et al., 2008). Moreover, there have been few positive responses from putative
pro-cognitive drugs in humans (Millan et al., 2012, Preskorn et al., 2014). The present study suggests that adjunctive sarcosine plus benzoate, but not sarcosine alone, can improve the cognitive and global functioning of patients with chronic schizophrenia,
even when their clinical symptoms cannot be improved. This finding lends support to the previous notion that NMDA-enhancement therapy can improve the
neurocognition of patients with schizophrenia (Kantrowitz et al., 2010, Lane et al., 2013) and further indicates that this pro-cognitive effect can be primary without improvement of the clinical outcome. Although it may not achieve a striking clinical difference, the effect of sarcosine plus benzoate on GAF was statistically significant
after 12 weeks. It has been suggested that the global functioning of patients with schizophrenia may be related to cognitive function rather than to clinical symptoms
(Carrión et al., 2011, Green et al., 2011). This result indicates that pro-cognitive effects may reflect in the improvement of global functioning.
This clincial trial is the first to examine the combination effect of a DAAO inhibitor and a GlyT-1 inhibitor on symptomatic or cognitive domains of
schizophrenia. The finding on its safety is akin to that of previous studies (Lane et al., 2005, Lane et al., 2006, Lane et al., 2013, Lane et al., 2010, Tsai et al., 2004), indicating add-on sarcosine and sarcosine plus benzoate are well tolerated. These mild side effects were likely coincidental observations because there was no
by the high doses that must be given or the relatively poor penetration of the CNS (Javitt, 2008). Therefore, combining two kinds of NMDA receptor-enhancing agents
is of a great interest in research (Labrie and Roder, 2010). For example, D-serine in combination with a DAAO antagonist produced greater ameliorative effects than either compound applied alone in animals (Hashimoto et al., 2009). Previous pre-clinical and pre-clinical studies supported compounds providing selective modulation of
the NMDA receptor D-serine/glycine site such as diminishing D-serine catabolism by inhibition of DAAO or stimulating the glycine modulatory sites by blocking
GlyT-1 could demonstrate beneficial effects on schizophrenia (Tsai and Lin, 2009). Our study supports that coadministration of GlyT-1 and DAAO inhibitors could provide a potential therapeutic approach. However, there has not yet been a preclinical study examining the combination effect of a DAAO inhibitor, such as
benzoate, and a GlyT-1 inhibitor, such as sarcosine in the NMDA receptor models of cognitive impairment associated with schizophrenia (CIAS). Further studies are warranted.
Although there was no significant improvement in global composite score from the sarcosine add-on treatment, the sarcosine group also displayed improved
reasoning and problem solving (p value of 0.04) when compared to the placebo group. Therefore, it remains possible whether a dose higher than the dose in the current study, to reach a higher level of NMDA activation, would generate a better response (Kantrowitz et al., 2010, Lane et al., 2008).
The current result shows that adjunctive sarcosine plus benzoate improved the cognitive function of patients but not their negative symptoms. The relationships between positive, negative and cognitive symptoms have been equivocal in both cross-sectional and longitudinal studies. While some studies suggest that cognitive deficits are modestly associated with negative symptoms (Berman et al., 1997, Savilla et al., 2008), no significant associations between changes in negative
symptoms and cognitive impairment have been raised (Bell and Mishara, 2006, Umbricht et al., 2014). Our current result supports the notion that negative symptoms and cognition should be viewed as independent targets for intervention (Nasrallah et al., 2014). As in benzoate alone treatment (Lane et al., 2013), the study
drugs in the current study did not affect social cognition. This may reflect the multi-determined nature of the social cognitive domain (Couture et al., 2006) and may be related to the inadequate study duration. The current results encourage further study of longer treatment duration and NMDA-enhancement therapy plus social
rehabilitation in patients with chronic schizophrenia.
