Effect of Oxidizer on the Galvanic Behavior of Cu/Ta Coupling during Chemical Mechanical Polishing
Szu-Jung Pan, Jui-Chin Chen, and Wen-Ta Tsai*,z
Department of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan
The effect of oxidizer addition, namely, H2O2, KIO3, and Fe共NO3兲3, on the galvanic behavior of the Cu/Ta coupling in 0.01 M Na2SO4+ 1 wt % Al2O3base slurry was studied. Both open-circuit potentials of the uncoupled Cu and Ta as well as galvanic current of the Cu/Ta were measured in static and under chemical mechanical polishing conditions to analyze the roles of these additives. The results showed that Fe共NO3兲3was more effective than H2O2and KIO3in promoting the passivation of Ta, which in turn caused the change of polarity between Cu and Ta. The effect of Cu/Ta area ratio on the galvanic behavior of the coupling was also investigated. The results showed that in Fe共NO3兲3-containing slurry, Ta was the anode with a Cu/Ta area ratio of 5:1 but became the cathode with an area ratio of 1:1.
© 2006 The Electrochemical Society. 关DOI: 10.1149/1.2186181兴 All rights reserved.
Manuscript submitted July 6, 2005; revised manuscript received January 25, 2006. Available electronically April 6, 2006.
To meet the requirement of global planarization, chemical me- chanical polishing共CMP兲 has emerged as a critical technique in Cu metallization processing.1Although Cu CMP exhibits some advan- tages over the conventional techniques, such as dry-etching, etc., there still exist some unfavorable factors, such as Cu corrosion, scratch and pit formations, etc., affecting the CMP efficiency. Sev- eral forms of corrosion can occur during Cu CMP. One of them is associated with the galvanic effect between the Cu and barrier in direct contact in damascene architecture in CMP slurry.2 If these materials in intimate contact have substantial difference in electro- chemical properties in CMP slurry, the galvanic effect will become important in affecting the CMP efficiency. Depending on the polar- ity between Cu and barrier metal, either Cu or barrier metal may suffer accelerated material loss in CMP slurry. The investigation of the galvanic behavior of different Cu/barrier couples is thus of great importance.
Some well-known galvanic couplings in microelectronic compo- nents include Al/Ti3 and those between Cu and their diffusion barriers, such as Ti, Ta, and TaN.4,5 In any specific CMP slurry, the electrochemical potential of the coupling falls between the re- spective open-circuit potentials 共OCPs兲 of the uncoupled compo- nents, as normally found in the galvanic corrosion systems. Steiger- wald et al.6indicated that due to the galvanic interaction between Cu and Ti, the removal rate of Ti in the Cu/Ti coupling in NH4OH base CMP slurry was higher than that of the uncoupled Ti. Similarly, Zeidler et al.7,8 found that the wet-etch rate of W–Ti alloy in the Cu/共W–Ti alloy兲 couple in H2O2-containing slurry was higher than that of the uncoupled W–Ti alloy. They also measured corrosion potentials 共Ecorr兲 and corrosion current densities 共icorr兲 for the Cu/共W–Ti alloy兲 coupling. The results revealed that the Ecorrof the coupling was between that of uncoupled Cu and W–Ti alloy, and the icorrof the coupling was higher than that of uncoupled Cu and W–Ti alloy. Obviously, the removal rate of metal during CMP is affected by the galvanic interaction between Cu and the barrier.
Ernur et al.9pointed out that the galvanic etch rate of Cu was affected by the nature of barrier metal. They also found that the direction of the current flow between Cu and W changed depending on the composition of solution used. The change in the polarity is certainly determined by the electrochemical characteristics of each metal in any specific slurry, which in turn affects the effectiveness of the CMP process. Besides the characteristics of the barrier metal and the slurry compositions, Huang et al.10indicated that increasing the area ratio for Cu to Ta would cause an increase in the corrosion rate of Ta, providing cathodic protection to Cu in NH4OH base slurry.
Nevertheless, the investigation on the galvanic behavior of Cu/Ta coupling in CMP slurry is still scarce. In this investigation, the ef-
fects of different oxidizers关H2O2, KIO3, and Fe共NO3兲3兴 on the gal- vanic behavior of Cu/Ta couples were explored and compared. The polarity change due to area ratio was also examined.
