行政院國家科學委員會專題研究計畫 成果報告
利用精密複分解聚合之技術合成新型奈米級功能性高分子 材料與其應用(3/3)
研究成果報告(完整版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 96-2221-E-011-014-
執 行 期 間 : 96 年 08 月 01 日至 97 年 07 月 31 日 執 行 單 位 : 國立臺灣科技大學化學工程系
計 畫 主 持 人 : 廖德章
計畫參與人員: 碩士班研究生-兼任助理人員:張鳳全 碩士班研究生-兼任助理人員:李威逸 大專生-兼任助理人員:傅俊偉
博士班研究生-兼任助理人員:黃慶成 博士班研究生-兼任助理人員:陳文祥
報 告 附 件 : 出席國際會議研究心得報告及發表論文
處 理 方 式 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢
中 華 民 國 97 年 11 月 01 日
行政院國家科學委員會補助專題研究計畫 □ 成 果 報 告
□期中進度報告
利用精密複分解聚合之技術合成新型功能性奈米級之高分子 材料與其應用
計畫類別:□ 個別型計畫 □ 整合型計畫 計畫編號:NSC 96-2221-E-011-014
執行期間:94 年 8 月 1 日至 97 年 7 月 31 日
計畫主持人:廖德章 共同主持人:
計畫參與人員:黃慶成、陳文祥、李威逸、張鳳全、傅俊偉
成果報告類型(依經費核定清單規定繳交):□精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣科技大學
中 華 民 國 97 年 10 月 31 日
目錄
摘要………. p.3
計畫緣由與目的………. p.5
儀器與設備………. p.7
實驗………. p.8
結果與討論………. p.13
結論………. p.22
計畫成果自評………. p.23
致謝………. p.23
參考文獻………. p.23
反應式、表格、圖………. p.25
摘要
利用原冰片烯甲醇和四種不同取代基醯氯製備四種含不同側鏈酯基基團(辛烷 酯基、癸烷酯基、苯甲酯基及十二烷酯基)之原冰片烯單體(NBMO、NBMD、NBMDO 和NBMB) 。 將 四 種 含 不 同 側 鏈 酯 基 之 原 冰 片 烯 單 體 與 釕 金 屬 觸 媒 {Cl2Ru(CHPh)[P(C6H11)3]2}進行精密複分解聚合。此四種聚合物之數目平均分子量 為112,000至201,000,廣分佈指數(PDI)介於1.4-2.2之間。
將原冰片烯甲基辛烷酯單體(NBMO)分別與原冰片烯甲基三甲基矽烷單體 (NBMTMS)和原冰片烯甲基酞酐單體(NBMPI)利用釕金屬觸媒進行精密複分解聚 合 反 應 , 合 成 新 型 隨 機 共 聚 合 物 , 再 分 別 進 行 水 解(Hydrolysis) 及 胂 解 (Hydrazinolysis)反應,製備側鏈含有功能性羥基和功能性胺基之原冰片烯隨機共聚 合物。其數目平均分子量介於62,100至153,000,廣分佈指數(PDI)介於1.73-6.21之 間,10%熱重損失溫度分別介於355-401℃之間,並可溶於一般有機溶劑如四氫呋 喃、氯仿、二氯甲烷、苯、二甲苯、甲苯等。
利用此功能性原冰片烯隨機共聚合物與甲基丙烯基氧乙基異氰酸酯(MOI)進 行反應,得到新型側鏈含酯基基團與可交聯基團之原冰片烯隨機共聚合物,進一 步利用甲基丙烯酸甲酯與側鏈含有酯基基團與可交聯基團之原冰片烯共聚合物在 自由基起始劑的作用下進行交聯反應,形成AB 交聯共聚合物,進一步比較AB交 聯共聚合物與聚甲基丙烯酸甲酯之熱性質,發現此AB交聯共聚合物在氮氣下之 10%熱重損失溫度為367 ℃,比聚甲基丙烯酸甲酯在氮氣下之10%熱重損失溫度 (325℃)增加了42 ℃。
將原冰片烯甲基辛烷酯單體(NBMO)與含保護基之原冰片烯甲基酞酐單體 (NBMPI),利用釕金屬觸媒進行精密複分解聚合反應,可獲得新型隨機與嵌段共聚 合物。此新型隨機與嵌段共聚合物經氫化、胂解、四級化後,可獲得高穩定性之 新型兩親媒性隨機與嵌段共聚合物(Nano-scale polymeric materials,奈米級之高分 子材料)。藉由改變親水基和疏水基含量之比例,製備一系列水溶性原冰片烯隨機 與嵌段共聚合物(Nano-scale polymeric materials,奈米級之高分子材料),並在水溶 液中自組裝形成微胞。由螢光光譜可確認微胞在溶液中的形成。微胞進一步可利 用掃描式電子顯微鏡(SEM)和穿透式電子顯微鏡(TEM)技術鑑定高分子奈米微粒 的形成。奈米級高分子微胞在銨鹽濃度50-75 mol% 及側鏈酯基基團濃度50-25 mol%有均勻之分布,其大小經掃描式電子顯微鏡(SEM)和穿透式電子顯微鏡 (TEM)測定為6.8至18.1奈米和5.0至13.7奈米(nm)。
關鍵詞:
精密複分解聚合,側鏈酯基基團,甲基丙烯基氧乙基異氰酸酯,可交聯聚合物,
兩親媒性,自組裝,奈米級之高分子材料
英文摘要
Four kinds of norborene monomers containing pendent ester group are prepared by reacting 5-(hydroxy methyl)bicyclo[2.2.1]hept-2-ene and four kinds of the substituents of the carboxylic acid chloride. Novel pendent ester group homopolymers of 5-(octanoate methyl)bicyclo[2.2.1]hept-2-ene (NBMO), 5-(decanoate methyl) bicycle [2.2.1]hept-2-ene (NBMD), 5-(dodecanoate methyl)bicyclo[2.2.1]hept-2-ene (NBMDO) and 5-(benzoate methyl)bicyclo[2.2.1]hept-2-ene (NBMB) have been synthesized by precision metathesis polymerization using Ru catalyst{Cl2Ru(CHPh)[P(C6H11)3]2}.
These four polymers with molecular weight( Mn ) between 112,000 to 201,000 and polydispersities ( Mw / Mn =1.4-2.2) were prepared.
Novel random copolymers of 5-(trimethylsiloxymethyl)bicyclo[2.2.1]hept-2-ene (NBMTMS) or 5-(phthalimide methyl)bicyclo[2.2.1]hept-2-ene (NBMPI) with NBMO have been synthesized by precision metathesis polymerization using Ru catalyst {Cl2Ru(CHPh)[P(C6H11)3]2}. After hydrolysis and hydrazinolysis of the random copolymers, the random copolymers containing functional hydroxyl group and functional amino group were also obtained. Molecular weight( Mn ) are between 62,100 to 153,000 and polydispersity index are between 1.73 and 6.21. The temperatures of 10 % weight loss of these random copolymers were in the range of 255
-355 ℃ and were highly soluble in common organic solvents such as THF, chloroform, dichloromethane, benzene, xylene and toluene. Novel cross-linkable random copolymers were prepared from random copolymers containing functional hydroxyl group and functional amino group with 2-methacryloyloxyethyl isocyanate (MOI).
Reaction of this multifunctionalized methacryloyl polymer with methyl methacrylate (MMA) under free-radical polymerization conditions has led to the formation of AB cross-linked system. The thermal stability of AB cross-linked material of poly(MMA) and random poly(NBMO-co-NBMOAC) [decomposition temperature (Td10%) =367 ℃ in nitrogen] was enhanced by 42 ℃ compared with poly(MMA) (Td10% = 325 ℃ in nitrogen).
Novel amphiphilic random and block copolymers were obtained by precision metathesis polymerization of ester-bridged monomer (Nano-scale polymeric materials) , NBMO and NBMPI by using Ru catalyst. Highly stable copolynorbornenes containing hydrophilic ammonium salt and hydrophobic alkyl ester group (Nano-scale polymeric materials) were obtained by ring-opening metathesis polymerization (ROMP), hydrogenation, hydrazinolysis, and subsequent quaternization. Various random copolymers with varying compositions of bicyclo[2.2.1]hept-5-ene-2-methyl octanoate (NBMO) and 5-(phthalimide methyl)-bicyclo[2.2.1]hept-2-ene (NBMPI) were synthesized to control the interaction and microstructure of aggregates via ROMP. The formation, structure, and morphologies of the aggregates were investigated. The association of polymer molecules in water were be confirmed by fluorescence spectroscopy. The size and morphologies of aggregates were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Nanometer-scale polymeric micelles having a uniform distribution of such random copolymers with 50-75 mol % of ammonium salts and 50-25 mol % of alkyl ester group were obtained, and their sizes were characterized by SEM (6.8-18.1 nm) and TEM (5.0-13.7 nm).