Different from most sarcosine or benzoate trials, the current study showed that all three groups have modest improvement in clinical manifestation; however, the
improvements by sarcosine or sarcosine plus benzoate treatment were similar to that of the placebo group. In comparison with two previous sarcosine trials which showed effects on clinical symptoms, including positive and negative symptoms, in chronic schizophrenia, the current subjects tended to be older [38.4 (mean value) y/o
vs. 31.8 y/o (Tsai et al., 2004) and 30.9 y/o (Lane et al., 2010)], have longer illness durations [14.7 years vs. 9.6 years (Tsai et al., 2004) and 9.5 years (Lane et al., 2010)], and have more hospitalizations [(3.6 vs. 2.8 (Lane et al., 2010)]. The age and
illness duration of the current study subjects were closer to those in the trial of sarcosine added to clozapine, which failed to reduce positive and negative symptoms of schizophrenia [38.4 y/o vs. 36.1 y/o and 14.7 years vs. 14.9 years] (Lane et al.,
2006), suggesting that adjunctive 2 g/day sarcosine reveals limited efficacy in older patients and those with longer illness duration.
A recent study (Lane et al. 2013) demonstrated that benzoate was beneficial for patients with schizophrenia. The age, illness duration, and previous number of hospitalizations (38.4 y/o, 13.7 years and 3.2, respectively) of the current study
subjects were similar to those (37.3 y/o, 14.7 years, and 3.6, respectively) in the previous trial of benzoate (Lane et al., 2013). However, the current study showed that sarcosine plus benzoate had limited effects in patients who had failed to achieve clinical improvement with multiple trials of antipsychotic agents. Without a group of only add-on benzoate, it remains unclear whether sarcosine plus benzoate may have generated overactive neurotransmission; thereby somewhat hampering the beneficial effect of benzoate per se. Studies using only add-on benzoate is necessary to clarify this issue.
Although the precise mechanisms are unknown, the involvement of
neuroimmune dysregulation and the glutamatergic system in the pathophysiology of schizophrenia is intriguing (Heresco-Levy et al., 2015, Steiner et al., 2012). Agents with both anti-inflammatory and glutamatergic transmission modification properties
are promising in treatment of schizophrenia (Hashimoto, 2014). It was reported that sodium benzoate can suppress the mevalonate pathway and reduce microglial and
astroglial inflammatory responses (Brahmachari et al., 2009). Whether patients can benefit from benzoate treatment through both the anti-inflammatory and NMDA receptor-mediated pathways deserves further investigation.
This study has several limitations. First, there were only three study arms:
placebo, sarcosine, and sarcosine plus benzoate. Therefore, comparisons of benzoate vs. the current three arms remain unclear. For example, whether sarcosine plus benzoate is superior or equal to benzoate alone is unknown. That is, whether there is a synergistic or additive effect from sarcosine plus benzoate remains to be
patients in a single psychiatric hospital; therefore the generalizability of the findings would be limited. Studies focusing on subjects who are younger, at an earlier stage of illness, or have less severe psychotic symptoms are necessary. Third, the changes in blood levels of amino acids such as glycine and D-serine after treatment were not measured. Further studies should be conducted. Fourth, the treatment duration of 12
weeks may have been insufficient for assessment of the efficacy and safety for patients with chronic schizophrenia. Fifth, the sarcosine plus benzoate group tended
to have more females and a shorter duration of illness, albeit insignificantly, which might have influenced the practice effect on cognitive testing. Sixth, we did not follow cognitive function at a time point post the trial to examine the sustained influence of NMDA-enhancement treatment after the subjects discontinued the
study drugs. Finally, the study was started before a commercial Chinese version of MCCB was available; therefore the T scores in the current study could not be compared with other studies directly. Further trials which refer to the normative data
for Chinese version of MCCB should be encouraged.
In conclusion, a combination of NMDA-enhancing agents (sarcosine and benzoate), but not sarcosine alone, can improve cognitive function in patients with chronic schizophrenia, even when their clinical symptoms cannot be improved.
These findings support the NMDA theory of cognitive impairment in schizophrenia (Hashimoto et al., 2013, Javitt et al., 2012, Krystal et al., 1994, Lin et al., 2014b, Paoletti et al., 2013). Future larger-sized studies in other racial populations are
warranted.
