This article was downloaded by: [National Chiao Tung University 國立交通大學]
On: 30 April 2014, At: 22:22
Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer
House, 37-41 Mortimer Street, London W1T 3JH, UK
Journal of the Air & Waste Management Association
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/uawm20
Simultaneous Sampling of Gas- and Aerosol-Phase TDI
with a Triple Filter System
Chuen-Jinn Tsai
a, Kai-Chung Cheng
a, Shankar G. Aggarwal
a, Tung-Sheng Shih
b& I.-Fu
Hung
ca
Institute of Environmental Engineering , National Chiao Tung University , Hsin-chu ,
Taiwan
b
Institute of Occupational Safety and Health , Council of Labor Affairs , Taipei , Taiwan
cDepartment of Nuclear Science , National Tsing Hua University , Hsin-chu , Taiwan
Published online: 22 Feb 2012.
To cite this article: Chuen-Jinn Tsai , Kai-Chung Cheng , Shankar G. Aggarwal , Tung-Sheng Shih & I.-Fu Hung (2003)
Simultaneous Sampling of Gas- and Aerosol-Phase TDI with a Triple Filter System, Journal of the Air & Waste Management
Association, 53:10, 1265-1272
To link to this article: http://dx.doi.org/10.1080/10473289.2003.10466279
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained
in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no
representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of
the Content. Any opinions and views expressed in this publication are the opinions and views of the authors,
and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied
upon and should be independently verified with primary sources of information. Taylor and Francis shall
not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other
liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or
arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic
reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any
form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at
http://
www.tandfonline.com/page/terms-and-conditions
Simultaneous Sampling of Gas- and Aerosol-Phase TDI with
a Triple Filter System
Chuen-Jinn Tsai, Kai-Chung Cheng, and Shankar G. Aggarwal
Institute of Environmental Engineering, National Chiao Tung University, Hsin-chu, Taiwan
Tung-Sheng Shih
Institute of Occupational Safety and Health, Council of Labor Affairs, Taipei, Taiwan
I.-Fu Hung
Department of Nuclear Science, National Tsing Hua University, Hsin-chu, Taiwan
ABSTRACT
A new triple filter system sampler/model is proposed for the precise and accurate simultaneous sampling and de-termination of gas- and aerosol-phase 2,4-toluene diiso-cyanate (TDI). The system consists of two front Teflon filters for sampling aerosol-phase TDI and a final coated glass fiber filter to collect gas-phase TDI. The aerosol-phase TDI is collected on the first Teflon filter, while the second Teflon filter is used to estimate gaseous TDI ad-sorbed by the first. According to the gas adsorption test of two Teflon filters in series, the TDI gas adsorption fraction of the two filters is almost the same. Results of the evap-oration test using pure TDI aerosols collected on the Tef-lon filter show that significant evaporation of the com-pound does not occur during sampling. These two findings allow the use of a model to estimate accurate gas-and aerosol-phase TDI concentrations. The comparison test with an annular denuder shows that the triple filter system can minimize the TDI sampling bias between the dual filter and the annular denuder systems.
INTRODUCTION
Toluene diisocyanate (TDI), especially 2,4-toluene diiso-cyanate, is a major isocyanate compound used commer-cially in surface coatings, polyurethane foams, adhesives, resins, elastomers, binders, and sealants.1The compound
has two –N⫽C⫽O functional groups attached to a parent toluene molecule. TDI is a semivolatile organic com-pound (SOC) and has a vapor pressure of 0.025 mmHg at 25 °C. Its health effects are well known.2,3Exposure to TDI
in the workplace may commonly result in occupational asthma caused by sensitization, contact dermatitis, and hypersensitivity pneumonitis (HP). The permissible ceil-ing exposure limit of the Occupational Safety and Health Administration (OSHA) is 0.02 ppm (0.14 mg/m3, at 25
°C, 1 atm) for this compound.4Several sampling methods
for use in the workplace have been reported.1,5–7
Cur-rently, OSHA 42 is the standard method using a single glass fiber filter coated with 1-(2-pyridyl)piperazine (1,2-PP) for total TDI sampling without separating the gaseous and particulate phases.1However, the respiratory
deposi-tion site of inhaled TDI and health effects depend upon the physical state (i.e., gas- or aerosol-phase) of airborne TDI.8Therefore, it is very important to know the correct
amount of gas- and aerosol-phase TDI present in a partic-ular workplace.