Keywords :
precision metathesis polymerization, pendent ester group, 2-methacryloyloxyethyl isocyanate, cross-linkable polymer, amphiphilic, self-assembly , Nano-scale polymeric
materials
計畫緣由與目的
烯類複分解聚合 (Olefin metathesis polymerization) 在高分子合成上是一個很 重要的方法。近年來在高分子材枓的合成上,環烯類精密複分解聚合與非環二烯
類及炔類的複分解聚合日趨重要。1隨著各種新型觸媒開發,對於帶有各式官能基
的高分子合成材枓也越來越多,成為重要的功能性高分子材枓合成方法之一。
有機金屬觸媒用於複分解聚合已有一段時日,但對有官能基之單體在聚合時 會有所限制,同時水份或氧氣的存在對聚合也相當敏感。例如鎢 (W) 、鈦 (Ti) 、 鉬 (Mo) 及釕 (Ru) 等金屬化合物為較常用的環烯類精密複分解聚合觸媒,其中釕 金屬觸媒 (Ru catalyst) 對水份或氧氣的安定性 (Stability) 或相容度 (Tolerance)
較佳,甚至可於水溶液中進行聚合反應。2例如 1996 年 Grubbs 等人新開發的釕
碳烯配位觸媒 {Cl2Ru(CHPh)[P(C6H11)3]2},3對環烯類精密複分解聚合非常有效,
尤其在空氣中還算安定,可進行具有官能基單體的聚合。另外亦具有高聚合速率 及高分子量之特性,一般而言均具有活性聚合 (Living polymerization) 的現象。
利用環烯類衍生物為單體所進行的精密複分解聚合之相關研究越來越多,主 要有側鏈型液晶,4-6兩步法 (Two step) 合成三嵌段 (Triblock) 共聚合物,7各種官
能基導入之聚合物。8, 9其中官能基的導入,使聚合物具有光電性質10及生化活性。
11
聚原冰片烯 (Polynorbornene) 及其衍生物是第一個藉精密複分解聚合之商業
化產品,為重要的工程塑膠之一。12因其透明性良好、耐衝擊性佳 (橡膠添加劑) 、
廣泛之溫度使用範圍、良好之機械物性和加工性。廣用於照明器具、機械、電子 零件、管件和食品包裝等。又其衍生物如酸及酯類的聚合物更被當作電子產業的
光阻劑。13-15國內有關精密複分解聚合的研究,據筆者所知還算很少,尤其在大學
之學術機構更少,對於此一極具潛力的高分子材料之合成方法更顯示出其重要性。
由於近年來奈米技術與奈米材料的重要性提升,高分子奈米材料中嵌段聚合 物又扮演重要角色,而精密複分解聚合技術有利於發展嵌段聚合物,若材料具兩 親媒性更可形成自組裝性奈米微粒材料。
本計畫主要是在高壓反應器中藉由 Diels-Alder 反應16-21合成出多種新的原冰 片烯衍生物,進一步合成出新型原冰片烯聚合物及新型原冰片烯共聚合物,並研 究其聚合行為與物性,對原冰片烯衍生物的精密複分解聚合相關領域有更深入的 了解與貢獻。
計畫第一年成功合成出四種含有側鏈酯基基團的原冰片烯衍生物,並進一步 利用釕金屬觸媒對此四種具有側鏈酯基基團的原冰片烯衍生物進行聚合。透過本 計畫第一年對所合成四種具有側鏈酯基基團之原冰片烯單體進行聚合行為研究,
並製備四種具有側鏈酯基基團的原冰片烯聚合物材料,相關物性及鑑定結果於後 詳述。
計畫第二年成功合成具有保護基團之原冰片烯單體,並進一步利用釕金屬觸 媒對此具有保護基團之原冰片烯單體與本計畫第一年所製備之具有側鏈酯基基團 的原冰片烯單體進行隨機共聚合反應,然後水解除去保護基團,分別製備出新型 含有功能性羥基及胺基之原冰片烯共聚合物材料,並研究其物性。此共聚合物進 一步與甲基丙烯基氧乙基異氰酸酯 (MOI) 進行縮合反應,製備側鏈含有酯基基團 和末端含可交聯基團之原冰片烯隨機共聚合物,並利用甲基丙烯酸甲酯與側鏈含
有酯基基團和末端含可交聯基團之原冰片烯隨機共聚合物進行 AB 交聯共聚合反
應。另外利用甲基丙烯酸甲酯進行自由基聚合製備得到聚甲基丙烯酸甲酯,進而
比較AB 交聯共聚合物與聚甲基丙烯酸甲酯之熱性質,其結果及數據於後詳述。
計畫第三年,利用精密複分解聚合,合成新型含酯基及酞酐基之原冰片烯隨 機和嵌段共聚合物。此新型原冰片烯隨機和嵌段共聚合物經氫化、胂解、四級化 後,可獲得具高穩定性之新型兩親媒性原冰片烯隨機和嵌段共聚合物 (Nano-scale polymeric materials, 奈米級之高分子材料) 。藉由改變親水基團和疏水基團含量 之比例,製備一系列水溶性原冰片烯隨機和嵌段共聚合物 (Nano-scale polymeric materials, 奈米級之高分子材料) ,並在水溶液中自組裝形成微胞 (Nano-scale polymeric materials, 奈米級之高分子材料) 。由螢光光譜可確認微胞在溶液中的 形成。微胞進一步可利用掃描式電子顯微鏡 (SEM) 和穿透式電子顯微鏡 (TEM) 技術鑑定,其結果於後詳述。
儀器與設備
(1) 紅外線光譜儀(IR),型號為 JASCO IR-9700,測試範圍為 4000-400cm-1,測
試法為利用KBr 打片測試而得。
(2) 13C 及1H NMR 核磁共振光譜儀,型號為 Bruker DRX-500,測試範圍為 125 MHz 到 500 MHz 之間。所使用的溶劑為重氫氯仿(CDCl3)。
(3) 示差熱分析儀(DSC),型號為 Du pont 9000 DSC system,設定升溫速率為每 分鐘10℃。
(4) 毛細管熔點測定儀,型號為 Model BüCHI 535。
(5) 元素分析儀(Elemental analyzer),型號為 Perkin-Elmer 2400。
(6) 熱重分析儀(TGA),型號為 ULVAC (Sinku-Riko) model 7000,升溫速率為每
分鐘10℃,氮氣流率為每分鐘 60 立方公分。
(7) 凝膠滲透層析儀(GPC),由五根 Waters (Ultrastyragel) 所構成 [300 × 7.7 mm (guard, 500, 103, 104, 105 Å in a series)],四氫呋喃為移動相,使用 RI 偵測器測 定樣品之分子量,聚苯乙烯(Polystyrene)作為標準品。
(8) 掃描式電子顯鏡 (SEM),型號為 Model BüCHI 535,放大倍率: 5x~30Kx。
(9) 穿透式電子顯微鏡(TEM),型號為 JEOL 2000FXII,放大倍率: 200x~800Kx。
(10) 螢光光譜儀(Spectrofluorophotometer),型號為 SHIMADZU RF-5301PC。
實驗:
第一年度
Synthesis of Ester-Containing Norbornenes
Ester derivatives of 5-(octanoate methyl)bicyclo[2.2.1]hept-2-ene (NBMO),5- (decanoate methyl)bicyclo[2.2.1]hept-2-ene(NBMD),5-(dodecanoate methyl) bicycle [2.2.1]hept-2-ene(NBMDO),and 5-(benzoatemethyl) bicyclo[2.2.1]hept-2-ene (NBMB) were prepared according to earlier literature
(Table 1).
22,23General Procedure of ROMP of Various Monomer(s)
A solution of catalyst was prepared by dissolving metathesis catalyst 1 (3 mg, 3.6 x 10-3 mmol) in 1 mL of anhydrous methylene chloride in an argon-filled dry box. The monomer(s) (0.8 mmol) were dissolved in 10 mL of methylene chloride and then degassed via a freeze-pump-thaw cycle. After degassing the monomer(s) solution completely, the catalyst solution was injected into the monomer(s) solution by a syringe.
The reaction was performed at 30 ℃ for 2h. The polymerization reaction was terminated by the addition of a small amount of ethyl vinyl ether (0.2 mL) and homo/copolymer were obtained by precipitating in excess methanol. The yields of the polymers were in the range of 72 and 94%.
第二年度
Random Copolymer of NBMO and NBMTMS [Scheme. 1 (a)].