Acknowledgements
This study is supported in part by Hospital Administration Commission,
Ministry of Health and Welfare, Taiwan (9814; 9914); Ministry of Science and
Technology (NSC-101-2314-B-039-030-MY3; MOST 103-2325-B-039-005);
Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of
Excellence (MOHW103-TDU-B-212-113002); Chang-Hua Hospital Program Grant
collection, management, analysis, and interpretation of the data; or preparation,
review, or approval of the manuscript.
Statement of Interest
Dr. Tsai is a director and shareholder of SyneuRx International Corporation,
which plans to develop glycine transporter-1 inhibitor and D-amino acid oxidase
inhibitors, including sodium benzoate, for the treatment of central nervous system
disorders. SyneuRx International Corporation was not involved in the funding or
execution of the study. All other authors report no biomedical financial interests or
potential conflicts of interest.
Figure legend:
Figure. Flow chart of the subjects throughout the study period.
75 schizophrenia patients were screened. 63 were eligible and randomized: 21 received placebo; 21, sarcosine; and 21, sarcosine plus benzoate. Participants were measured with Positive and Negative Syndrome Scale (PANSS) and Global
Assessment of Functioning (GAF) every 3 weeks. Cognitive domains recommended by the Measurement and Treatment Research to Improve Cognition in
Schizophrenia (MATRICS) committee, were measured at weeks 0 and 12. Side-effect assessments including the Simpson-Angus Rating Scale, the Abnormal Involuntary Movement Scale (AIMS), the Barnes Akathisia Scale and Udvalg for
Kliniske Undersogelser (UKU) Side-effects Rating Scale, were conducted at weeks 0, 3, 6, 9, and 12. Routine laboratory tests were arranged at the baseline and
endpoint. Totally, 49 of the participants completed the trial: 16 received placebo; 16, sarcosine; and 17, sarcosine plus benzoate.
References
Allott K, Liu P, Proffitt T-M, Killackey E. 2011. Cognition at illness onset as a predictor of later functional outcome in early psychosis: Systematic review and methodological critique. Schizophr Res 125: 221-235.
American Psychiatric Association. 1994. Diagnostic and stastistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Press. Bado P, Madeira C, Vargas-Lopes C, Moulin T, Wasilewska-Sampaio A, Maretti L,
et al. 2011. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology (Berl) 218: 461-470.
Balu DT, Coyle JT. 2014. Chronic D-serine reverses arc expression and partially rescues dendritic abnormalities in a mouse model of NMDA receptor
hypofunction. Neurochem Int 75: 76-78.
Barnes TR. 1989. A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672-676.
Bell MD & Mishara AL. 2006. Does negative symptom change relate to neurocognitive change in schizophrenia? Implications for targeted treatments. Schizophr Res 81: 17-27.
Berman I, Viegner B, Merson A, Allan E, Pappas D & Green, A I. 1997. Differential relationships between positive and negative symptoms and
neuropsychological deficits in schizophrenia. Schizophr Res 25: 1-10. Brahmachari S, Jana A & Pahan K. 2009. Sodium benzoate, a metabolite of
cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol 183: 5917-5927.
Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, et al. 2007. The Cognitive and Negative Symptoms in Schizophrenia Trial
(CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry 164: 1593-1602.
Burnet PW, Anderson PN, Chen L, Nikiforova N, Harrison PJ, Wood MJ. 2011. D-amino acid oxidase knockdown in the mouse cerebellum reduces NR2A mRNA. Mol Cell Neurosci 46: 167-175.
2011. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry 168: 806-813.
Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. 1998. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry 155: 1214-1220. Corbera S, Wexler BE, Ikezawa S & Bell MD. 2013. Factor Structure of Social
Cognition in Schizophrenia: Is Empathy Preserved? Schizophr Res Treatment. 2013, 409205.
Couture SM, Penn DL, Roberts DL. 2006. The functional significance of social cognition in schizophrenia: a review Schizophr Bull 32: s44-s63.
D'Souza DC, Radhakrishnan R, Perry E, Bhakta S, Singh NM, Yadav R, et al. 2013. Feasibility, safety, and efficacy of the combination of d-serine and
computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology 38: 492-503.
