Effects of Forming Temperature on Structure and Chemical Properties
of Calcium Phosphate Cement
Ching-Wen Lou
1, b, Yung-Yu Chang
1, Ming-Chun Sie
1, Wen-Cheng Chen
2and
Jia-Horng Lin
3, 4, 5, a1Institute of Biomedical Engineering and Materials Science, Central Taiwan University of Science
and Technology, Taichung 40601, Taiwan, R.O.C.
2Advanced Medical Devices and Composite Laboratory, Department of Fiber and Composite
Materials, Feng Chia University, Taichung City 407, Taiwan, R.O.C.
3Laboratory of Fiber Application and Manufacturing, Department of Fiber and Composite Materials,
Feng Chia University, Taichung City 40724, Taiwan, R.O.C.
4School of Chinese Medicine, China Medical University, Taichung 40402, Taiwan, R.O.C. 5Department of Fashion Design, Asia University, Taichung 41354, Taiwan, R.O.C.
ajhlin@fcu.edu.tw, b cwlou@ctust.edu.tw Keywords: hydroxyapatite , Calcium phosphate cement, XRD, FTIR.
Abstract. With similar components to human bone, hydroxyapatite (HAp) has been commonly
used in dental and orthopedics practice. Calcium phosphate cement (CPC) can be made into porous HAp scaffolds through reaction; therefore, this study aims to examine how reaction temperatures influence CPC. CPC powder and NaCl are blended, and after diammonium phosphate (DAP) is added, they are infused into a mold for a hydration reaction under a saturated vapor pressure to form CPC scaffolds. XRD and FTIR are used to determine the influence of reaction temperatures on crystallization and functional groups. A scanning electron microscope (SEM) observes the structures of the CPC scaffolds. Test results show that when the temperature of a saturated vapor pressure is 70 ℃, the CPC scaffolds have significant crystalline conversion and functional group peaks. SEM observation indicates that the temperature of a saturated vapor pressure does not affect the formation of the CPC scaffolds’ structure.
Introduction
As of late, hydroxyapatite (HAp) with a porous structure has gained considerable attention, and has been widely applied in biomedical engineering, such as cell loading, drug-releasing agent, and hard tissue scaffolds; especially bone implants, which simulate the micro-porous and macro-porous structures of the mineral phase of living bones. Calcium phosphate ceramics (CPC) exist in various varieties, including HAp, tricalcium phosphate, and calcium phosphate cement. These ceramics are characterized by biocompatibility, bone bonding, and bone regeneration, and are commonly used in non- or low-load applications. Like other calcium phosphates, CPC has low mechanical properties. CPC is able to exhibit hardening at a low temperature, and thus the combination of protein and drugs with CPC matrices is feasible. Furthermore, the addition of different porogens enables the
CPC scaffolds to have greater porosity and various pore sizes, which makes CPC scaffolds more feasible for biomedical engineering applications. [2]. This study aims to examine the influence of different reaction temperatures on the structure and chemical properties of the CPC scaffolds. After the XRD analyses are identified by referring to the peaks of Joint Committee on Powder Diffraction Standard (JCPDS), it is confirmed that CPC scaffolds have turned into HAp after the reaction. In addition, the increasing temperature of a saturated vapor pressure helps the conversion of CPS. The analyses of FTIR test demonstrate the presence of P-O bonding, and furthermore, the comparison of FTIR and XRD test results can obtain HAp with a higher percentage for applications.
Experimental Material
Tetracalcium phosphate (TTCP) is purchased from Choneye Pure Chemicals, Taiwan, R.O.C. Dicalcium phosphate anhydrous (DCPA) is purchased from Alfa Aesar, A Johnson Matthey Company, U.S.A. (NH4)2HPO4 (diammonium phosphate, DAP) is purchased from Choneye Pure Chemicals, Taiwan, R.O.C. NaCl is purchased from Shimakyu’S Pure Chemical, Japan.
Procedure
CPC powder, composed of TTCP and DCPA, is blended with NaCl for two minutes, after which DAP solvent is added. The resulting mixture is poured into a stainless steel mold, processed at 50, 60, and 70 ℃of a saturated vapor pressure for 96 hours, and then demolded. The yielded products are dried at 45℃ in an oven for 2 hours, immersed in deionized water for 12 hours to remove NaCl, and then dried at 45℃ in an oven again for 12 hours to form CPC.
Tests
X-Ray Diffraction (XRD) The X-ray diffractometer (36 MXP3, Mac Science, Japan) uses copper as
the target (Cu Kα = 0.154 nm), and is operated at a voltage of 40kV and a current of 30mA. An X-ray diffractometer measures the crystalline phase of the samples and scans are performed between 20 values of 15 and 45 degrees at a scan speed of 0.5o/min. The results of XRD then refer to JCPDS spectrum for a comparison of HAp (JCPDS 9-432) and TTCP (JCPDS 25-1137). Fourier
Transform Infrared Spectroscopy (FT-IR) An ATR stage is mounted in a Fourier transform infrared
spectroscopy (FT-IR, IR Affinity-1, Shimadzu Corporation, Japan), and samples are placed on the stage and then pressed with a knob. The scan range is 600- 4000 cm-1 and twenty samples are examined. The result is then compared with the spectrum to determine the presence of functional groups or bonding. Sem Observation Samples are fixed on an aluminum stage for 30 seconds of gold sputtering, after which samples are placed in the SEM sample room. An SEM (HITACHI S3000N, Japan) with an accelerating voltage of 10 keV is used for structural observation and photo shooting.
