Natural frequency analysis of tooth stability under
various simulated types and degrees of alveolar vertical
bone loss
C-H Wang1, H-W Liu2, K-L Ou3, N-C Teng4, J-J Yu4, and H-M Huang3* 1
Department of Prosthodontics, College of Dentistry, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
2
Department of Dentistry, Taipei City Hospital, Zhongxiao Branch, Taipei, Taiwan, Republic of China 3
Graduate Institute of Biomedical Materials and Engineering, Taipei Medical University, Taipei, Taiwan, Republic of China
4
School of Dentistry, Taipei Medical University, Taipei, Taiwan, Republic of China
The manuscript was received on 9 January 2008 and was accepted after revision for publication on 9 May 2008.
DOI: 10.1243/09544119JEIM394
Abstract: The aim of this study was to test natural teeth stability under various simulated types and degrees of alveolar vertical bone loss, as well as to assess the role that the surrounding bone played for maintaining tooth stability. A three-dimensional finite element model of the human maxillary central incisor with surrounding tissue, including periodontal ligament, enamel, dentin, pulp, and alveolar bone, was established. One side and multiple vertical bone loss were simulated by means of decreasing the surrounding bone level apically from the cemento-enamel junction in 1 mm steps incrementally downward for 10 mm. Natural frequency values of the incisor model with various types and degrees of bone loss were then calculated. The results showed that, with one-sided bone resorption, the model with labial bone loss had the lowest natural frequency decreasing rates (8.2 per cent). On the other hand, in cases of multiple bone loss, vertical bone resorption at the mesial and distal sides had more negative effects on tooth stability compared to vertical bone losses on facial and lingual sides. These findings suggest that the natural frequency method may be a useful, auxiliary clinical tool for diagnosis of vertical periodontal diseases.
Keywords: tooth, natural frequency, finite element, modal testing
1 INTRODUCTION
There are two major patterns of alveolar bone loss, i.e. horizontal and vertical alveolar bone loss [1]. Horizontal alveolar bone loss is the simultaneous loss in height of all walls surrounding the tooth roots. The assessment of pocket depth and the attachment level is the basis of diagnosis in period-ontics involving alveolar bone loss. However, the estimation of pocket depth or attachment level by the probing technique, is questionable due to its lack of accuracy [2–6].
To overcome the inaccuracy problems of probing, several researchers attempted to apply vibrational methods or tools to evaluate periodontal conditions. In this regard, several groups documented the mechanical mobility characteristics of teeth after vibration of central incisors with a horizontal force [7, 8]. However, the most important vibrational parameter, natural frequency, was not discussed in their reports.
Natural frequency is a function of the stiffness and mass of a structure and is related to the boundary conditions of an object [9, 10]. Potential applications of natural frequency analysis in dental implant research were investigated by a number of research groups [11–15]. Their results demonstrated that nat-ural frequency can be an important parameter in the
*Corresponding author: Graduate Institute of Biomedical Mat-erials and Engineering, Taipei Medical University, 250 Wu-Hsing Street, Taipei 110, Taiwan, Republic of China. email: hhm@ tmu.edu.tw
frequencies of the anterior teeth in vivo [9, 10]. They found that the mean natural frequency of anterior teeth significantly decreased in value when the tooth periodontium attachment loss was greater than 4 mm. These findings suggested that natural fre-quency values might be a useful parameter for assessing periodontal conditions clinically. To ex-plain in detail the mechanisms that change in periodontal conditions can affect a tooth’s natural frequency, Kojima and Fukui calculated the natural frequencies of the central incisor, canine, premolar, and molar using the finite element method [18]. Their conclusion indicated that the root length in alveolar bone is the major factor that affects the natural frequency of a tooth.
Vertical alveolar bone loss is the phenomenon that bone loss with a vertical intra-bony defect is surrounded by high walls of unaffected bone [19]. In this type of bone loss, the positions of bone loss could be on one, two, three, or all sides of the tooth. Several researchers investigated the prevalence and severity of vertical defects and found that the prevalence of vertical defects was 38 and 61 per cent for the populations of dentally aware indivi-duals and the indiviindivi-duals seeking dental care, respectively [20, 21]. In clinical diagnosis, radio-graphy is the major diagnostic method for checking alveolar bone loss. However, the use of two-dimensional characteristics of X-ray photography makes it more difficult to interpret the underlying buccal and lingual bone [4]. Their lack of fine resolution leaves a possibility for only qualitative measurement.