Errico F, Napolitano F, Squillace M, Vitucci D, Blasi G, de Bartolomeis A, et al. 2013. Decreased levels of D-aspartate and NMDA in the prefrontal cortex
and striatum of patients with schizophrenia. J Psychiatr Res 47: 1432-1437. FirstWord Pharma. 2014. Roche's bitopertin fails to meet main goals of late-stage
schizophrenia trials ; January 21st, 2014:[Available from:
http://www.firstwordpharma.com/node/1180713#axzz3En2j9x4U. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. 2010.
International consensus study of antipsychotic dosing. Am J Psychiatry 167: 686-693.
Green MF, Kern RS, Braff DL, Mintz J. 2000. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the "right stuff" ?
Schizophr Bull 26: 119-136.
Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. 2004. Approaching a consensus cognitive battery for clinical trials in
schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 56: 301-307.
Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. 2005. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference.
Schizophr Bull 31: 882-887.
Green MF, Schooler NR, Kern RS, Frese FJ, Granberry W, Harvey PD, et al. 2011. Evaluation of functionally meaningful measures for clinical trials of
cognition enhancement in schizophrenia. Am J Psychiatry 168: 400-407. Guy W. 1976. ECDEU Assessment Manual for Psychopharmacology: U.S. Dept. of
Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs.
Guy W. 2000. Clinical Global Impressions (CGI) Scale. Modified From: Rush J, et al.: Psychiatric Measures.Washington DC: APA.
Hashimoto K. 2014. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets 18: 1049-1063.
Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, et al. 2009. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of
D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry 65: 1103-1106.
Hashimoto K, Malchow B, Falkai P, Schmitt A. 2013. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 263: 367-377.
Heresco-Levy U, Durrant AR, Ermilov M, Javitt DC, Miya, K, Mori, H. 2015. Clinical and electrophysiological effects of D-serine in a schizophrenia patient positive for anti-N-methyl-D-aspartate receptor antibodies. Biol Psychiatry 77: e27-e29.
Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. 2004. High-dose glycine added to olanzapine and risperidone for the treatment of
schizophrenia. Biol Psychiatry 55: 165-171.
Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. 2005. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine
for treatment-refractory schizophrenia. Biol Psychiatry 57: 577-585. Javitt, DC. 2008. Glycine transport inhibitors and the treatment of schizophrenia.
Biol Psychiatry 63: 6-8.
Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, et al. 2001. Adjunctive high-dose glycine in the treatment of schizophrenia. Int J
Neuropsychopharmacol 4: 385-391.
Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. 2012. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38: 958-966.
Kantrowitz JT, Malhotra AK, Cornblatt B. 2010. High dose D-serine in treatment of schizophrenia. Schizophr Res 121: 125-130.
Kasper, S. 2006. Optimisation of long-term treatment in schizophrenia: Treating the true spectrum of symptoms. Eur Neuropsychopharmacol 16: Suppl 3, s135-s141.
Kay SR, Fiszbein A, Opler LA. 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261-276.
Kern, RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, et al. 2008. The MATRICS Consensus Cognitive Battery, Part 2: Co-Norming and
Standardization. Am J Psychiatry 165: 214-220.
Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, et al. 2011. The MCCB Impairment Profile for Schizophrenia Outpatients:Results from the MATRICS Psychometric and Standardization Study. Schizophr Res
126: 124-131
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in
humans. Arch Gen Psychiatry 51: 199-214.
Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. 2005. Sarcosine or D-serine add-on treatment for acute exacerbatiadd-on of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry 62: 1196-1204.
Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, et al. 2006. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry 60: 645-649.
Lane HY, Lin CH, Green MF, Hellemann G, Huang CC, Chen PW, et al. 2013. A randomized, double-blind, placebo-controlled add-on treatment of benzoate,
a D-amino acid oxidase inhibitor, for schizophrenia. JAMA Psychiatry 70: 1267-1275.
Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. 2010. A randomized, double-blind, placebo-controlled comparison study of sarcosine
(N-methylglycine) and d-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol 13: 451-460.
Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, et al. 2008. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a
randomized, double-blind study. Biol Psychiatry 63:9-12.