The 5-(trimethylsiloxy methyl)bicyclo[2.2.1]hept-2-ene (NBMTMS) (44–46 ℃ /2 mmHg, endo/exo = 82:18) was prepared by the reaction of 5-(hydroxy methyl) bicyclo[2.2.1]hept-2-ene with an equimolar solution of trimethylchlorosilane and triethylamine in diethyl ether.24 A solution of catalyst was prepared by dissolving metathesis catalyst {Cl2Ru(CHPh)[P(C6H11)3]2} (5 mg, 6.1x10-3 mmol) in 1 mL of anhydrous methylene chloride in an argon-filled dry box. The monomers, NBMO (0.2 g, 0.8 mmol) and NBMTMS (157 mg, 0.08 mmol), were dissolved in 10 mL of methylene chloride and then degassed via a freeze-pump-thaw cycle. After degassing the monomer solution completely, the catalyst solution was injected into the monomer solution by a syringe. The polymerization was performed at 30 ℃ for 2 h. The color of the solution changed from pink to yellow after 10 min when the reaction was performed at 30 ℃.
The reaction was terminated by the addition of a small amount of ethyl vinyl ether (0.2 mL) and random copolymer [random poly(NBMO-co-NBMTMS)] was obtained by precipitating in excess methanol (300 mL). The white-colored polymer was further purified by dissolving it in methylene chloride and re-precipitating it with methanol and drying it overnight under vacuum at ambient temperature. The yield of random poly(NBMO-co-NBMTMS) was 86%. The IR spectrum of random poly (NBMO
-co-NBMTMS) exhibited bands at 1730 and 1102 cm
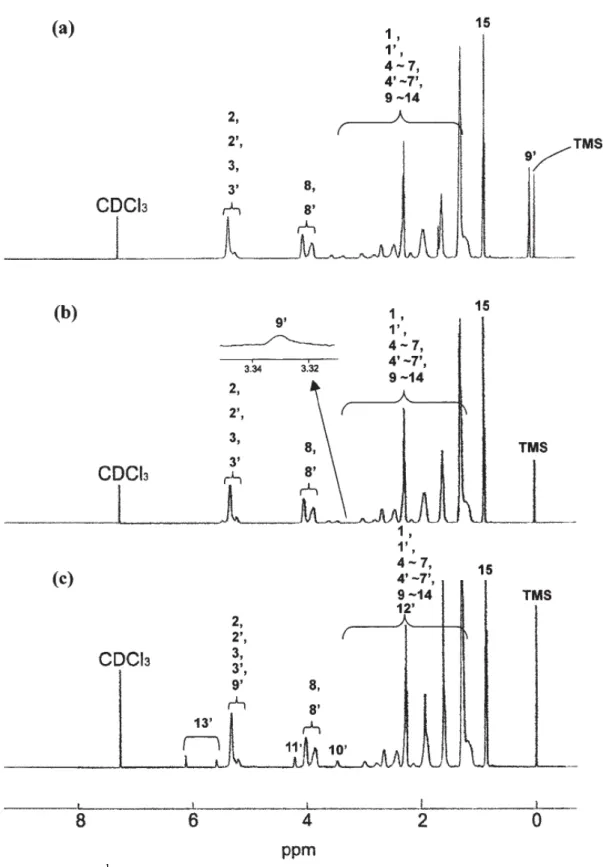
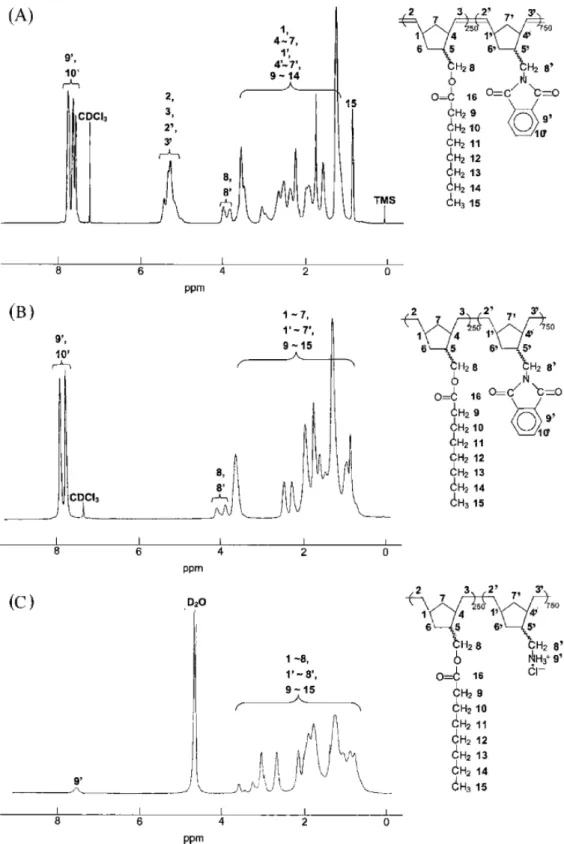
-1 due to stretching of carbonyl andO-Si(CH3)3 groups, respectively. 1H NMR (500 MHz, CDCl3, ppm): 0.07–0.09 (H9’;9H), 0.87–0.89 (H15; 3H), 1.20–3.00 (H1, H10, H4,H4’, H5, H5’, H6, H6’, H7, H7’, and H9–H14; 26H), 3.32–4.03 (H8 and H8’; 4H) and 5.22–5.34 (H2, H2’, H3, and H3’; 4H). 13C NMR (125 MHz, CDCl3, ppm): 0.41 (C9’), 14.50 (C15), 23.02 (C14), 25.44 (C13), 29.37 (C12), 29.57 (C11), 32.10 (C10), 34.87 (C9), 36.27–47.00 (C1, C1’, C4, C4’, C5, C5’, C6, C6’, C7, and C7’), 66.49–67.35 (C8 and C8’), 129.86–135.25 (C2, C2’, C3, and C3’) and 174.27 (C16) [Figure 1 a].
Random Copolymer of NBMO and NBMPI [Scheme. 1 (b) and 2(f)].
The 5-(phthalimide methyl)bicyclo[2.2.1]hept-2-ene (NBMPI) was prepared by azotropic removal of water from an equimolar solution of aminocontaining norbornene[5-(amino methyl)bicyclo[2.2.1]hept-2-ene] and phthalic anhydride in xylene.22 The melting point was about 99–100℃.25 A solution of catalyst was prepared by dissolving{Cl2Ru(CHPh)[P(C6H11)3]2} catalyst, (5 mg, 6.1x10-3 mmol) in 1 mL of anhydrous methylene chloride in an argon-filled dry box. The monomers NBMO (0.2 g, 0.8 mmol) and NBMPI (20.3 mg, 0.08 mmol) were dissolved in 10 mL of methylene chloride and then degassed via a freeze-pump thaw cycle. After degassing the solution completely, the catalyst solution was injected into the monomer solution by a syringe.
The polymerization was performed at 30℃ for 2 h. The color of the solution changed from pink to yellow after 10 min when the reaction was performed at 30℃. The reaction was terminated by the addition of a small amount of ethyl vinyl ether (0.2 mL) and random copolymer [random poly(NBMO-co-NBMPI)] was obtained by precipitating in excess methanol (300 mL). The white-colored polymer was further purified by dissolving in methylene chloride and re-precipitating with methanol and drying overnight under vacuum at ambient temperature. The yield of random poly(NBMO-co-NBMPI) was 92% [ Mn =8.67 x 104; polydispersity index (PDI) = 2.91]. 1H NMR (500 MHz, CDCl3, ppm): 0.86–0.89 (H15; 3H), 1.10–3.00 (H1, H10, H4, H4’, H5, H5’, H6, H6’, H7, H7’, and H9–H14; 26H), 3.87–4.03 (H8 and H8’; 4H), 5.21–5.33 (H2, H2’, H3, and H3’; 4H), 7.69 (H10’; 2H), 7.81 (H9’; 2H). 13C NMR (125 MHz, CDCl3, ppm): 14.03 (C15), 22.55 (C14), 24.97 (C13), 28.89 (C12), 29.10 (C11), 31.63 (C10), 34.40 (C9), 35.79–46.50 (C1, C10, C4, C4’, C5, C5’, C6, C6’, C7, and C7’), 66.01–66.90 (C8 and C8’), 123.06 (C9’), 129.41–134.76 (C2, C2’, C3, and C3’), 132.08 (C11’),133.74 (C10’), 168.44 (C12’), 173.78 (C16).
Hydrolysis of Random Poly(NBMO-co-NBMTMS) [Scheme. 1 (a) and 2(b)].