Although vibration analysis has previously been used for assessing the problems of horizontal alveolar bone loss [9, 10, 17], information suggesting whether vertical alveolar bone loss influences the natural frequency value of a tooth is still unavailable. Because the natural frequencies of teeth can provide a meaningful clinical index as to the dentition’s relative periodontal condition, it appears that a comprehensive analysis of the natural frequencies for natural teeth with vertical alveolar bone loss is soon likely to be conducted. Accordingly, in this
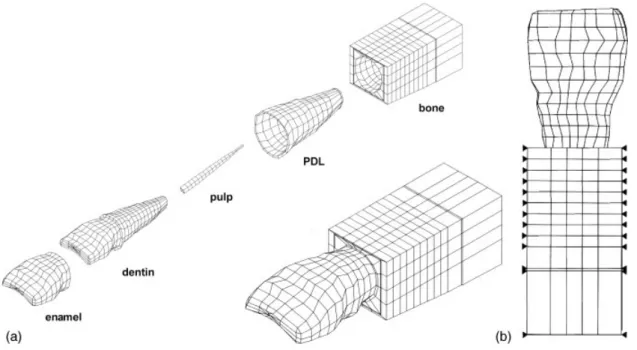
model of the human maxillary central incisor and periodontium, containing enamel, dentin, pulp, periodontal membrane, compact bone, and spongy bone, was built, using a computed tomographic (CT) image-reconstruction technique. CT was performed on non-carious human maxillary central incisors at a resolution of 1 mm. Basic anatomic measurements of the teeth were acquired from the CT images to ensure that the geometries and dimensions of the incisor model were in the normal range, then, the incisor contour lines from each CT image were replotted at the same intervals and a three-dimensional solid model was reconstructed and meshed using commercial finite element software (ANSYS, Swanson Analysis System, Houston, PA, USA). The model consisted of a total of 2312 nodes and 2142 three-dimensional solid elements, includ-ing a majority of eight-node hexahedral elements and a small percentage of four-node pyramids, and five-node tetrahedral elements (Fig. 1(a)). The ana-tomic structures, including the 23.5 mm long tooth and the 0.25 mm thickness of the periodontal membrane, were selected as in previous studies [8, 17]. The alveolar process was located 2 mm apically from the cemento-enamel junction (CEJ) [22]. The bone around the upper central incisors was thicker on the lingual side than on the labial side. The interior architecture and regeneration of surround-ing bone was ignored dursurround-ing simulation.
The material properties of the model were assumed to be homogeneous, isotropic, and linearly elastic. The specific values of the material properties are listed in Table 1 and were adapted from previous reports [17, 18, 23]. According to the findings of a previous study [17], the first vibration mode (funda-mental mode) of a human maxillary central incisor is a single bending mode vibrating along the lingual– labial direction. Furthermore, considering that al-veolar bone cannot vibrate in the mesial–distal direction, the boundary conditions of this model were chosen to fix the mesial–distal side of the bone at all nodes (Fig. 1(b)). Because the boundary con-dition has the same effect on the fundamental and
higher mode natural frequencies, it was proposed that it is appropriate to take the fundamental mode as representative in the assessment of periodontal conditions [17]. Therefore, in this study, only the first natural frequency and associated mode shape of the model were calculated.
2.2 Validation of the finite element model In this study, a modal testing experiment was performed to assess the natural frequency of human maxillary central incisors as reported previously [9, 17]. Briefly, ten extracted non-carious incisors were collected for use in similar in vitro tests. The tested teeth were fixed by a metal clamp stand with a torque of 20 N cm and lubricated with normal saline solution during the entire testing process. The nat-ural frequencies of each tested tooth were recorded with the clamping level varied from 2 to 6 mm in
2 mm steps downward from the CEJ of the tooth. The tested teeth were excited to vibration by an impulse force with an impulse force hammer (Model GK291C80, PCB Piezotronics, Inc., Buffalo, New York, USA), and signals of response obtained as the displacement signals were acquired through a piezoelectric accelerometer (Model 352B22, PCB Piezotronics, Inc., New York, USA), which was at-tached to the labial side of the teeth. To validate the FE model, the mean natural frequencies of the teeth were calculated and compared with the results from the finite element method.