Levin R, Dor-Abarbanel AE, Edelman S, Durrant AR, Hashimoto K, Javitt DC, et al. 2015. Behavioral and cognitive effects of the N-methyl-D-aspartate receptor co-agonist D-serine in healthy humans: Initial findings. J Psychiatr
Res. 61: 188-195.
Lin CH, Chen PK, Chang YC, Chuo LJ, Chen YS, Tsai GE, et al. 2014a. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer
disease: A randomized, double-blind, placebo-controlled trial. Biol Psychiatry 75: 678-685.
effects of COMT and TPH2 on social cognition. Psychiatry 76: 273-294. Lin CY, Tsai GE, Lane HY. 2014b. Assessing and treating cognitive impairments in
schizophrenia: current and future. Curr Pharm Des 20:5127-5138.
Lingjærde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. 1987. The UKU side effect rating scale: A new comprehensive rating scale for psychotropic drugs and a
cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand 76: 1-100.
Ma WF, Tsai GE, Chang JP-C, Lane HY. 2012. Reliability and validity of three Chinese-version tasks of Mayer–Salovey–Caruso Emotional Intelligence
Test. J Clin Nurs 19: 2656-2658.
Matsuura A, Fujita Y, Iyo M. & Hashimoto K. 2015. Effects of sodium benzoate on pre-pulse inhibition deficits and hyperlocomotion in mice after
administration of phencyclidine.Acta Neuropsychiatr. 27: 159-167. Mayer JD, Salovey P, Caruso DR, Sitarenios G. 2003. Measuring emotional
intelligence with the MSCEIT V2.0. Emotion 3: 97-105.
McBain CJ, Kleckner NW, Wyrick S, Dingledine R. 1989. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Mol Pharmacol 36: 556-565.
McCleery A, Ventura J, Kern RS, Subotnik KL, Gretchen-Doorly D, Green MF, et al. 2014. Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr Res
157: 33-39.
Meltzer HY. 2013. Update on typical and atypical antipsychotic drugs. Annu Rev Med 64: 393-406.
Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. 2012. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11: 141-168.
Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman J, Keefe R. 2008. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry 165: 978-987.
Nasrallah HA, Keefe RSE & Javitt DC. 2014. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry 13: s1-s11.
Paoletti P, Bellone C, Zhou Q. 2013. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383-400.
Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. 2012. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different
Endogenous Coagonists. Cell 150: 633-646.
Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt D. 2014. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on
event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract 20: 12-24.
Savilla K, Kettler L & Galletly C. 2008. Relationships Between Cognitive Deficits, Symptoms and Quality of Life in Schizophrenia. Aust N Z J Psychiatry 42: 496-504.
Sawa A. 2009. Cortical Development and Glutamatergic Dysregulation in Schizophrenia. Biol Psychiatry 66: 530-532.
Schultz SK, Andreasen NC. 1999. Schizophrenia. Lancet 353: 1425-1430.
Sergi MJ, Green MF, Widmark C, Reist C, Erhart S, Braff DL, et al. 2007. Social cognition and neurocognition: effects of risperidone, olanzapine, and
haloperidol. Am J Psychiatry 164: 1585-1592.
Simpson GM, Angus JW. 1970. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11-19.
Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, et al. 2012. Bridging the gap between the immune and glutamate hypotheses of
schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol
Psychiatry 13: 482-492.
Sternberg S. 1996. High-speed scanning in human memory Science 153: 652-654. Tsai GE, Lane HY, Yang P, Chong MY, Lange N. 2004. Glycine transporter I
inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 55: 452-456.
Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. 2009. Curr Pharm Des 16: 522-537.
Tsai GE, Yang P, Chung LC, Lange N, Coyle JT. 1998. D-serine added to
antipsychotics for the treatment of schizophrenia. Biol Psychiatry 44: 1081-1089.
Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, et al. 2014. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: A randomized, double-blind, proof-of-concept
study. JAMA Psychiatry 71: 637-646.