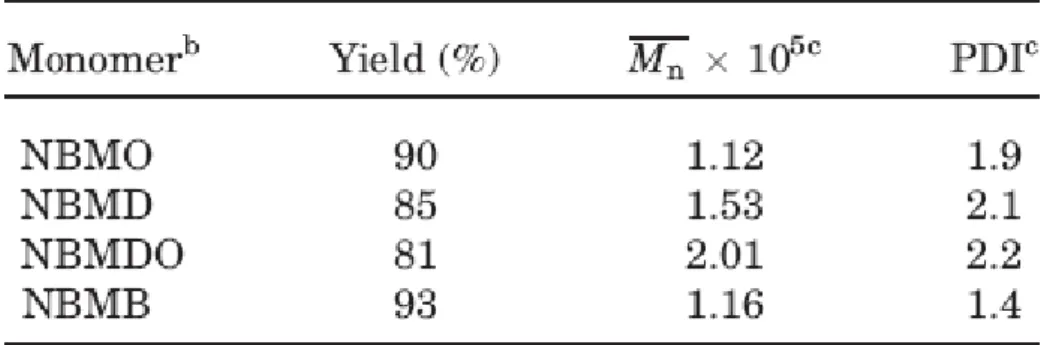
Random poly(NBMO-co-NBMTMS) (0.2 g, 0.8 mmol) was hydrolyzed in 15 mL of tetrahydrofuran under HCl(aq) catalyzed conditions [1 mL of 1 N HCl(aq)], and reaction mixture was stirred at room temperature for 3 h. The random copolymer with hydroxyl group, random poly(NBMO-co-NBMOH), was then precipitated in excess methanol (yield = 81%). The IR spectrum of random poly(NBMO-co-NBMOH) exhibited a broad band at 3481 cm-1 due to hydroxyl group and a band at 1723 cm-1 due to stretching of carbonyl group. 1H NMR (500 MHz, CDCl3, ppm): 0.87–0.89 (H15; 3H), 1.16–3.00 (H1, H10, H4, H40, H5, H5’, H6, H6, H7, H7’ and H9–H14; 26H), 3.33 (H9’; 1H), 3.86–4.03 (H8 and H8’; 4H), 5.21–5.47 (H2, H2’, H3 and H3’; 4H). 13C NMR (125
MHz, CDCl3, ppm): 14.09 (C15), 22.62 (C14), 25.03 (C13), 28.96 (C12), 29.17 (C11), 31.69 (C10), 34.46 (C9), 35.83–44.39 (C1, C10, C4, C40, C5, C50, C6, C60, C7 and C7’), 66.09–66.98 (C8 and C8’), 129.54–134.84 (C2, C2’, C3 and C3’), 173.88 (C16)
[Figure 1 b].
Hydrazinolysis of Random Poly(NBMO-co-NBMPI) [Scheme. 1 (b) and 2 (g)].
Random poly(NBMO-co-NBMPI) (0.4 g, 1.60 mmol) was suspended in 15 mL of ethanol in Schlenk tube. To the above mixture, 5 mL of hydrazine monohydrate was added. The mixture was degassed via a freeze-pump-thaw cycle, then the tube was heated to 100℃. Hydrazinolysis was performed for 12 h, and then the solution was cooled to room temperature. The random copolymer with amine group, random poly(NBMO-co-NBMA) was precipitated in excess methanol (yield = 83%). 1H NMR (500 MHz, CDCl3, ppm): 0.87–0.90 (H15; 3H), 1.10–3.00 (H1, H10, H4, H4’, H5, H5’, H6, H6’, H7, H7’ and H9–H14; 26H), 3.87–4.04 (H8 and H8’; 4H), 5.22–5.46 (H2, H2’, H3, H3’ and H9’; 6H). 13C NMR (125 MHz, CDCl3, ppm): 14.07 (C15), 22.59 (C14), 25.01 (C13), 28.94 (C12), 29.14 (C11), 31.67 (C10), 34.45 (C9), 35.80–44.19 (C1, C10, C4, C4’, C5, C5’, C6, C6’, C7 and C7’), 66.06–66.90 (C8 and C8’), 129.50–134.83 (C2, C2’, C3 and C3’), 173.87 (C16).
Modification of Random Poly(NBMO-co-NBMOH) and Random Poly(NBMO–
co-NBMA)
[Scheme. 1 (a) and (b)].
Random poly(NBMO-co-NBMOH) and random poly(NBMO-co-NBMA) were modified by reaction with MOI. A mixture of 0.25 g of polymer and 0.025 g of MOI was dissolved in 15 mL of tetrahydrofuran (THF), and continuously stirred at room temperature for 4 h. The modified random copolymers were then precipitated in excess methanol. The yields of modified random copolymers, random poly(NBMO-co-NBMO AC) and random poly(NBMO-co-NBMUAC) were in the range of 79 and 84%.
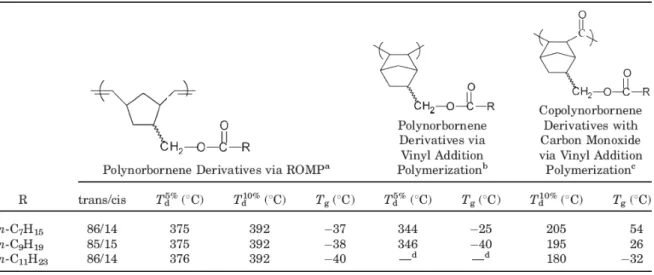
Random Poly(NBMO-co-NBMOAC) [Scheme. 1 (a) and 2 (d)].
The IR spectrum of random poly(NBMO-co-NBMOAC) exhibited an absorption band at 1645 cm
-1due to C=C of vinylic group of acryloyl moiety.
1H NMR (500 MHz, CDCl
3, ppm): 0.87–0.89 (H
15; 3H), 1.20–3.00 (H
1, H
1’,H
4, H
4’, H
5, H
5’, H
6, H
6’, H
7, H
7’, H
12’, and H
9–H
14; 29H), 3.48 (H
10’; 2H), 3.86–4.03 (H
8and H
8’; 4H), 4.22 (H
11’; 2H), 5.21–5.34 (H
2, H
2’, H
3,H
3’, and H
9’; 5H), 5.59 and 6.12 (H
13’; 2H).
13C NMR(125 MHz, CDCl
3, ppm): 14.09 (C
15), 18.33 (C
12’), 22.62 (C
14), 25.03 (C
13), 28.96 (C
12), 29.17 (C
11), 31.69 (C
10), 34.46 (C
9), 35.82–46.50 (C
1,C
1’, C
4, C
4’, C
5, C
5’, C
6, C
6’, C
7, C
7’and C
10’), 63.74 (C
11’), 66.09–66.95 (C
8and C
8’), 126.00(C
13’), 129.51–136.00 (C
2, C
2’, C
3, C
3’and C
14’), 167.24 (C
15’), 173.88 (C
16’and C
16) [Figure 1 c].
Random Poly(NBMO-co-NBMUAC) [Scheme. 1 (c) and 2 (h)].
The IR spectrum of random poly(NBMO-co-NBMUAC) exhibited an absorption band at 1640 cm-1 due to C=C of vinylic group of acryloyl moiety. 1H NMR (CDCl3, ppm):
0.87–0.88 (H15; 3H), 1.20–3.00 (H1, H10, H4, H4’, H5,H5’, H6, H6’, H7, H7’, H13’, and H9–H14; 29H),3.50 (H11’; 2H), 3.88–4.04 (H8 and H8’; 4H), 4.22(H12’; 2H), 5.22–5.34 (H2, H2’, H3, H3’, H9’, and H10’; 6H), 5.60 and 6.13 (H14’; 2H). 13C NMR(125 MHz, CDCl3, ppm): 14.08 (C15), 18.31(C13’), 22.60 (C14), 25.01 (C13), 28.94 (C12), 29.14(C11), 31.67 (C10), 34.45 (C9), 36.80–44.42 (C1,C1’, C4, C4’, C5, C5’, C6, C6’, C7, and C7’), 40.11(C11’), 63.70 (C12’), 66.06–67.00 (C8 and C8’),126.00 (C14’), 129.50–134.85 (C2, C2’, C3, C3’, and C15’), 167.24 (C16’), 173.86 (C17’ and C16).
Saponification and Modification of Poly(NBMO) [Scheme. 1 (c) and 2 (c,e)].
A 100-mL two-necked flask equipped with a gas inlet (for Argon) was charged with 10 mL of THF containing 0.4 g (1.6 mmol) of poly(NBMO) and 0.22 g (1.6 mmol) of potassium carbonate (K
2CO
3). Methanol (1 mL) was added to solution with stirring for 15 min at room temperature.The reaction mixture was then poured into excess methanol (300 mL) and the random poly(NBMO-co-SNBMOH) was precipitated. Subsequently, random poly(NBMO-co-SNBMOH) was reacted with MOI. The yield of modified random poly(NBMO-co-SNBMOAC) was 94%. In the
1H NMR (500 MHz, CDCl
3) spectrum of random poly(NBMO-co-SNBMOAC), the vinylic proton peaks of crosslinkable acryloyl moiety were observed at δ (ppm) 5.60 and 6.13.
AB Cross-linked Systems of Random Poly(NBMO-co-NBMOAC) and Poly(MMA) Random poly(NBMO-co-NBMOAC), 20 wt % based on MMA, was dissolved in 0.8 g of MMA and benzoyl peroxide; 1 wt % based on MMA, was added to the polymer solution as a free radical initiator. The solution of random poly(NBMO-co-NBMOAC) and MMA was degassed three times via a freeze-pump-thaw cycle and then heated at 90 ℃ for 16 h. The MMA underwent polymerization and cross-linking reaction simultaneously with random poly(NBMO-co-NBMOAC).