2.3 Simulation of vertical bony defects
After the reliability of the finite element model was verified, three types of vertical bony defects, one-wall, two-one-wall, and three-wall bone resorption, were simulated using the finite element model. The degree of bone loss was defined in millimetres from the CEJ to the alveolar bone crest depending on where the bone loss occurred. Because the geometric dimension of alveolar bone between labial and lingual sides were not symmetric, vertical bony defects involving various walls were also simulated in each type of bony defect. In each situation, compact bone and spongy bone were reduced apically from 3 to 10 mm in 1 mm steps from the CEJ (Fig. 2). The natural frequencies were then computed and compared in correlation with the Fig. 1 (a) The finite element model used in this study consisted of enamel, dentin, pulp, PDL,
cancellous bone, and cortical bone. (b) The boundary conditions of this model were set to fix the mesial–distal side of the bone (closed triangles)
Table 1 Mechanical properties used in the finite element model Young’s modulus (GPa) Density (g/cm3) Poisson’s ratio Enamel 77.9 3.0 0.33 Dentin 16.6 2.2 0.31 Pulp 0.006 89 1.0 0.45 PDL 0.05 1.1 0.45 Compact bone 10 1.4 0.26 Cancellous bone 0.5 1.4 0.38
bone level. In this study, the natural frequency de-creasing rate (NFDR) was defined as the natural frequency value change rate when the bone level was varied from its original status to 10 mm down from the CEJ.
3 RESULTS
The first natural frequencies of the ten maxillary central incisors tested in vitro ranged from 1900 to 2400 Hz, with an average of 2194 ¡ 166 Hz. Figure 3 shows that the frequency decreased significantly when the clamping level was lowered horizontally. There was a linear relationship between the natural frequency and the attachment level, which was expressed mathematically as Y 5 2341.78X + 2837 (r 5 20.99, p , 0.01), where X was the periodontal attachment level (mm) and Y was the correspond-ing natural frequency (Hz). To validate the finite element model, modal testing experiments were simulated on human incisors. The first mode of natural frequency of the incisor with normal attach-ment is 2700 Hz, which was slightly larger than that of in vitro results. Nonetheless, a similar decreasing tendency in natural frequency (Y 5 2339.75X + 3352) was found compared to the in vitro test, when the clamping level was lowered horizontally. The first mode shapes of the model are plotted in Fig. 4. It is a single bending mode vibrating along the labial– lingual direction.
The finite element model was then used to cal-culate the natural frequencies when the tooth had various types of vertical bone loss. These results demonstrated that the decrease in natural frequency value of a tooth corresponded to the amount of bone loss. The results obtained from the simulations of the one-sided bone resorption are shown in Fig. 5. In the figure, regardless of the bone loss involved on a given side, the natural frequency value decreased correlated with the pocket depth. The natural fre-quency values of the incisors with 10 mm one-sided bone loss were in the range 2300–2500 Hz, depend-ing on where the bone loss occurred. The model of Fig. 2 Examples of the finite element models with
various types of 2 mm vertical bone loss: (a) one-sided bone resorption on the facial side, (b) two-sided bone resorption on the mesial– lingual sides, (c) three-sided bone resorption on the mesial–lingual–distal sides
Fig. 3 Changes of natural frequency on horizontal bony defects. Solid and dashed lines represent the data from the finite element method and in vitro modal testing experiments respectively
Fig. 4 The first vibration mode shapes of the finite element model with normal periodontal condi-tions plotted from (a) three-dimensional and (b) side views. The vibrational mode shapes are compared with their original shapes (dashed line). The symbols F and L denote facial and lingual sides respectively
labial bone loss had the lowest NFDR (8.2 per cent) compared with that of lingual bone loss (14.1 per cent), mesial bone loss (12.7 per cent), and distal bone loss (11.7 per cent).