Van den Berghe-Snorek S, Stankovich MT. 1985. Thermodynamic control of D-amino acid oxidase by benzoate binding. J Biol Chem 260: 3373-3379. Verrall L, Burnet PW, Betts JF, Harrison PJ. 2010. The neurobiology of D-amino
acid oxidase and its involvement in schizophrenia. Mol Psychiatry 15: 122-137.
Weiser M, Heresco-Levy U, Davidson M, Javitt DC, Werbeloff N, Gershon AA, et al. 2012. A multicenter, adon randomized controlled trial of low-dose
d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry 73: e728-e734.
Tables
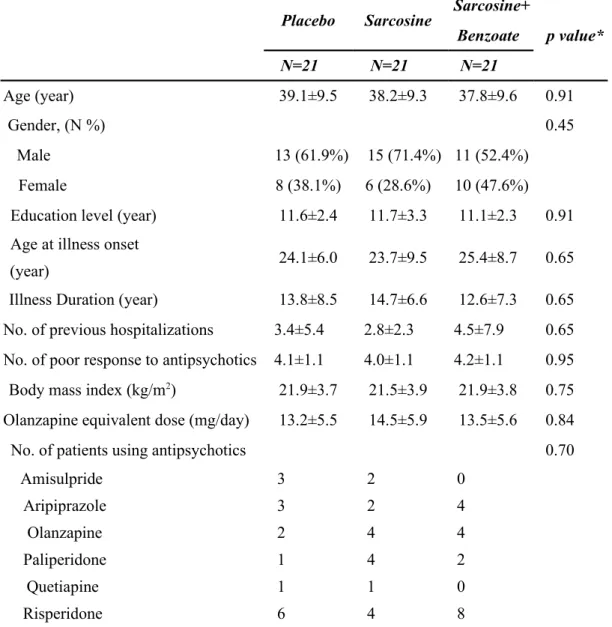
Table 1. Baseline characteristics of the 63 patients randomly assigned to three treatment groups
Placebo Sarcosine Sarcosine+
p value* Benzoate N=21 N=21 N=21 Age (year) 39.1±9.5 38.2±9.3 37.8±9.6 0.91 Gender, (N %) 0.45 Male 13 (61.9%) 15 (71.4%) 11 (52.4%) Female 8 (38.1%) 6 (28.6%) 10 (47.6%)
Education level (year) 11.6±2.4 11.7±3.3 11.1±2.3 0.91 Age at illness onset
(year) 24.1±6.0 23.7±9.5 25.4±8.7 0.65
Illness Duration (year) 13.8±8.5 14.7±6.6 12.6±7.3 0.65 No. of previous hospitalizations 3.4±5.4 2.8±2.3 4.5±7.9 0.65 No. of poor response to antipsychotics 4.1±1.1 4.0±1.1 4.2±1.1 0.95 Body mass index (kg/m2) 21.9±3.7 21.5±3.9 21.9±3.8 0.75 Olanzapine equivalent dose (mg/day) 13.2±5.5 14.5±5.9 13.5±5.6 0.84
No. of patients using antipsychotics 0.70
Amisulpride 3 2 0 Aripiprazole 3 2 4 Olanzapine 2 4 4 Paliperidone 1 4 2 Quetiapine 1 1 0 Risperidone 6 4 8
Zotepine 5 4 3
* Kruskal-Wallis tests for continuous variables, Pearson’s chi-square (χ2) tests for gender variable and Fisher's Exact test for No. of patients using antipsychotics
Table 2. Clinical measures over the 12-week treatment with the comparisons of changing rates among the three treatment groups of for all the 63 patients.