There are two methods currently in use to separate TDI from its aerosol counterpart—the dual filter system (ISO-CHEK) and the annular denuder method.8 In the
dual filter system, air first goes through a front Teflon filter, which traps the aerosol-phase TDI while allowing the gas-phase TDI to pass through. The gas-phase TDI is collected by a glass fiber filter coated with 9-(N-methyl-aminomethyl) anthracene (MAMA).9,10 Teflon filters are
known to adsorb some gaseous SOCs;11thus, the
adsorp-tion of gaseous TDI by the Teflon filter of the dual filter system may occur, resulting in an overestimation of the aerosol-phase TDI concentration. In the annular denuder system, air first enters an impactor (D50⫽ 2.5 m) as an
elutriator, followed by an annular denuder tube to adsorb gaseous TDI. The final stage of the sampler is a 37-mm IMPLICATIONS
Accurate determination of gas- and aerosol-phase TDI in the ambient air of a workplace is of great importance be-cause the respiratory deposition site of inhaled TDI and health effects depend upon the physical state of the toxi-cant. A new triple filter system is proposed and shown to be accurate for simultaneous sampling and determination of gas- and aerosol-phase TDI concentrations.
filter holder containing a glass fiber filter to collect the aerosol-phase TDI. The coating and extraction procedure of the annular denuder is somewhat complicated.
The surface-area–normalized gas/Teflon partition co-efficient (Kp,s, m
3/m2) has been applied to estimate the
concentration of gaseous SOCs adsorbed by the Teflon filter11as
Kp,s⫽ 关Cp/Cg兴/af (1)
where Kp,sis the ratio of particle-phase concentration (Cp,
ng/g) to gas-phase concentration (Cg, ng/m
3) normalized
by the specific surface area of the Teflon filter (af, m 2/g). It
can be correlated by the subcooled liquid vapor pressure (PL
o, torr)12,13as
Log Kp,s⫽ mr,sLog PLo⫹ br,s (2)
where mr,s and br,s are constants that can be calculated
from experimental results of the amount of TDI adsorbed on the Teflon filter at different temperatures plotted in the form of logKp,sversus logPLo. By knowing the actual
temperature, Kp,sfor a sampling event can be obtained.
Gaseous TDI concentration adsorbed on the Teflon filter (Cp) can be calculated by multiplying Kp,s by Cg and af.
However, PLoand afare species- and temperature-dependent
and, hence, must be determined by experiment.
This research is intended to confirm the speculation that the front Teflon filter of the dual filter system ad-sorbs gaseous TDI, leading to an overestimation of aerosol-phase TDI concentration. A new triple filter system is introduced, and a model is proposed to overcome the drawbacks of the dual filter and annular denuder systems. The triple filter system consists of two front Teflon filters and a final coated glass fiber filter in a series. Based on the amount of TDI collected by each of the three filters, a model to calculate the exact amount of gas- and aerosol-phase TDI is developed.
EXPERIMENTAL METHODS
The triple filter system shown in Figure 1 consists of two Teflon filters (37 mm in diameter, Zefluro membrane, polytetrafluoroethylene, Gelman Laboratory) in series fol-lowed by a 1,2-PP coated glass fiber filter (37-mm in di-ameter, type A/E, SKC). The first Teflon filter collects aerosol-phase TDI, while the second Teflon filter cali-brates the gas-phase TDI adsorbed by the first. The glass fiber filter is used to collect the remaining gas-phase TDI. The surface area of the Teflon filter was measured using the Brunauer–Emmett–Teller method for three fil-ters (for each filter, three measurements were taken) and found to be 6.75⫾ 0.41 m2/g. The tests were performed at
relative humidity (RH)⫽ 53.2%, temperature ⫽ 19.8 °C. A typical 37-mm-diameter Teflon weighed 159.96 mg. After loading the filter into the holder of the sampler, the exposed diameter of the filter was reduced to 22 mm.