第三年度
Preparation of Block Copolymer of NBMPI and NBMO via Living ROMP
Block copolymer of monomers, NBMPI and NBMO, was obtained by ROMP by using Ru catalyst 1. The monomer(1.24 g, 4.9 mmol ) ( [NBMPI] / [catalyst 1] = 500), NBMPI was dissolved in 10 mL of anhydrous methylene chloride and then degassed via a freeze-pump-thaw cycle. After complete degassing, the catalyst solution [2 mg of Ru catalyst 1 (2.45 x 10-3 mmol) in 0.5 mL of anhydrous methylene chloride] was injected into the monomer solution by a syringe. The pink solution was vigorously stirred at ambient temperature for 1 h. Prepolymer [poly(NBMPI)] with Mn = 1.24 x 105 and PDI=1.4 was determined using gel permeation chromatography (GPC). Additional monomer solution of NBMO (1.23 g, 4.9 mmol)([NBMO] / [catalyst 1] = 500) in methylene chloride was injected to the still-living reaction mixture and the solution was stirred for another 2 h at 30℃ . The reaction was terminated by the addition of a small amount of ethyl vinyl ether. After termination, the solution was stirred for an additional 5 min, and the block copolymer, poly(NBMPI-b-NBMO)-2, was precipitated in excess of methanol and the white polymer was further purified by dissolving in methylene chloride and reprecipitating in methanol.
Hydrogenation, Hydrazinolysis, and Quaternization of Poly(NBMPI-b-NBMO) (Scheme 3)
Hydrogenation of poly(NBMPI-b-NBMO)-3 was conducted in a typical experiment by using p-toluenesulfonhydrazide as hydrogenating agent as described in the previous reaction. The yield of poly(HNBMPI-b-HNBMO)-3 was 88%. In the 1H NMR (500 MHz, CDCl3) spectrum of poly(HNBMPI-b-HNBMO)-3, the peak at δ (ppm) 5.2–5.6 (olefinic) present in the reactant NMR spectrum had disappeared. The yield of quaternized block copolymer, poly(HNBMQ-b-HNBMO)-3, was 75%.
結果與討論:
第一年度
Characterization of Various Ester-bridged Polynorbornenes
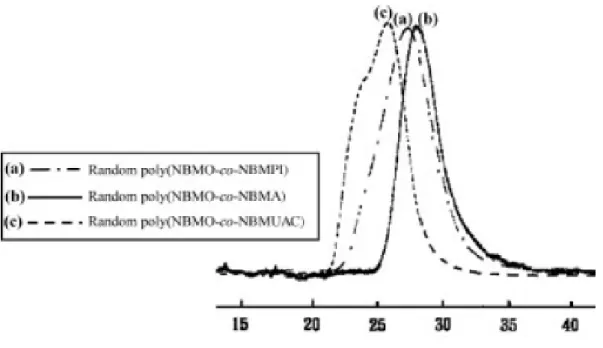
Ester-bridged polynorbornenes with high molecular weights and yields were observed in ROMP of the ester-containing monomers such as NBMO, NBMD, NBMDO, and NBMB (Table 2). The broad PDI values were observed indicating that ROMP of ester-containing monomers was performed in nonliving manner. It might be attributable to the functional ester group tolerance of Ru catalyst.26 The 1H NMR spectrum of poly(NBMO) shows resonances due to the olefinic resonance protons of the polymer at δ (ppm) 5.21 and 5.33. The ratio of cis-to-trans contents in the polymer was found to be 14 : 86. In the 13C NMR spectrum of poly(NBMO), the signals at δ (ppm) 35.82–46.55 are ascribed to the carbons in cyclic structure of the polymer. The various olefinic carbon signals of the ring opened polymer backbone appeared between δ (ppm) 129.47 and 134.86. Polymers [poly(NBMO), poly(NBMD), poly(NBMDO), and poly(NBMB)]
were highly soluble in common organic solvents such as THF, chloroform, methylene chloride, and benzene, but partially soluble in dimethylsulfoxide (DMSO),
N,N-dimethylacetamide (DMAc) and N,N-dimethylformamide(DMF). Breunig et al
27 and Liaw et al.28 reported that the length of alkyl side chains of polynorbornene and copolynorbornene with carbon oxide via vinyl addition polymerization had a very important role on the glass-transition temperature (Tg). Interestingly, polynorbornenes containing various alkyl ester groups showed little effect on Tg values (about -39℃) via ROMP in this study (Table 3), which is attributed to the soft main chain of polynorbornene, indicating that the soft main chain of polynorbornene via ROMP had a more important role for Tg values than that of polynorbornene and copolynorbornene with carbon oxide via vinyl addition polymerization. Furthermore, ROMP-type polynorbornenes with alkyl ester side chains such as poly(NBMO), poly(NBMD), and poly(NBMDO) exhibited glass transition at a lower temperature than poly(NBMB) with phenyl ester side chain (56℃). However, they underwent decomposition at a higher temperature of 10 % weight loss than poly(NBMB) (372 ℃ in N2). Poor thermal stability of poly(NBMB) might be attributed to the thermal degradation by molecular rearrangement involving five-membered cyclic acetoxonium type of ion formed by π electrons of benzene adjacent to ester carbonyl.29 These types of rearrangements are often rationalized on the basis of low-energy transitions.29 The other polymers, poly(NBMO), poly(NBMD) and poly(NBMDO), do not posses any π electrons for facilitating similar type of rearrangements and hence fragmentation is difficult in these cases. Similar behavior of polymer with phenyl side chain was observed by Breunig and Risse.22第二年度
Characterization of Random Poly(NBMO-co-SNBMOH) , Poly(NBMO–co- SNBMOAC), Random Poly(NBMO-co-NBMTMS), Random Poly(NBMO-co- NBMOH), and Random Poly(NBMO-co-NBMOAC)
After saponification and modification of poly(NBMO), poly(NBMO-co-SNBMOH) and poly(NBMO-co-SNBMOAC) could be obtained. However, the time for preparation of an organosoluble random poly(NBMO-co-SNBMOH) should be controlled to avoid poor solubility and shorter than 15 min. In the 1H NMR (500 MHz, CDCl3)spectrum of
organosoluble random poly(NBMO-co-SNBMOAC), the vinylic proton peaks of crosslinkable methacryloyl moiety were observed at δ (ppm) 5.60 and 6.13. The content of crosslinkable moiety in the organosoluble random poly(NBMO-co-SNBMOAC) was calculated to be 3.6% by 1H NMR. Random poly(NBMO-co-SNBMOAC) was soluble in common organic solvents such as THF, chloroform, dichloromethane, 1,2-dichlorobenzene, benzene, toluene, and xylene. It is difficult to control the reaction time to get organosoluble and crosslinkable polymer by saponification of poly(NBMO).
Another route should be considered by ring-opening metathesis copolymerization of NBMO and NBMTMS. Random poly(NBMO-co-NBMTMS) could be obtained via ROMP of NBMO and NBMTMS. The 1H NMR spectrum of random poly(NBMO-co-NBMTMS) shows resonances due to the olefinic resonance protons of the polymer at δ (ppm) 5.21–5.47 [Figure 1a]. The content of trimethylsilyl moiety in the random poly(NBMO-co-NBMTMS) was calculated to be 12% by 1H NMR.