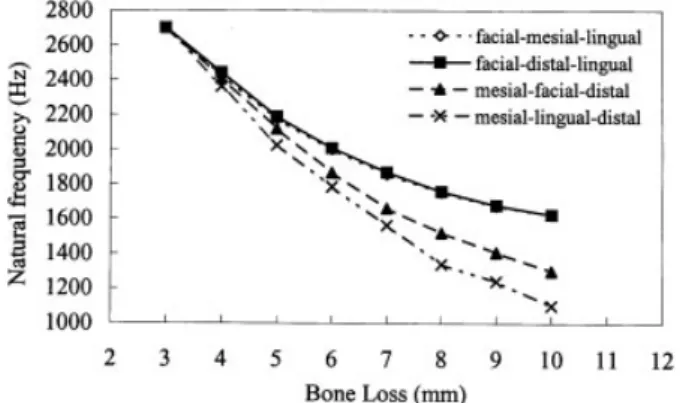
The natural frequency values of the incisors with 10 mm of bone loss were reduced to 1800–2200 Hz (Fig. 6) and 1100–1650 Hz (Fig. 7) for the two-sided and three-sided bone resorption models respec-tively. In the case of two-sided resorption, the NFDR of the tooth model was roughly divided into two groups. When bone loss involved the labial side, the NFDR of the models (22.1 per cent for labial–mesial bone loss and 21.7 per cent for labial–distal bone loss) was smaller compared to cases of bone resorption that occurred at lingual sites (30.5 per cent for lingual–mesial bone loss and 30.1 per cent for lingual–distal bone loss). In the simulations of three-sided bone loss, bone resorption involving the facial and lingual sides demonstrated close NFDR values (39.8 per cent and 39.7 per cent for labial– mesial–lingual and labial–lingual–distal bone loss, res-pectively), and was lower than that of the other two
situations involving bone resorption of the labial– distal–mesial side (51.8 per cent) and mesial–lingual– distal side (59.2 per cent).
4 DISCUSSION
In previous reports, an electronic mobility measure-ment device (Periotest, Siemens AG, Bensheim, Ger-many) was used to measure tooth mobility. Unfortu-nately, several variables influenced the results of the mobility measurement device [24]. For example, tooth mobility is strongly related to the excitation direction and application force and the reading from the method does not always correspond precisely to a clinical diagnosis [24]. The major advantage of natural fre-quency analysis is that the measurement method, modal testing, is a non-invasive and non-destructive method of investigation, and the application of such a technique is not affected by the amount or the site of the force applied. For modal testing, shaking force and transient impulse force are two basic types of stimuli to determine natural frequency of a structure. In the present in vitro tests and numerical simulations, the impulse force method was carried out to measure the fundamental frequency of the teeth. Accordingly, strain rate effects were neglected.
To verify a finite element model, experimental testing of the model is the best method [25]. Here a series of modal testing experiments was used to test the model. Comparison of the data plotted in Fig. 3 revealed similar qualitative results between modal testing experiments and finite element simulations. The natural frequencies of the teeth decreased linearly when the exposed heights were increased. These results are consistent with the findings of a previous study [17] and are in agreement with Fig. 5 Changes in the natural frequency value of the
model with various types and degrees of one-sided bone loss. Legends indicated the bone resorption sites
Fig. 6 Changes in the natural frequency value of the model with various types and degrees of two-sided bone losses. The legend indicates the bone resorption sites
Fig. 7 Changes in natural frequency values of the model with various types and degrees of three-sided bone losses. The legend indicates the bone resorption sites
Considering the mechanical structure of a tooth, its vibration characteristics can be explained using beam theory, as reported previously [9, 10, 17]. Mechani-cally, a tooth is free to vibrate at one end but is fixed at the other end, behaving like a cantilever beam. The natural frequency of a cantilever beam is correlated to boundary conditions and the effective vibrating length [13, 17]. In this regard, the frequency alteration of the tooth involved with periodontal destruction is due to the changes in the boundary condition and the effective vibrating length of the model. In addition, both mass and size of a tooth affect its natural frequency, and because these two factors are varied among individuals, a natural frequency value for periodontal disease judgement is not appropriate. Accordingly, a normalized value (NFDR) was defined as a parameter for assessing the alteration in tooth periodontal health.