Scale Treatment
Group
Baseline* Week 3 Week 6 Week 9 Week 12 Difference in Score Changing Rate (±SE) vs. Placebo ** t Value P Value Difference in Score Changing Rate (±SE) vs. Sarcosine ** t Value P Value Mean ± SD Mean ± SD Mean ± SD Mean ± SD Mean ± SD PANSS Total Placebo 86.1±12.3
(N=21) 84.5±12.5 (N=19) 82.4±11.4 (N=16) 80.9±10.9 (N=16) 80.1±11.1 (N=16) -0.04 ±0.08 -0.53 0.60 Sarcosine 87.1±10.9 (N=21) 84.7±10.8 (N=19) 82.0±8.9 (N=18) 80.5±10.2 (N=16) 80.5±10.1 (N=16) 0.04 ±0.08 0.53 0.54 S+B 84.9±8.0 (N=21) 82.7±8.4 (N=19) 81.6±8.8 (N=19) 79.3±9.6 (N=17) 78.6±9.7 (N=17) -0.07 ±0.07 -1.00 0.32 -0.11 ±0.07 -1.53 0.13 PANSS Subscale Positive Placebo 19.6±3.9 19.3±3.6 18.6±3.6 18.3±3.4 18.1±3.5 -0.01 ±0.03 -0.26 0.80 Sarcosine 20.3±3.3 19.9±2.9 19.0±2.5 18.8±2.7 18.9±2.6 0.01 ±0.03 0.26 0.80 S+B 18.9±3.1 18.3±3.2 17.8±3.0 17.7±2.4 17.5±2.3 -0.02 ±0.03 -0.68 0.50 -0.02 ±0.03 -0.94 0.35 Negative Placebo 22.6±3.9 22.0±4.0 21.7±3.5 21.4±3.5 21.1±3.6 -0.03 ±0.02 -1.17 0.24 Sarcosine 22.8±3.8 21.8±3.3 21.2±2.8 20.7±3.1 20.6±3.1 0.03 ±0.02 1.17 0.24 S+B 22.3±4.2 21.7±4.7 21.6±4.7 20.8±4.7 20.7±4.6 0.02 ±0.02 1.02 0.31 -0.00 ±0.02 -0.17 0.86 General Placebo 44.0±7.2 43.7±7.4 42.2±6.8 41.3±6.6 41.0±6.8 -0.01 ±0.05 -0.15 0.88
psychopathology Sarcosine 44.0±6.1 43.0±6.0 41.8±5.3 41.0±6.1 41.1±6.2 0.01 ±0.05 0.15 0.88 S+B 43.7±3.6 42.6±3.8 42.1±4.1 40.9±4.5 40.4±4.6 -0.07 ±0.05 -1.54 0.13 -0.08 ±0.05 -1.70 0.09 GAF Placebo 48.9±7.6 49.8±7.1 51.8±5.3 52.0±5.2 52.4±5.2 -0.06 ±0.06 -1.00 0.32 Sarcosine 47.0±7.2 47.2±6.6 48.6±5.9 48.7±6.8 48.8±7.0 0.06 ±0.06 1.00 0.32 S+B 47.0±7.1 48.5±6.7 49.1±6.7 51.1±6.3 51.4±6.5 0.16 ±0.06 2.85 0.005 0.10 ±0.06 1.85 0.066 CGI Placebo 4.0±0.5 3.9±0.6 4.0±0.5 3.9±0.6 3.9±0.6 0.01 ±0.01 0.91 0.36 Sarcosine 4.1±0.5 4.0±0.5 3.9±0.5 4.0±0.5 3.9±0.5 -0.01 ±0.01 -0.91 0.36 S+B 3.8±0.5 3.8±0.5 3.8±0.5 3.7±0.6 3.7±0.6 0.00 ±0.01 0.70 0.48 0.01 ±0.01 1.63 0.11 *Clinical severity at baseline was similar among the three treatment groups by Kruskal-Wallis tests (PANSS-Total, P=0.75; PANSS-Positive, P=0.54; PANSS-Negative, P=0.77; PANSS-General psychopathology, P=0.87; GAF, P=0.41; CGI, P=0.09)
** Mixed-model repeated measure (MMRM) methods analysis with treatment, visit and treatment-visit interaction as fixed effects and intercept as random effect; baseline value as the covariance. An autoregressive AR(1) covariance matrix was fit to the within-patient repeated measures. P values were based on two-tailed tests.
PANSS: Positive and Negative Syndrome Scale GAF: Global Assessment Function;
CGI: The Clinical Global Impression - Severity scale S+B: Sarcosine+Benzoate
Lin et al Sarcosine plus Benzoate in Schizophrenia Patients
Table 3. Cognitive function measured with the 7 domains recommended by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Committee over the 12-week treatment among three treatment groups of the 49 completers, who had both baseline and endpoint cognitive assessments.