Hydrolysis of random poly(NBMO-co-NBMTMS) was performed under acidic conditions to remove the trimethylsilyl protecting groups and to unmask the alcohol functionalities. Characterization of the hydrolyzed random poly(NBMO-co-NBMOH) by the 1H NMR spectroscopy [Figure 1b] indicated disappearance of signals at δ (ppm) 0.07–0.09 due to –O-Si-(CH3)3 and appearance of a new broad signal at δ (ppm) 3.33 due to hydroxyl group confirmed the complete removal of the trimethylsilyl group from the polymer. After modification of random poly(NBMO-co-NBMOH) by reacting with MOI, random poly(NBMO-co-NBMOAC) [Scheme 1(c)] was obtained. In the 1H NMR (500 MHz, CDCl3) spectrum of random poly(NBMO-co-NBMOAC)[Figure 1c], resonance signals of vinylic protons of MOI moiety were observed at δ (ppm) 5.59 and 6.12. In the 13C NMR spectrum of random poly(NBMO-co-NBMOAC), the signal at δ (ppm) 126.00 [-O-C(O)-C(CH3)=CH2] was observed due to the carbon of vinylene units of the methacryloyl group of the side chains. The IR spectrum of random poly(NBMO-co-NBMOAC)showed a new absorption band at 1645 cm-1 due to C=C vinylic group, and band due tohydroxyl group had disappeared in the IR spectrumof product. The content of methacryloylmoiety in the random poly(NBMO-co-NBMOAC) was calculated to be 12% by 1H NMR. The chemical transformation from hydroxy group to methacryloyl group has been achieved completely.All the polymers were highly soluble in common organic solvents such as THF, chloroform, dichloromethane, 1,2-dichlorobenzene, benzene, toluene, xylene and insoluble in DMSO. GPC curves of random poly(NBMO-co-NBMTMS),random poly(NBMO-co-NBMOH), and random poly(NBMO-co-NBMOAC) were broad and unimodal molecular weight distribution with a shoulder. The GPC curve of random poly(NBMO-co-NBMTMS) with broader PDI value( Mn = 1.48 x 105, PDI = 4.32) than poly(NBMO) (PDI =1.9, Table 2) was observed. It might be attributable to the difference in the reactivity of two nonliving monomers, NBMO and NBMTMS. The GPC curve of random poly(NBMO-co-NBMOH) showed a clear decrease in polymer molecular weight and PDI value ( Mn = 1.45 x105, PDI = 2.91) after deprotection, consistent with loss of the bulky trimethylsilyl group. Furthermore, random poly(NBMO-co-NBMOH) was modified by reacting with MOI and the random poly(NBMO-co-NBMOAC) with flexible ethyl methacrylate group side chain was obtained. Random poly(NBMO-co-NBMOAC)( Mn = 1.53 x105, PDI = 4.37 ) with relative high molecular weight and broad GPC curve containing a shoulder might be attributable to incorporation of the flexible ethyl methacrylate group in side chain and some sort of aggregation phenomenon caused by hydrogen bonding interaction between amide and carboxyl groups.25,26 Random poly(NBMO-co-NBMTMS), random poly(NBMO-
co-NBMOH), and random poly(NBMO-co-NBMOAC) exhibited 10% weight loss at
382, 395, and 355 ℃, respectively. After deprotection of trimethylsilane group, the resulting hydroxyl-containing poly(NBMO-co-NBMOH) exhibited good thermal stability, which might be attributable to primary alcohol side groups. These behaviors were similarly observed by Valenti and Wagener.27 Random poly (NBMO-co-NBMOAC) showed relative poor thermal stability, which may be attributed to the ester thermal decomposition of flexible ethyl methacrylate group in the side chain of polymer.28
Characterization of Random Poly(NBMO-co-NBMPI), Random Poly(NBMO-co- NBMA ), and Random Poly(NBMO-co-NBMUAC)
As amino-containing monomer [5-(amino methyl)-bicyclo[2.2.1]hept-2-ene] is in tolerant monomer for ROMP using Ru catalyst (I), the amino group of the monomer was protected by converting it to 5-(phthalimidemethyl)bicyclo[2.2.1]hept-2-ene, NBMPI, by reaction with phthalic anhydride. After ROMP of NBMO and NBMPI, random poly(NBMO-co-NBMPI) was obtained. Hydrazinolysis of random poly (NBMO-co-NBMPI) afforded a new organosoluble random copolynorbornene containing ester-bridged alkyl group and amino (-NH2) side chains. The 1H NMR spectrum of random poly(NBMO-co-NBMPI) shows resonances due to the olefinic resonance protons of the polymer at δ(ppm) 5.22–5.34. The content of phthalimide moiety in the random poly(NBMO-co-NBMPI) was calculated to be 13% by 1H NMR.
The GPC curve of random poly(NBMO-co-NBMPI) with broader PDI value ( Mn =1.06 x105, PDI =3.20, Figure 2b) than poly(NBMO) (PDI =1.9, Table 2) might be attributable to the difference in the reactivity of living monomer (NBMPI) and nonliving monomer(NBMO). After hydrazinolysis of random poly(NBMO-co-NBMPI) completely, the signals of hydrogens at δ (ppm) 7.69–7.81 present in the 1H NMR spectrum of random poly(NBMO-co-NBMA) ( Mn =6.21 x104, PDI =1.73, Figure 2b) due to the aromatic group of phthalimide had disappeared and a broad signal at δ (ppm) 5.55 due to amino group appeared. The signals of vinylic proton of crosslinkable side chain in the 1H NMR spectrum of methacryloyl modified random poly(NBMO-co-NBMUAC)were observed at δ (ppm) 5.60 and 6.13. In the 13C NMR spectrum, a signal at δ (ppm) 126.00 [-O-C(O)-C(CH3)=CH2] was observed due to the carbon of methacryloyl group in the side chain. Random poly(NBMO-co-NBMUAC) ( Mn =2.84 x 105, PDI = 4.12, Figure 2c) with relative high molecular weight and broad GPC curve containing a shoulder might be attributable to incorporation of the flexible ethyl methacrylate group in side chain and some sort of aggregation phenomenon caused by hydrogen bonding interaction between urea and carboxyl groups.30,31 The content of methacryloyl moiety in the random poly(NBMO-co-NBMUAC) was calculated to be 13% by 1H NMR. The chemical transformation from amino group to methacryloyl group has been achieved completely.The random poly(NBMO-co-NBMPI) with protective group (NBMPI) exhibited better thermal stability than the deprotected random poly(NBMO-co-NBMA).
The 10% decomposition temperatures of the random poly(NBMO-co-NBMPI),random poly(NBMO-co-NBMA), and random poly(NBMO-co-NBMUAC) were 401, 394, and 358 ℃ , respectively. Random poly(NBMO-co-NBMPI) exhibited better thermal stability than random poly(NBMO-co-NBMA) because of the incorporation of aromatic phthalimide group. To improve the solubility and convert to crosslinkable, the random poly(NBMO-co-NBMPI) was modified by deprotecting the groups. After deprotection, random poly(NBMO-co-NBMA) still exhibited good thermal stability. A highly
organosoluble and crosslinkable copolymer, random poly(NBMO-co-NBMUAC), was obtained after modification with MOI. The modified copolymer exhibited lower thermal stability, which might be attributed to the ester thermal decomposition of flexible ethyl methacrylate group in the side chain of polymer.32 Similar solubility of random poly(NBMO-co-NBMPI), random poly(NBMO-co-NBMA), and random poly (NBMO-co-NBMUAC) was shown and the polymers were highly soluble in common organic solvents, such as THF, chloroform, dichloromethane, 1,2-dichlorobenzene, benzene, xylene, toluene, and DMAc but partially soluble in DMSO and DMF.
AB Cross-linked Systems of Random Poly(NBMO-co-NBMOAC) and Poly(MMA)
The incorporation of random poly(NBMO-co-NBMOAC) into poly(MMA) by forming networked polymer was considered to be a strategy to improve thermal stability and chemical resistance of poly(MMA). Polymerization of MMA in the presence of random poly(NBMO-co-NBMOAC) afforded poly(MMA) crosslinked with random poly(NBMO-co-NBMOAC), thus yielding AB cross-linked material.29 The solubility of the cross-linked polymer was insoluble in THF, ethanol, acetone, benzene, toluene, and chloroform. The thermal stability of AB cross-linked material of poly(MMA) and random poly(NBMO-co-NBMOAC) [decomposition temperature (Td10%=367 ℃ in nitrogen] was enhanced by 42 ℃ compared with poly(MMA) (Td10% = 325 ℃ in nitrogen).第三年度
Amphiphilic block copolymer has attracted attention because it can form various nanostructures, such as micelles or nanoparticles by self assembling in the selective solvent or monolayers by adsorption at the air–liquid interface.33–36 In general, structures of the micelles or nanoparticles strongly depend on the hydrophilic–
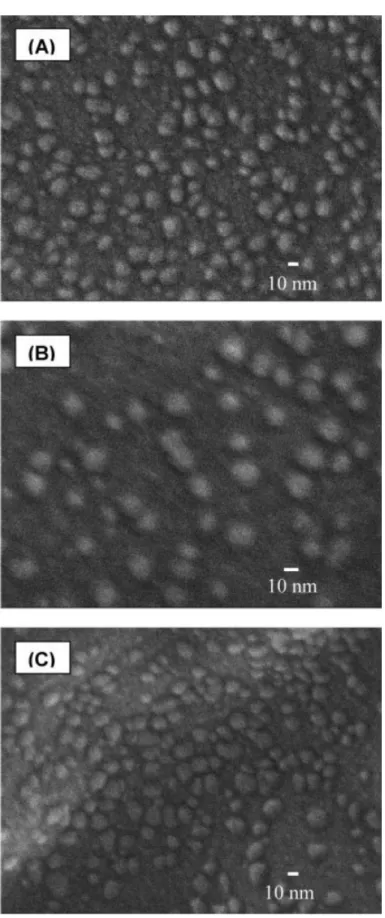
hydrophobic balance and polymer architecture as well as the polymer concentration, salt concentration, temperature, solvent pH, and so on.33–36 To comprehensively investigate the self-assembling behavior of amphiphilic block copolymers, it is necessary to prepare polymer samples with well-controlled molecular weight of hydrophobic and hydrophilic segment and sufficiently high chemical stability. The morphology of nanoparticles derived from amphiphilic block copolymer, poly(HNBMQ-b-HNBMO)-1, was characterized by scanning electron microscope (SEM). Diameters of nanoparticles of poly(HNBMQ-b-HNBMO)-1 (x = 500, y = 100 in theory and x =491, y = 270 by GPC,
Figure 4) (15–25 nm) [Figure 3B] and poly(HNBMQ-b-HNBMO)-3 (x = 50, y=50 in
theory and x = 51, y = 31 by GPC, Scheme 3) (11–20 nm)[Figure 3C] were in the range of 11–25 nm by SEM. SEM micrographs of block copolymers with higher hydrophobic and hydrophilic segmental lengths such as poly(HNBMQ-b-HNBMO)-2 indicated slightly larger nanoparticles (15 – 25 nm). Another block copolymer, poly(HNBMQ-b-HNBMO)-3, with shorter hydrophobic and hydrophilic segmental lengths showed slightly smaller nanoparticles (11–20 nm) in their SEM micrograph.Chacterization of Various Random Copolymers [Random Poly(NBMO-co-NBMPI)].