It was reported that the first vibration frequency corresponds to tooth mobility [7, 18]. Therefore, tooth mobility increases while the natural frequency de-creases with an increase in alveolar bone loss [18]. Although multiple defects were observed only in a minor proportion of teeth [20], they were simulated in this study to assess the roles that alveolar bone played in the maintenance of tooth stability. Clinically, an attachment loss reaching 4 mm was noted as moder-ate periodontal disease requiring treatment [10]. Therefore, the natural frequency change rate of the tooth with bone loss from 3 to 4 mm should be a useful indicator for clinical diagnosis. Table 2 showed that these values varied from 2 to 12 with the variation in degree and location of the bone defect.
For a beam-like structure, the vibration frequency and vibration mode shape strongly correlate with its area moment of inertia. Because the area moment of inertia of an incisor at the mesial and distal surfaces is much smaller than the value for the facial and lingual surfaces, the natural frequency correspond-ing to the bendcorrespond-ing vibration in the mesial–distal direction is much larger than the frequency vibration in the facial–lingual direction. This explains the simulated results shown in Fig. 4: the first vibration
similar results. When one-sided bone resorption occurred, the finite element model with bone defects of the facial side revealed less NFDR than the other three situations. This is because, anatomically, the geometric size of the lingual surrounding bone is larger than the bone of the facial side. Therefore, with equal bone resorption depths, teeth with bone defects in the lingual side lose more bone compared to the opposite side, which results in a larger red-uction in tooth stability.
In Fig. 7, the tooth with bone resorption occurring on the mesial–lingual–distal or mesial–facial–distal sides demonstrated larger NFDR values compared to analogue teeth with facial–mesial–lingual or facial– distal–lingual bone defects. In analogy with the above analysis, the decrease in natural frequency value strongly correlated to the increase in tooth mobility. Therefore, bone structures on the mesial sides are more important for maintaining tooth stability compared to bone on the facial and lingual sides. In addition, although clinical findings indicate that vertical defects were more frequent on mesial than on distal surfaces [20, 21], these results showed that mesial and distal bone resorption have similar effects on tooth stability (Figs 6 and 7).
In conclusion, the natural frequency value of a maxillary central incisor is affected by the degrees Table 2 The natural frequency change rate of the model when the bone loss level increased from 3 to 4 mm
Bone loss side* One sided (%) Two sided (%) Three sided (%)
F 1.9 — — L 3.4 — — M 4.8 — — D 4.1 — — M-F — 6.4 — D-F — 5.7 — M-L — 8.0 — D-L — 7.3 — F-M-L — — 10.2 F-D-L — — 9.6 M-F-D — — 10.8 M-L-D — — 12.6
*F, L, M, and D denote facial, lingual, mesial, and distal resp-ectively.
and types of vertical bone resorption. In the situation of one-side bone loss, surrounding bone on the facial side plays a less important role in maintaining tooth stability compared to the bone on the other three sides. In multiple defects, mesial and distal bone is more important than facial and lingual bone.
ACKNOWLEDGEMENTS
This study was supported by Grant 95004-62-171 from Tapei City Hospital, Taipei, Taiwan.
REFERENCES
1 Tsao, Y. P., Nelva, R., Al-Shammari, K., Oh, T. J., and Wang, H. L. Factors influencing treatment outcomes in mandibular class II furcation defects. J. Periodontol., 2006, 77(4), 641–646.
2 Armitage, G. C., Svanberg, G. K., and Lo¨e, H. Microscopic evaluation of clinical measurements of connective tissue attachment level. J. Clin. Periodontol., 1977, 4(3), 173–190.
3 Polson, A. M., Caton, J. G., Yeaple, R. N., and Zander, H. A. Histological determination of probe tip penetration into gingival sulcus of humans using an electronic pressure-sensitive probe. J. Clin. Periodontol., 1980, 7(6), 479–488.
4 Rimes, R. J., Mitchell, C. N. T., and Willmot, D. R. Maxillary incisor root resorption in relation to the ectopic canine: a review of 26 patients. Eur. J. Orthod., 1997, 19(1), 79–84.
5 Robinson, P. J. and Vitek, R. M. The relationship between gingival inflammation and resistance to probe penetration. J. Periodontal Res., 1979, 14(3), 239–243.
6 Saglie, R., Johansen, J. R., and Flotra, L. The zone of completely and partially destructed periodontal fibers in pathological pockets. J. Clin. Periodontol., 1975, 2(4), 198–202.