The sampler used as a reference standard in this work is the annular denuder sampler (inner diameter d1 ⫽ 4
mm, outer diameter d2 ⫽ 6 mm, length h ⫽ 75 mm),
which is shown in Figure 2. In the sampler, the gas-eous TDI is adsorbed by the denuder tube, while the aerosol-phase TDI is collected by the after-glass-fiber filter that is not coated. The diffusion coefficient of the de-nuder for TDI was estimated to be 0.061 cm2/sec at 25 °C
following the method in Tucker and Nelken.14The
pen-etration of TDI through the denuder was calculated to be 0.019 (or efficiency is 98.1%) at a 0.5 L/min flow rate using the Possanzini equation.15
Sample Preparation and Analysis
The coating and extraction reagents recommended in the literature were used.1Before sampling, the 37-mm glass
fiber filters were coated with 0.5 mL of 0.2 mg/mL 1,2-PP/ methylene chloride (CH2Cl2) solution and then dried by
a nitrogen (N2) stream. After sampling, the filters were
placed into a vial and extracted with 4 mL of 10/90 (v/v) dimethyl sulfoxide/acetonitrile solution with ultrasonica-tion for 30 min. Teflon filters were not coated before sampling. After sampling, the filters were immediately placed into extraction vials containing 0.5 mL of 0.2 mg/mL 1,2-PP/CH2Cl2solution and 4 mL of 10/90 (v/v)
dimethyl sulfoxide/acetonitrile solution. Samples in the Figure 1. The triple filter system.
Tsai, Cheng, Aggarwal, Shih, and Hung
vials were then derivatizated and desorbed by ultrasoni-cation for 30 min.
The denuder tube (URG-2000 –15T, URG) was coated with 1 mL of 2 mg/mL 1,2-PP/CH2Cl2solution. The
de-nuder was capped and rolled by a shaker (Vortex-2 Genie, Scientific Industries) for 5 min to evenly coat the denuder wall. The CH2Cl2was then evaporated with an N2stream,
leaving behind a dried coating of 1,2-PP on the denuder wall. After sampling, 2 mL of 10/90 (v/v) dimethyl sulfox-ide/acetonitrile solution was added into the denuder, and the tube was capped and shaken by a shaker for 5 min for extraction. Each extract was then decanted into a sample vial containing 2 mL of 10/90 (v/v) dimethyl sulfoxide/ acetonitrile solution and kept for the analysis. Amber glassware was used throughout the experiment.
Before the analysis, the extracts were clarified by pass-ing them through a 0.45-m pore size polyvinyl filter (Millipore Millex-HV). Samples were analyzed by high-performance liquid chromatography (HPLC) (LC-10AT, Shimadzu) within 24 hr of sampling. A fluorescence de-tector (RF-551, Shimadzu) was used, and the excitation and emission wavelengths were set at 240 and 370 nm, respectively. The output of the detector was sent to a personal computer for on-line recording of the data. The precision of the analysis was determined to be good with a relative standard deviation of less than 5.1%. The recov-ery test of adsorbed TDI on the coated glass fiber filter showed that the recovery efficiency was 96.6 ⫾ 2.2%. MDL (method detection limit) was determined to be 0.09 ppb (0.9 g/m3, at 25 °C, 1 atm) in the equivalent TDI
gaseous concentration when the sampling flow rate was 1 L/min and sampling time was 15 min.
The analysis was done with a 4.6 mm⫻ 25 cm col-umn with 5-m silica packing (RP-8, Phenomenex). The mobile phase consisted of 60% acetonitrile and 40% 0.05 M aqueous solution of ammonium acetate, which was adjusted to pH⫽ 6.2 with glacial acetic acid. The flow rate was 1 mL/min. Sample injection volumes were 10 L. These conditions separated the TDI 1,2-PP– urea from ex-cess 1,2-PP and sample impurity, with the retention time of the TDI derivative being⬃8.7 min.
Generation of TDI
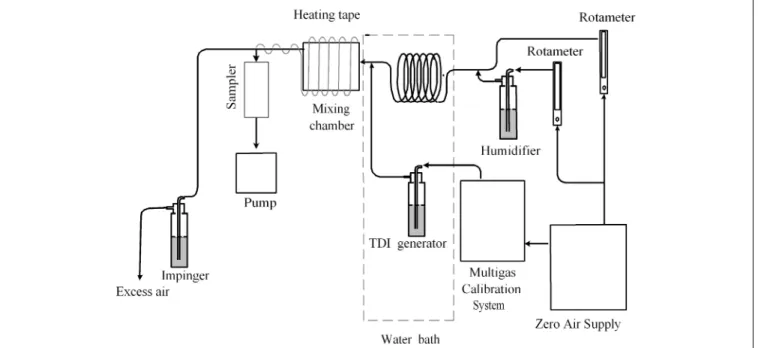
The experimental setup is shown in Figure 3. Gas- and aerosol-phase TDI (or mixed-phase TDI) were generated by introducing clean air into a liquid TDI-containing im-pinger with a flow rate of 30 mL/min (at 30 ⫾ 1 °C) adjusted by a dynamic gas calibration system (Thermo Model 146, Thermo Environmental Instruments, Inc.). The flow rate of dilution air was adjusted by a rotameter to generate the required TDI concentration for the test.