Random poly(NBMO-co-NBMPI)s exhibited unimodal molecular weight distribution curves. When the content of NBMPI increased from 50 to 80 mol % in the random copolymerization system, the nature of GPC curve becomes a broad molecular weight
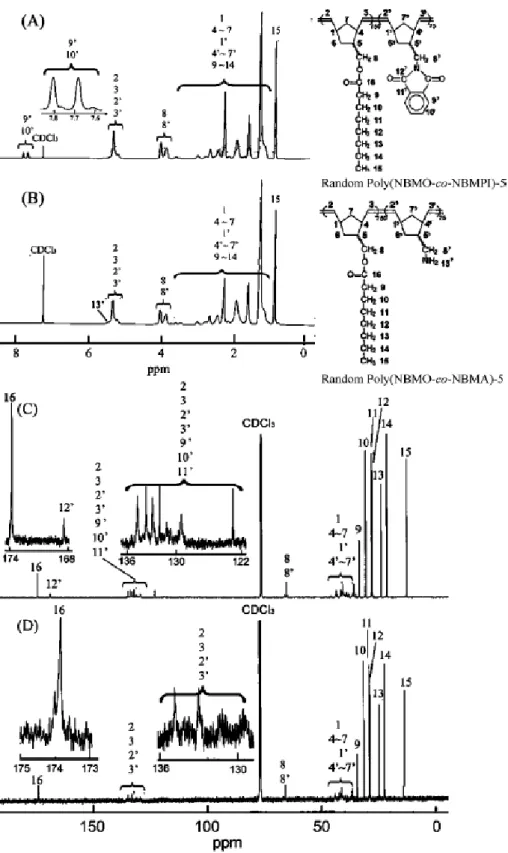
distribution with a shoulder. It might be due to the difference in the reactivity of propagating polymer metallacarbene toward living monomer, NBMPI, 25 and nonliving monomer, NBMO. The 1H NMR spectrum of random poly(NBMO-co-NBMPI)-3 showed new broad signals due to olefinic protons between δ (ppm) 5.29 and 5.45
(Figure 4A). In the
13C NMR spectrum, the signals at δ (ppm) 36.68-44.09 are ascribed to the carbons in the cyclic structure of random poly(NBMO-co-NBMPI)-3. The resonance signals of olefinic carbons of the polymer main chain appeared between δ (ppm) 128.27 and 135.07. The molar fraction of random poly(NBMO-co-NBMPI) was estimated from the relative peak area of phthalic aromatic proton resonance and olefinic proton resonance. The molar fractions of NBMPI (fNBMPI(exp)) for poly(NBMO-co- NBMPI)-1, -2, -3, and -4 were calculated to be 48.7, 67.2, 74.9, and 76.6 mol %, respectively. To obtain an organosoluble amino-containing copolymer, the content of amino pendent group was limited to be 13.2 mol %. The molar fraction of NBMO (fNBMO(exp)) for random poly(NBMO-co-NBMPI)-5 was estimated to be 86.8 mol % from the relative peak area of methyl proton resonance and olefinic proton resonance.After hydrazinolysis of poly(NBMO-co-NBMPI)-5, an organo-soluble poly(NBMO-co -NBMA)-5 with 13.2 mol % amino-containing segment was obtained. The content of alkyl ester group (fNBMO(exp)
= 86.8 mol%) was still remained in the poly(NBMO-co -NBMA)-5 after hydrainolysis of poly(NBMO-co-NBMPI) (f
NBMO(exp) = 86.8 mol %) and confirmed by the 1H NMR and 13C NMR spectra (Figure 5). In the 1H NMR and13C NMR spectra of random poly(NBMO-co-NBMA)-5, the signals due to protons of alkyl ester (H9-H15 in Figure 5) at δ (ppm) 0.87 (H15), 1.28 (H11-H14), 1.59 (H10), and 2.26 (H9) and the signals due to carbons of alkyl ester (C9-C16 in Figure 4) at δ (ppm) 36.80 (C9), 34.45 (C10), 29.14 (C11), 28.94 (C12), 25.01(C13), 22.59 (C14), 14.07 (C15), and 173.8 (C16) were still remained. The thermal stability of various polymers such as poly(NBMO) and random poly(NBMO-co-NBMPI) was compared. Better thermal stability of random poly(NBMO-co-NBMPI) having phthalimide groups was exhibited than poly(NBMO). Likewise, the influence of thermal stability on the hydrogenation was performed. For instance, the order of the temperatures at which 10% weight loss is poly(NBMO) (393 °C) < poly(HNBMO) (401 °C) < random poly(NBMO-co-NBMPI) (410 °C) < random poly(HNBMO-co-HNBMPI) (426 °C). Rapid weight loss of poly(NBMO-co-NBMPI)s does not occur until >400 °C under nitrogen, and all copolymers were decomposed in a single step. Random poly(NBMO-co-NBMPI)s similarly have one Tg value at about 35 °C, which is quite different from poly(NBMO) (Tg = -37°C) and poly(NBMPI) (Tg = 144 °C). After hydrogenation, the resonance signals of olefinic protons in the 1H NMR spectrum of reactant between δ (ppm) 5.29 and 5.45 disappeared completely (Figure 4B). The aromatic resonance signals of the hydrogenated random copolymer between δ (ppm) 7.60 and 7.78 still appeared which indicated the remaining phthalimide group. The hydrazinolysis of random poly(HNBMO-co-HNBMPI) was carried out by using hydrazine monohydrate, and then the quaternized random copolymer, random poly(HNBMO-co-HNBMQ)-3, was obtained by the reaction with HCl(g) (Scheme 3).1H NMR (500 MHz, CDCl3) δ (ppm):
7.4-7.7 (NH3+: 3H), due to hydrogens of quaternary ammonium ion (Figure 1c).
Random poly(HNBMO-co-HNBMQ) with the molar fraction of HNBMQ segment feed in the experiment (fHNBMQ(th)) = 67-80 mol % ; fHNBMQ(exp) = 67.2-76.6 mol %) was soluble in water, methanol, and ethanol. The critical micelle concentrations (cmc) of random poly(HNBMO-co-HNBMQ)-2 (fHNBMQ(th) = 67 mol %; fHNBMQ(exp) = 67.2 mol % by 1H NMR; Scheme 3) and random poly(HNBMO-co-HNBMQ)-3 (fHNBMQ(th) =75 mol
%; fHNBMQ(exp) = 74.9 mol % by 1H NMR; Scheme 3) were observed at 0.1 and 0.3 g dL-1 in aqueous solution by fluorescence spectra.37 Unfortunately, random poly(HNBMO-co-HNBMQ) with fHNBMQ(th) = 50 mol % (fHNBMQ(exp) = 48.7 mol % by
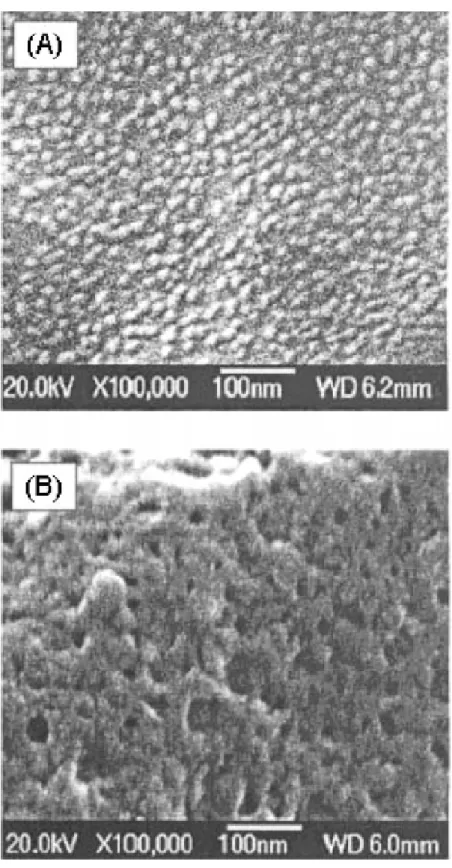
1H NMR; Scheme 3) was soluble in ethanol and only partially soluble in water and methanol; hence, cmc could not be measured in aqueous solution. The cmc of poly(HNBMO-co-HNBMQ)-4 (fHNBMQ(th) = 80 mol %; fHNBMQ(exp) = 76.6 mol % by 1H NMR; Scheme 3) could not be observed due to relatively high ammonium salt content and low surface activity. It is interesting to find the individual micelles of random copolymers with composition range from 50 to 75 mol % ammonium salt (fHNBMQ) in SEM and TEM micrographs as isolated nanometer-scale spherical, and their average diameters were in the range of 6.8-18.1 nm in SEM and 5.0-13.7 nm in TEM micrographs. Nanometer-scale polymeric micelles having a uniform distribution of such random copolymers with 50-75 mol % of ammonium salts and 50-25 mol % of alkyl ester group were obtained. These results indicate that the intrapolymer aggregates formed by the random copolymer might be due to the formation of unimer (Figure 6A) depending on the molar ratio of hydrophobic group/hydrophilic group. When ammonium salt content (fHNBMQ) was increased more than 80 mol% (fHNBMQ(exp) = 76.6 mol% by 1H NMR), the micelle morphology changed from spherical to network-like aggregates (Figure 6B) which is due to hydrophobically cross-linked chains of interpolymers.