7 Noyes, D. H. and Solt, C. W. Measurement of mechanical mobility of human incisors with sinusoidal forces. J. Biomech., 1973, 6(5), 439–442. 8 Oka, H., Yamamoto, T., Saratani, K., and
Kawa-zoe, T. Application of mechanical mobility of periodontal tissues to tooth mobility examination. Med. Biol. Engng Comput., 1989, 27(1), 75–81. 9 Huang, H. M., Yeh, C. Y., Lee, S. Y., Wang, M. S.,
Pan, L. C., and Chen, C. C. Factors influencing the dynamic behaviour of human teeth. Med. Biol. Engng Comput., 2001, 39(2), 176–181.
10 Huang, H. M., Lee, S. Y., Yeh, C. Y., Wang, M. S., Chang, W. J., and Lin, C. T. Natural frequency analysis of periodontal conditions in human anterior teeth. Ann. Biomed. Engng, 2001, 29(10), 915–920. 11 Barewall, R. M., Oates, T. W., Meredith, N., and
Cochran, D. L. Resonance frequency measurement
of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int. J. Oral Maxillofac. Implants, 2003, 18(5), 641–651.
12 Huang, H. M., Lee, S. Y., Yeh, C. Y., and Lin, C. T. Resonance frequency of dental implant stability with various bone quality: a numerical approach. Clin. Oral Implant Res., 2002, 13(1), 65–74. 13 Huang, H. M., Chiu, C. L., Yeh, C. Y., Lin, C. T.,
Lin, L. H., and Lee, S. Y. Early detection of implant healing process using resonance frequency analy-sis. Clin. Oral Implant Res., 2003, 14(4), 437–443. 14 Huang, H. M., Cheng, K. Y., Chen, C. F., Ou, K. L.,
Lin, C. T., and Lee, S. Y. Design and examination of a stability-detecting device for dental implants. Proc. IMechE, Part H: J. Engineering in Medicine, 2005, 219(H3), 203–211.
15 Meredith, N., Shagaldi, F., Alleyne, D., Sennerby, L., and Cawley, P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin. Oral Implant Res., 1997, 8(3), 234–243. 16 Okazaki, M., Fukumoto, M., and Takahashi, J.
Damped oscillation analysis of natural and artifi-cial periodontal membrane. Ann. Biomed. Engng, 1996, 24(2), 234–240.
17 Lee, S. Y., Huang, H. M., and Lin, C. Y. In vivo and in vitro natural frequency analysis of periodontal conditions, an innovative method. J. Periodontol., 2000, 71(4), pp. 632–640.
18 Kojima, Y. and Fukui, H. Calculation of natural frequencies of teeth supported with periodontal ligament. Dent. Mater. J., 2007, 26(2), 254–259. 19 Schwartz, M., Lamster, I. B., and Fine, J. B.
Clinical guide to periodontics, 1995, pp. 96–97 (W.B. Saunders, Philadelphia, Pennsylvania). 20 Baljoon, M., Natto, S., and Bergstrom, J.
Occur-rence of vertical bone defects in dentally aware individuals. Acta Odontol. Scand., 2003, 61(1), 47– 51.
21 Persson, R. E., Hollender, L. G., Laurell, L., and Persson, G. R. Horizontal alveolar bone loss and vertical bone defects in an adult patient popula-tion. J. Periodontol., 1998, 69(3), 348–356.
22 Carranza, F. A. and Newman, M. G. Clinical periodontology, 1996, pp. 44–45 (W. B. Saunders, Philadelphia, Pennsylvania).
23 Huang, H. M., Tsai, C. Y., Lee, H. F., Lin, C. T., Yao, W. C., Chiu, W. T., and Lee, S. Y. Damping effects on the response of maxillary incisor subjected to a traumatic impact force: a nonlinear finite element analysis. J. Dent., 2006, 34(4), 261–268.
24 Derhami, K., Wolfaardt, J. F., Faulkner, G., and Grace, M. Assessment of the Periotest device in baseline moblity measure of craniofacial implants. Int. J. Oral Maxillofac. Implants, 1995, 10(2), 221– 229.
25 Holmes, D. C. and Loftus, J. T. Influence of bone quality on stress distribution for endosseous implants. J. Oral Implantol., 1997, 23(3), 104–111.