Pure gaseous TDI was used to test the adsorption of gaseous TDI by Teflon filters. It was generated by passing the mixed-phase TDI through a Teflon filter that was placed in the mixing chamber. Pure aerosol-phase TDI was used to test whether TDI aerosols would evaporate once they were collected on a Teflon filter. Pure TDI aerosols were generated when the mixed-phase TDI was allowed to enter the denuder tube, which was placed after the mixing chamber so that gaseous TDI was adsorbed on the denuder tube completely and only aerosol-phase TDI was passed through the tube. The adsorption capacity of pure TDI gas by the denuder tube was also determined by placing a coated glass fiber filter behind the tube. The test results showed that the adsorption capacity of the de-nuder tube was high enough for the tube to generate pure aerosol-phase TDI. Detailed test results will be described later.
Compressed air passed through a zero air supply sys-tem (Thermo Model 111, Thermo Environmental Instru-ments, Inc.) supplying clean and dry air before the TDI generating system. The air was conditioned to a temper-ature of⬃30 °C by a water bath and heating tape applied to the pipes. The humidity of the airstream was reduced by the zero air supply system for tests at low RH condi-tions (RH⬇ 10%). For tests at high RH (RH ⬇ 80%), the RH of the dried air was increased by pumping the dilution air partially into de-ionized water in an impinger with a flow rate of⬃5 L/min controlled by a rotameter. The flow rate of each of the tested samplers was controlled by a portable air-sampling pump (GilAir3, Gillian Instrument Corp.). The flow rate of pumps and the airflow through the rotameters were calibrated by a bubbler calibrator Figure 2. The annular denuder system.
(PN#800268, Gillian Instrument Corp.) before the exper-iment.
RESULTS AND DISCUSSION
Adsorption Test of Gaseous TDI on Teflon Filters
To study adsorption of gaseous TDI on the Teflon filters with respect to incoming amount of gaseous TDI, the triple filter system (see Figure 1), consisting of two Teflon filters in series followed by a coated glass fiber filter, was placed in the experimental setup (see Figure 3). The pure gaseous TDI of 5–15 ppb was introduced into the system at a flow rate of 1 L/min. The experiment was performed repeatedly for an incoming gaseous TDI amount ranging from 0 to 9273 ng. To take the effect of humidity into consideration, the tests were performed at two different RHs (i.e., 83.4% at 30.1 °C [high humidity] and 10.3% at 30.6 °C [low humidity]).
The adsorption tests of gaseous TDI by the Teflon filters were carried out with TDI concentrations of 14.3⫾ 2.9 ppb (low humidity) and 16.1⫾ 1.2 ppb (high humid-ity), respectively. For the first Teflon filter, the incoming amount of TDI was calculated from the amount deter-mined in the three filters (i.e., (M1⫹ M2⫹ M3)). For the
second filter, the incoming TDI was calculated as (M2⫹ M3). Results in Figure 4 show that the amount of gaseous
TDI adsorbed on each Teflon filter is almost the same with respect to the incoming amount of the gas and does not depend on RH. However, the adsorbed TDI increases ini-tially with an increase in the incoming amount of TDI and finally becomes a constant when the Teflon filter is saturated at the incoming TDI of⬃3000 ng. That is, after attaining the saturation condition, there will be no further
change in the adsorbed amount of the TDI gas on the Teflon filters. Before saturation, the fraction of TDI gas adsorbed by the filter (␣ ⫽ 0.21) can be calculated by the slope of the regression line from the plot of adsorbed gaseous TDI versus incoming gaseous TDI to the filter.
Collection Efficiency Test of the Annular Denuder System
The annular denuder system was used as a reference sam-pler for this study. It was also used to adsorb all gaseous TDI when only pure aerosol-phase TDI was to be gener-ated for the evaporation test of TDI aerosols, which will be Figure 3. Experimental setup for TDI generation and sampling.
Figure 4. Gaseous TDI adsorbed by the Teflon filters of the triple filter system (before equilibrium, slope (␣) ⫽ 0.21; the amount of TDI ad-sorbed⫽ 645 ng after equilibrium).