Cognitive Domain Treatment Group
Baseline* Endpoint Difference in Score Changing Rate (±SE) vs. Placebo **
t Value P Value
Difference in Score Changing Rate (±SE) vs. Mean ± SD Mean ± SD Speed of processing Placebo 21.9±8.4 24.2±9.1 (N=16) (N=16) Sarcosine 22.1±8.6 25.8±10.3 0.93 ±2.19 0.43 0.67 (N=16) (N=16) S+B 25.1±5.7 30.7±6.7 3.02 ±2.17 1.39 0.17 (N=17) (N=17) Attention / Vigilance Placebo 40.0±11.4 40.7±15.0 Sarcosine 35.3±15.2 35.3±12.5 -3.51 ±4.55 -0.77 0.44 S+B 33.9±16.8 39.4±10.8 3.46 ±4.53 0.76 0.45 Working memory Placebo 33.5±9.4 35.5±8.9 Sarcosine 30.2±10.4 33.0±10.5 1.90 ±2.50 0.76 0.45 S+B 33.7±9.7 39.6±12.3 3.91 ±2.48 1.57 0.12
Verbal learning and memory
Placebo 36.5±10.4 40.2±9.1
Sarcosine 33.3±8.5 35.1±8.8 -2.99 ±2.63 -1.44 0.26
S+B 37.3±7.8 43.6±9.7 2.72 ±2.61 1.04 0.30
Visual learning and memory Placebo 35.2±6.5 38.4±6.8 Sarcosine 35.0±7.6 38.6±.11.7 0.72 ±3.50 0.21 0.84 S+B 38.5±9.5 45.8±12.1 5.04 ±3.09 1.44 0.16 Reasoning and problem solving Placebo 36.7±15.1 35.2±13.6 Sarcosine 37.2±17.4 42.1±17.7 8.12 ±3.86 2.11 0.04 S+B 33.9±17.1 41.1±13.5 7.71 ±3.83 2.01 0.05 Social cognition Placebo 40.8±4.9 41.4±8.0 Sarcosine 42.4±5.1 43.3±4.6 0.82 ±2.41 0.34 0.74 S+B 43.8±7.5 43.6±9.2 0.91 ±2.40 0.38 0.71 Neurocognitive composite † Placebo 19.1±12.4 22.5±13.2 Sarcosine 15.7±16.5 21,1±17.0 1.50 ±3.10 0.49 0.63 S+B 18.6±13.3 30.8±14.6 8.41 ±3.07 2.73 0.009 Global composite score ‡ Placebo 20.0±11.2 23.1±12.8 Sarcosine 17.4±14.9 22.4±15.6 1.50 ±2.76 0.54 0.59 25
Lin et al Sarcosine plus Benzoate in Schizophrenia Patients
S+B 20.4±11.4 31.1±14.0 7.77 ±2.74 2.84 0.007 Note:
All cognitive raw scores of patients were standardized to T scores based the data of 78 healthy comparison participants. For cognitive domains that included more than one measure, the summary score for the domain was calculated by summing the T scores of the tests included in that domain and then standardizing the sum to a T score (Kern et al., 2008).
†For assessing neurocognitive function, a composite t score including all 6
neurocognitive domains, excluding social cognition, was calculated by standardizing the sum of the t scores.
‡For assessing the global cognitive function, an overall composite t score including all 7 domains was calculated by standardizing the sum of the t scores.
*Cognitive performance at baseline was similar among the three treatment groups by Kruskal-Wallis tests (Speed of processing p=0.17;
Attention / Vigilance, p=0.69; Working memory, p=0.64; Verbal learning and memory, p=0.48; Visual learning and memory, p=0.50;
Reasoning and problem solving, p=0.77; Social cognition, p=0.38; Neurocognitive composite, p=0.80; Global composite score, p=0.75
** Mixed-model repeated measure (MMRM) methods, adjusted with age, gender, education, treatment and visit times. P values were based on two-tailed tests.