Results and Discussion XRD Analyses
Figure 1 shows the influence of various reaction temperatures of the saturated vapor pressure on crystalline conversion of CPC scaffolds. Hydroxyapatite is a chief material of human bones, and it
has good osteophilic properties and osteoconduction, and as a result is a good substitute for impaired bones. The peak of TTCP appears at 28.4° and 29.3° [3], and that of HAp appears at 26.2° and 32.2° [4]. According to the results of the previous study, CPC scaffolds reach a complete hardening after they solidify for 4 days [5]. An increasing temperature of a saturated vapor pressure results in a decreased peak of TTCP, but a greater peak of HAp; the bandwidth of which becomes narrow and sharp, indicating that CPC gradually converts into HAp and the yield of HAp thus increases.
Figure 1. The XRD crystalline diffractions of CPC scaffolds as related to various reaction temperatures of a saturated vapor pressures. The CPC scaffolds are hardened 4 days.
FTIR Analyses
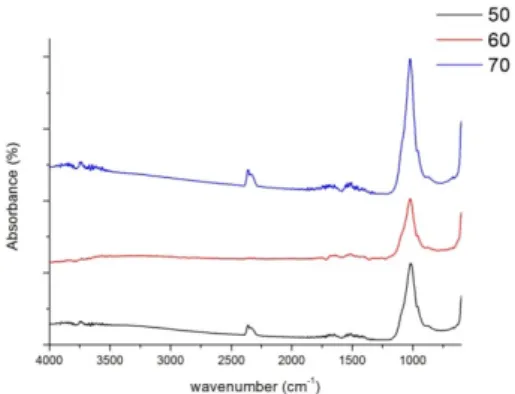
Figure 2 shows the functional groups of CPC scaffolds as related to various temperatures of the saturated vapor pressure. Distinct peaks are present at 1022, 960, and 606 cm-1; the functional groups of which are P-O bonding, a possible result of the influence of phase transition. The peaks become more distinct as a result of increasing temperature of a saturated vapor pressure. The results are then compared to the XRD analyses so as to determine the generation of HAp.
SEM Observation
Figure 2. The FTIR analyses of CPC scaffolds as related to various reaction temperatures under a saturated vapor pressures. The CPC scaffolds are hardened 4 days.
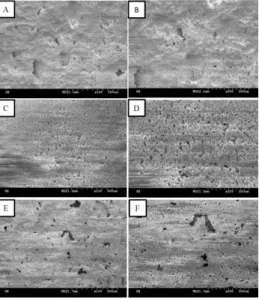
Figure 3 shows the SEM images of porous CPC scaffolds that are made at various temperatures of saturated vapor pressure and NaCl with a grain size of 210 µm serves as the porogen. Regardless of various temperatures of saturated vapor pressure, all CPC scaffolds have a porous structure, which does not shrink and disintegrate with the temperatures. The proper pore size is between 100
and 135 µm [6], and the pore size of CPC scaffolds all fall between 20-100 µm. The big pores are good for cell growth and attachment, and the small pores are good for fluid to flow.
Figure 3. SEM images of CPC scaffolds made of a temperature of a saturated vapor pressure: A) 50 ℃; 100, B) 50 ; ℃ ×200, C) 60 ; 100, D) 60 ; ×200, E) 70 ℃ ℃ ℃; 100, and F) 70 ; ℃ ×200. The CPC scaffolds are hardened for 4 days. The grain size of NaCl is 210 µm.
Conclusion
This study successfully crates porous CPC scaffolds. The test results show that the variations in the temperature of a saturated vapor pressure influence the crystalline conversion and the function groups’ generation of CPC scaffolds. The comparison of XRD and FTIR analyses manifests the
generation of HAp, which occurs optimally at 70℃ of a saturated vapor pressure. SEM images
exclude the temperature of saturated vapor pressure as a factor that influences the forming structure.
Acknowledgement
The authors would especially like to thank National Science Council of the Taiwan, for financially supporting this research under Contract NSC 100-2622-E-166-001-cc3.
References
[1] I. Sopyan and J. Kaur: Ceram. Int. Vol. 35 (2009), p. 3161.
[2] X. Miao, Y. Hu, J. Liu and A.P. Wong: Mater. Lett. Vol. 58 (2004), p. 397.
[3] R.R. Fan, L.X. Zhou, W. Song, D.X. Li, D.M. Zhang, R. Ye, Y. Zeng and G. Guo: Int. J. Biol. Macromol. Vol. 59 (2013), p. 227.
[4] G. Tripathi and B. Basu: Ceram. Int. Vol.38 (2012), p. 341.
[5] C.C. Huang. Fiber Reinforced Composite of Artificial Bone Prepared Techniques and Characteristics, Central Taiwan University of Science and Technology Institute of Biomedical Engineering and Material Science, (2013)
[6] S.J. Lee, I.W. Lee, Y.M. Lee, H.B. Lee and G. Khang: J. Biomat. Sci-Polym. E. Vol. 15 (2004), p. 1003.