Tsai, Cheng, Aggarwal, Shih, and Hung
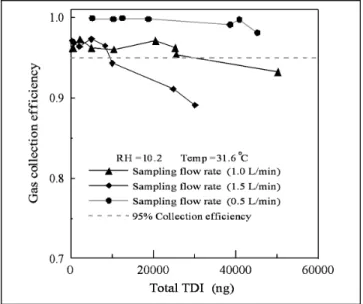
described in the next section. Therefore, it is important to determine the gas adsorption efficiency and capacity of the denuder tube. For this test, the device consisted of a denuder tube followed by a glass fiber filter coated with 1,2-PP, arranged in the sampler’s position as shown in the experimental setup (see Figure 3). The tests were carried out by introducing the gaseous TDI into the denuder at three different flow rates of 0.5, 1, and 1.5 L/min, respec-tively. The test condition was fixed during the whole experiment (RH⫽ 11.2% and temperature ⫽ 30.6 °C). The collection efficiency of the annular denuder is the ratio of TDI collected by the annular denuder to that collected by the annular denuder and the filter.
The results of the collection efficiency tests are shown in Figure 5. It shows that the collection efficiency of the annular denuder is highest at the flow rate of 0.5 L/min and decreases with an increase in the amount of gaseous TDI sampled. The test gas-phase TDI concentration was 53.3⫾ 25.4 ppb (0.386 ⫾ 0.184 ng/m3) (at RH⫽ 11.2%,
30.6 °C). If 95% collection efficiency is used as the criteria below which breakthrough occurs, the adsorption capac-ity of the denuder tube for TDI gas is⬎43, 27, and 10 mg, for the flow rates of 0.5, 1, and 1.5 L/min, respectively. The adsorption capacity was high enough when the de-nuder tube was used to generate pure aerosol-phase TDI.
Evaporation of Aerosol-Phase TDI during Sampling
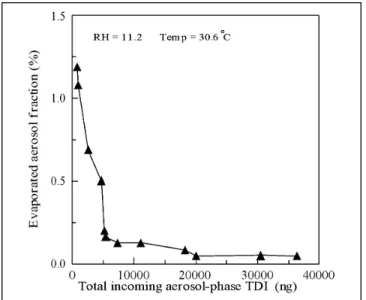
To test possible evaporation of aerosol-phase TDI col-lected on the Teflon filter during sampling, a test system (see Figure 2) consisted of a denuder tube followed by a Teflon filter and a glass fiber filter coated with 1,2-PP was placed in the experimental setup (see Figure 3). The mixed-phase (gas and aerosol) TDI was supplied with a
sampling flow rate of 0.5 L/min (RH ⫽ 10.2% and tem-perature⫽ 31.6 °C); the gaseous TDI was removed by the denuder tube allowing only aerosol-phase TDI to be col-lected on the Teflon filter. The gaseous TDI evaporated from aerosol-phase TDI was then collected by the coated glass fiber filter. The fraction of evaporation of the col-lected aerosol-phase TDI on the Teflon filter is the ratio of TDI collected by the glass fiber filter to that collected by both filters.
Figure 6 shows the fraction of evaporated aerosol-phase TDI as a function of total aerosol-aerosol-phase TDI. The test aerosol-phase TDI concentration was 74.4⫾ 22.4 ppb (0.539⫾ 0.162 ng/m3) (at RH⫽ 10.2%, 31.6 °C), which
was calculated by the amount determined by both filters (Teflon and glass fiber filters). The percentage of aerosol evaporated is less than 1.25% over a range of 700-3600 ng aerosol-phase TDI. The percentage is shown to be very low, indicating that evaporation of aerosol TDI is almost negligible.
Model of the Triple Filter System
Figure 7 illustrates the triple filter system. The masses collected by each of the three filters are as follows:
M1⫽ Ma1⫹ Mg1 (3)
M2⫽ Mg2⫹ Mag (4)
M3⫽ Mg3 (5)
where M1is TDI collected on the first Teflon filter (ng); Ma1is aerosol TDI collected on the first Teflon filter (ng); Mg1is gaseous TDI adsorbed by the first Teflon filter (ng);
M2is TDI collected on the second Teflon filter (ng); Mg2is
gaseous TDI adsorbed by the second Teflon filter (ng); Mag
is aerosol evaporated from the first Teflon filter (ng); M3is
TDI collected on the glass fiber filter (ng); and Mg3 is
gaseous TDI collected by the glass fiber filter (ng). The actual amount of gas- and aerosol-phase TDI, Mg
and Ma, are as follows:
Ma⫽ Ma1⫹ Mag (6)
Mg⫽ Mg1⫹ Mg2⫹ Mg3 (7)
The results of the evaporation test show that aerosol-phase TDI does not evaporate during sampling. According to this finding, eqs 4 and 6 can be written as
M2⫽ Mg2 (8)
Ma⫽ Ma1 (9)
Figure 5. Collection efficiency of gaseous TDI by the annular denuder tube vs. total incoming gaseous TDI.