Micelles observation of block polymers
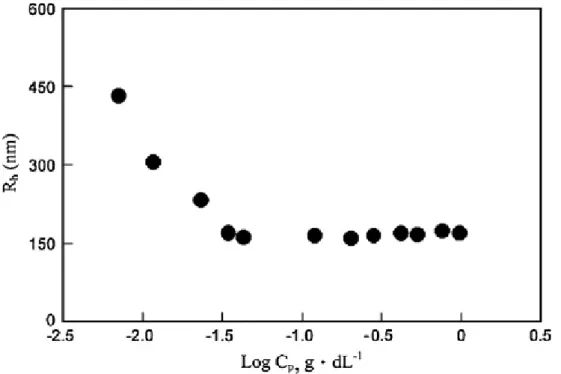
DLS (dynamic light scattering) measurement of micelles in ethanol was performed and hydrodynamic radius was calculated as shown in Figure 7. The hydrodynamic radius (Rh) of poly(HNBMQ100-b-HNBMO100) (fHNBMQ(th) =50 mol% ; fHNBMQ(exp)= 51 mol%
by GPC) in ethanol decreases as the concentration of poly(HNBMQ100-b-HNBMO100) increases, and then reaches a steady value about 160 nm at 3.2 x10-2 g dL-1. In our previous study,38 the poly(HNBMO) was insoluble in ethanol. However, quaternized block copolymer poly(HNBMQ100-b-HNBMO100) could be soluble in ethanol.
Therefore, the difference of solubility resulted from the quaternized hydrophilic segments of polymer. In general, the polymer allows each segment to interact with its favored environment. The polymer can form aggregates in which the hydrophobic segments were oriented within the micelle and the hydrophilic segments were exposed to the solvent. If the size of micelles was controlled by the concentration of hydrophobic segments, larger size of micelles could be expected to form at higher concentration.
However, the results are contradictory from DLS measurement, in which the size of micelles keeps constant beyond concentration of 3.2 x 10-2 g dL-1. Accordingly, the hydrodynamic radius of the cationic polymer in ethanol solution could result from electrostatic repulsive forces balance between intra- and intermicelles39. As hydrophobic segments aggregate, the cations will be located at the surroundings of the micelles. When polymer concentration is increased, the repulsive electrostatic forces between micelles increase and then reduce the size of micelles resulted so that the repulsive forces within intramicelles increase. Consequently, the size of micelles might reach a stable size due to the balance of electrostatic repulsive forces of intra- and inter-micelle. Figure 8 shows the TEM micrographs of the micelles of poly(HNBMQ100-b-HNBMO100), which was prepared from a polymer ethanol solution at the concentration of 0.02 g dL-1. The dark core regions correspond to hydrophobic segment of HNBMO in poly(HNBMQ100-b-HNBMO100). It can be seen from Figure 8.
that poly(HNBMQ100-b-HNBMO100) micelle is roughly spherical in shape. The average diameter of cores calculated from the TEM micrograph (Figure 8) is ca. 224 nm (radius 112 nm). From the DLS result (Figure 7), the hydrodynamic radius of poly (HNBMQ100-b-HNBMO100) in ethanol at 0.02 g dL-1 is 240 nm. The hydrodynamic diameter of micelles in ethanol solution measured by DLS is more than twice as large as the diameter calculated from TEM. This variation could be resulted from the influence
of the solvent.
Hydrophobic microdomains of polymers formed by alkyl ester groups
Pyrene is one of the few condensed aromatic hydrocarbons, which shows significant fine structure (vibronic bands) in its monomer fluorescence spectra in solution phase.
Hence, pyrene molecule is a useful fluorescence probe for characterization of molecular assemblies of numerous associating polymers 18. Relative intensities of the vibronic bands of pyrene fluorescence are known to show a significant dependence on the microenvironmental polarity around pyrene; the relative intensity of the third to first vibrational peaks (I3/I1) in fluorescence spectra of pyrene reflects the polarity in local media where pyrene exists, i.e., I3/I1 ratio being larger in less polar media 40. Therefore, the I3/I1 ratio can be used to discuss environmental effects on pyrene fluorescence.
Furthermore, excimer emission was investigated by fluorescence measurement to elucidate the solution’s behavior. Kramer reported that as the degree of hydrophobic association increases, interaction between isolated, covalently bound fluorescent hydrophobes allows the formation of dimeric, sandwich-like conformations that subsequently lead to excimer formation.19 That is, excimer formation becomes more favorable as the distance between fluorescent pyrene molecules decreases. The emission ratio of IE/IM reflects intra-/interchain interactions of pyrene groups in polymer solution, where IE/IM is the ratio of intensities of excimer and monomer fluorescence 19,20. To a saturation solution of pyrene (ca. 2 mM), trace amount of polymer was introduced.
Fluorescence measurement was carried out for the dilute solutions (3.16 x10-5 ~ 3.16 g dL-1). In Figure 9, the I3/I1 ratios for all random copolymers are plotted against log(polymer concentration) [log Cp] in water (Figure 9A) and in cosolvent [ethanol/methylene chloride = 2/1 (v/v)] (Figure 9B). In the polymer aqueous, the I3/I1
ratios for all copolymers are around 0.6 at lower polymer concentration, which are practically the same as that for pyrene in water. This indicates that there is no formation of polymeric aggregates in aqueous solution.41 As the polymer concentration is gradually increased, the I3/I1 ratio begins to increase significantly at a certain polymer concentration. Polymer with higher content of HNBMO (i.e., lower content of HNBMQ) exhibited higher influence on the ratio of intensity (I3/I1) of pyrene molecule. In the case of random poly(HNBMQ500-r-HNBMO250)( fHNBMQ = 67 mol%), the I3/I1 ratio begins to increase at a polymer concentration (Cp) of 5.6 x 10-4 g dL-1, which is defined as a critical aggregation concentration (CAC). As the polymer concentration is near 0.1 g dL-1, the value of I3/I1 reached a constant value (ca. 0.9). The similar phenomena also appeared for other copolymers. From Figure 9A, the CAC of random poly(HNBMQ750-r-HNBMO250) and random poly(HNBMQ1000-r-HNBMO250) was 3.2 x10-3 and 7.9 x 10-3 g dL-1, respectively. The results indicate that the increase in the ratio of hydrophilic ammonium salt/hydrophobic ester-linkage alkyl chain increases the concentration of onset of micelle formation, which is due to hydrophobic association.
The values of I3/I1 in ethanol/methylene chloride [2/1 (v/v)] cosolvent are around 0.97 at lower polymer concentration (Figure 9B), which is higher than in water due to the lower polarity of cosolvent. The CAC values of random poly(HNBMQ500-r-HNBMO250) and random poly(HNBMQ750-r-HNBMO250) in cosolvent are higher than in water, indicating that aggregation of unimers is easier in water than in cosolvent. However, random poly(HNBMQ1000-r-HNBMO250), which is hydrophobically cross-linked chains has lower CAC in cosolvent than in water. This result indicates the micropolarity related with the morphology of polymer aggregation. The aggregates of the intra- and inter-polymer, consisting of hydrophobic microdomains surrounded by charged segments, may be viewed as polymer micelles, such as unimers or unicore multipolymer micelles. The formation of the polymer micelles may occur at a certain
![Figure 9 . Plots of log Cp vs. I3/I1 ratio for random poly(HNBMQ-r-HNBMO)s in (A) water and (B) cosolvent [ethanol:methylene chloride= 2:1 (v/v)]](https://thumb-ap.123doks.com/thumbv2/9libinfo/9128528.412628/40.892.151.731.128.979/figure-plots-random-hnbmq-cosolvent-ethanol-methylene-chloride.webp)