From the experimental results of the adsorption tests of gaseous TDI on the Teflon filters placed in series (if the incoming stream is purely gaseous TDI), it has been shown that the fraction of gas adsorbed by the first [␣1⫽ Mg1/(Mg1⫹ Mg2⫹ Mg3)] and the second [␣2⫽ Mg2/(Mg2⫹ Mg3)] Teflon filters is almost the same (␣1 ⫽ ␣2 ⫽ ␣),
regardless of RH. Thus, the gas adsorbed by the first and second Teflon filters is
Mg1⫽ ␣Mg (10)
Mg2⫽ M2⫽ ␣共Mg⫺ Mg1兲 (11)
Similarly, the remaining gas collected on the final coated glass fiber filter can be represented as
Mg3⫽ M3⫽ 共1 ⫺ ␣兲共Mg⫺ Mg1兲 (12)
M2⫹ M3⫽ Mg⫺ Mg1 (13)
From eqs 10 and 13, we have
M2⫹ M3⫽ 共1 ⫺ ␣兲Mg (14)
The fraction of gas adsorbed by the Teflon filter,␣, can be calculated as
␣ ⫽ Mg2/共Mg2⫹ Mg3兲 ⫽ M2/共M2⫹ M3兲 (15)
From eq 15, we can obtain gaseous TDI, Mg, as
Mg⫽ 共M2⫹ M3兲2/M3⫽ 共M22⫹ 2M2M3⫹ M32兲/M3
⫽ 共M22⫹ M2M3兲/M3⫹ M2⫹ M3
or
Mg⫽ 共M2/M3兲共M2⫹ M3兲 ⫹ M2⫹ M3 (16)
After comparing eq 16 with eq 7, we have
Mg1⫽ 共M2/M3兲共M2⫹ M3兲 (17)
From eqs 3 and 9, we have
Ma⫽ M1⫺ Mg1 (18)
From eq 17, we can substitute the value of Mg1 ⫽ (M2/ M3)(M2⫹ M3) in eq 18 and obtain aerosol-phase TDI, Ma, as Ma⫽ M1⫺ 共M2/M3兲共M2⫹ M3兲 (19)
These equations only apply to unsaturated conditions of the Teflon filters. After attaining the saturation condition (see Figure 4), no further change in the adsorbed amount of TDI gas on filters occurs, and the amount adsorbed by the first and second filters becomes the same (Mg1⫽ Mg2
⫽ M2). Thus, from eqs 7 and 18, we have
Mg⫽ 2M2⫹ M3 (20)
Ma⫽ M1⫺ M2 (21)
However, eqs 20 and 21 can also be derived from the fact that as sampling goes on beyond saturation (1 L/min,10 min, 12.6 ppb [0.091 ng/m3]), the amount of gas collected
on the coated glass fiber filter increases continuously, and eventually M3is significantly greater than M2, so we can
replace M2⫹ M3by M3in eqs 16 and 19 and obtain eqs 20
and 21. The study reported by Hart and Pankow16using a Figure 6. Percentage of evaporated aerosol-phase TDI collected on
the Teflon filter.
Figure 7. The triple filter system.
Tsai, Cheng, Aggarwal, Shih, and Hung
two filter-polyurethane foam (PUF) adsorbent system for SOC sampling also supports eqs 20 and 21.
On the basis of our experimental results and the previous discussion, we can say that eqs 16 and 19 can be applied to estimate the accurate concentrations of gas-and aerosol-phase TDI whether the Teflon filters are sat-urated or not. The model can also be explained from Pankow’s theory.11–13
The Kp,sof the first Teflon filter can be calculated as
关Mg1/共W1⫻ af1⫻ 10⫺ 6兲兴/关共Mg1⫹ Mg2⫹ Mg3兲/共Q ⫻ t兲兴 (22)
where W1is the mass of the first Teflon filter (g); Q is the
sampling flow rate (m3/min); t is the sampling time (min);
and af1is the specific surface area of the first Teflon filter
(m2/g).
Similarly, the Kp,sof the second Teflon filter can be
calculated as
关Mg2/共W2⫻ af2⫻ 10⫺ 6兲兴/关共Mg2⫹ Mg3兲/共Q ⫻ t兲兴 (23)
In the triple filter system, the Teflon filters are positioned in series and sampling the same compound at exactly the same humidity, temperature, and time.
According to eq 2, PL o, m
r,s, and br,sof the two Teflon
filters are also the same so that Kp,sof the first Teflon filter
must be equal to that of the second Teflon filter. That is 关Mg1/共W1⫻ af1⫻ 10⫺ 6兲兴/关共Mg1⫹ Mg2⫹ Mg3兲/共Q ⫻ t兲兴
⫽ 关Mg2/共W2⫻ af2⫻ 10⫺ 6兲兴/关共Mg2⫹ Mg3兲/共Q ⫻ t兲兴
(24)
If the mass and specific surface area of the two Teflon filters are the same (W1⫽ W2;af1⫽ af2), then eq 24 can
be simplified as follows:
Mg1/共Mg1⫹ Mg2⫹ Mg3兲 ⫽ Mg2/共Mg2⫹ Mg3兲 (25)
This indicates that the fraction of TDI gas adsorbed by the first and second Teflon filters is the same, as predicted by our experiment. If we proceed further, then we can get eqs 20 and 21.
Comparison Test with the Reference Annular Denuder System
To confirm the ability of the triple filter system to sample the aerosol- and gas-phase TDI concentrations accurately, a comparison test using the reference annular denuder ‹ Figure 8. Results of comparison test between the triple filter and annular denuder systems: (a) total TDI, (b) aerosol-phase TDI, and (c) gaseous TDI. The data of the dual filter system are deduced from those of the triple filter system.
system was conducted in the laboratory. Both samplers were arranged in parallel after the mixing chamber in the experimental setup (see Figure 3). The experiment used the mixed-phase TDI stream of 10 different concentra-tions ranging from 2 to 60 ppb (0.434 ng/m3) at a flow
rate of 1 L/min. The humidity and temperature of the test were 82.7% and 29.8 °C, respectively.
Figures 8a-c show the comparison results of total TDI, aerosol-phase TDI, and gaseous TDI, respectively, col-lected by the triple filter and annular denuder systems. The data indicated as “dual filter system” in the figures were obtained from the sampling data of the triple filter system by using Ma⫽ M1and Mg⫽ M2⫹ M3(where Mais
the actual amount of TDI collected on the first Teflon filter, Mgis the total amount of gaseous TDI collected, and M1, M2, and M3are the amount of TDI collected on filters
-1, -2, and -3, respectively). Figure 8a shows that the total amount of TDI collected by the triple filter system is in good agreement with the total amount collected by the annular denuder system with R2⫽ 0.95. Figure 8b proves
the significant overestimation of TDI aerosol calculated from the dual filter system. But after the calibration with the proposed model for the triple filter system, the error can be minimized. The total amount of mixed-phase TDI is 1.03 times that of the denuder system. Similarly, differ-ences between the measured concentrations of the dual filter system and the denuder system also can be mini-mized by introducing the model of the triple filter system for gaseous TDI, as shown in Figure 8c.
CONCLUSIONS
The triple filter system sampler and the model proposed in this study can be applied for the accurate, simultaneous sampling and determination of gas- and aerosol-phase TDI in the ambient air. Results of the triple filter system are found to be in good agreement with the results of the reference annular denuder system. The triple filter system is simple and is shown to be much more accurate than the dual filter system in simultaneous sampling of gas- and aerosol-phase TDI. According to the experimental results, sampling duration and flow rate for the field sampling can be selected to be 15 min and 1 L/min, respectively, for the triple filter system. In the future, it will be worthwhile to study the triple filter system in the field for the effects of sampling time and other co-existing gases on the accuracy of TDI sampling.
ACKNOWLEDGMENTS
The authors are thankful to the Taiwan Institute of Occu-pational Safety and Health (IOSH) for financial support through contract IOSH90-A101.
REFERENCES
1. Streicher, R.P.; Christopher, M.R.; Key-Schwartz, R.; Schlecht, P.C.; Ellen, M. NIOSH Manual of Analytical Methods; National Institute for Occupational Safety and Health: Washington, DC, 1998; pp 115-132. 2. NIOSH Pocket Guide to Chemical Hazards; National Institute for
Occu-pational Safety and Health: Washington, DC, 1990; pp 90-117. 3. NIOSH Occupational Respiratory Diseases; National Institute for
Occu-pational Safety and Health: Washington, DC, 1986; pp 86-102. 4. NIOSH Criteria for a Recommended Standard—Occupational Exposure to
Diisocyanates; National Institute for Occupational Safety and Health:
Washington, DC, 1978.
5. Purnell, C.J.; Walker, R.F. Methods for the Determination of Atmo-spheric Organic Isocyanates: A Review; Analyst 1985, 110, 893-905. 6. Streicher, R.P.; Kennedy, E.R.; Lorberau, C.D. Strategies for the
Simul-taneous Collection of Vapours and Aerosols with Emphasis on Isocya-nate Sampling; Analyst 1994, 119, 89-97.
7. Levine, S.P. Hillig, K.J.D.; Dharmarajan, V.; Spence, M.W.; Baker, M.D. Critical Review of Methods of Sampling, Analysis, and Monitoring for TDI and MDI; Am. Ind. Hyg. Assoc. J. 1995, 56, 581-589.
8. Rando, R.J.; Poovey, H.G. Dichotomous Sampling of Vapour and Aero-sol of Methylene-bis-(phenylisocyanate) [MDI] with an Annular Dif-fusional Denuder; Am. Ind. Hyg. Assoc. J. 1994, 55, 716-721. 9. ISO-CHEK Isocyanate Sampling Cassette for 2, 4-TDI, 2, 6-TDI, 1, 6-HDI,
and MDI; Omega Specialty Instrument Co.: Chelmsford, MA, 1996.
10. Lesage, J.; Goyer, N.; Desjardins, F.; Vincent, J.Y.; Perrault, G. Workers’ Exposure to Isocyanate; Am. Ind. Hyg. Assoc. J. 1992, 53 (2), 146-153. 11. Mader, B.T.; Pankow, J.F. Gas/Solid Partitioning of Semivolatile Or-ganic Compounds (SOCs) to Air Filters. 1. Partitioning of Polychlori-nated Dibenzodioxins, PolychloriPolychlori-nated Dibenzofurans and Polycyclic Aromatic Hydrocarbons to Teflon Membrane Filters; Atmos. Environ.
2000, 34, 4879-4887.
12. Pankow, J.F. Review and Comparative Analysis of the Theories on Partitioning between the Gas and Aerosol Particulate Phase in the Atmosphere; Atmos. Environ. 1987, 21, 2275-2283.
13. Pankow, J.F. An Absorption Model of Gas/Aerosol Partitioning In-volved in the Formation of Secondary Organic Aerosol; Atmos. Environ.
1994, 28 (2), 189-193.
14. Tucker, W.A.; Nelken, L.H. Diffusion Coefficient in Air and Water. In
Handbook of Chemical Property Estimation Methods; Lyman, W.J., Reehl,
W.F., Rosenblatt, D.H., Eds.; McGraw-Hill: New York, 1982; pp 17.6-17.11.
15. Possanzini, M.; Febo, A.; Liberti, A. New Design of a High-Performance Denuder for Sampling of Atmospheric Pollutants; Atmos. Environ.
1983, 17, 2605-2610.
16. Hart, K.M.; Pankow, J.F. High-Volume Sampler for Particle and Gas Sampling. 2. Use of Backup Filter to Correct for the Adsorption of Gas-Phase PAHs to the Front Filter; Environ. Sci. Technol. 1994, 28, 655-661.
About the Authors
Chuen-Jinn Tsai is a professor, Kai-Chung Cheng is a graduate student, and Shankar G. Aggarwal is a postdoc-toral fellow with the Institute of Environmental Engineering at National Chiao Tung University in Hsin-chu, Taiwan. Tung-Sheng Shih is a director with the Institute of Oc-cupational Safety and Health at the Council of Labor Af-fairs in Taipei, Taiwan. I.-Fu Hung is a professor in the Department of Nuclear Science at National Tsing Hua Uni-versity in Hsin-chu, Taiwan. Address correspondence to: Chuen-Jinn Tsai, National Chiao Tung University, 5 Poai Street, Hsin-chu, Taiwan; fax: 886-3-5727835; e-mail: cjtsai@mail.nctu.edu.tw.