Experimental
Electrochemical measurements.— A pure Cu 共99.99% purity兲 rod with 2.5 cm diameter was used. This rod was sliced into disks, each with a thickness of 0.8 cm. Each disk was mounted in epoxy resin with one side exposed and with the other connected to a copper wire for electric conduction. The exposed surface area of each Cu disk specimen was 4.9 cm2. Similar processes were followed to prepare Ta共99.95% purity兲 specimens. Two different exposed sur- face areas, namely, 4.9 and 0.98 cm2, were chosen for Ta specimens.
These specimens were then ground with SiC paper to 800-grit finish before electrochemical tests. Some of the Cu and Ta specimens were connected with copper wires to investigate the galvanic char- acteristics between them. This coupling was referred to as the Cu/Ta specimen.
The electrochemical tests were conducted in the base slurries consisting of 0.01 M Na2SO4 supporting electrolyte and with 1 wt % Al2O3abrasive particles added. The average particle size of Al2O3 powders was 0.05m. Three different oxidizers, namely, 10 vol % H2O2, 0.1 M KIO3, and 0.1 M Fe共NO3兲3, were added in the base slurries. The pH values of the slurries containing the above oxidizers were 4.8, 5.7, and 1.5, respectively.
Electrochemical tests were conducted either in static or under
*Electrochemical Society Active Member.
zE-mail: wttsai@mail.ncku.edu.tw
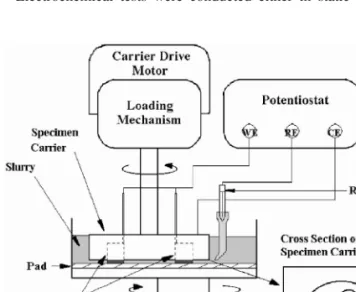
Figure 1. Schematic diagram of CMP apparatus for OCP and galvanic cur- rent measurements of the Cu/Ta coupling.
Journal of The Electrochemical Society, 153共6兲 B193-B198 共2006兲
0013-4651/2006/153共6兲/B193/6/$20.00 © The Electrochemical Society B193
CMP conditions. For the latter test, a polisher consisting of an upper carrier and a lower platen with the respective diameters of 7.6 and 20.3 cm was used. The schematic diagram showing the components of this polisher is illustrated in Fig. 1. As shown in this figure, a loading device was attached to the upper carrier. A tank holding the slurry during polishing was bound on the lower platen and a Rodel IC-1400 pad for polishing was also attached to it. During polishing, the lower platen rotated at a speed of 100 rpm, and a downward stress of 3 psi was applied to the specimens which were held in the upper carrier. The inset in Fig. 1 shows the geometry of the speci- men arrangement in the carrier. Cu and/or Ta specimens were loaded in positions A and B, respectively. An auxiliary specimen made of epoxy resin was put in position C for weight balance purposes.
When galvanic behavior was to be investigated, the Cu and Ta specimens were connected to each other by an external Cu wire.
An EG&G M362 potentiostat was used for the OCP measure- ment. The OCPs were recorded under static conditions and during CMP. A saturated calomel electrode and the carrier 共made of 304 SS兲 were used as the reference electrode and the counter electrode, respectively. The galvanic current共Ig兲 flowing from Cu to Ta in each slurry during and after CMP was measured using a zero resistance ammeter. The sign of Igwas taken as positive if Cu was the anode and vice versa.
Results and Discussion
Effect of oxidizer on the OCPs of uncoupled Cu and Ta .— The respective half-cell reactions and potentials for H2O2/H2O, IO3−/I2, NO3−/N2, and Fe3+/Fe2+are given below11
H2O2+ 2H++ 2e−= 2H2O
E = 1.776 − 0.0591 pH + 0.0295 log关H2O2兴 关1兴 2IO3−+ 12H++ 10e−= I2+ 6H2O
E = 1.175 − 0.0709 pH + 0.0591 log关IO3−兴2/PI
2 关2兴
2NO3−+ 12H++ 10e−= N2+ 6H2O E = 1.246 − 0.0709 pH + 0.00591 log关NO3−兴2/PN
2 关3兴
Fe3++ e−= Fe2+
E = 0.771 + 0.0591 log关Fe3+/Fe2+兴 关4兴 The effects of these oxidizers on the OCPs of uncoupled Cu and Ta in 0.01 M Na2SO4+ 1 wt % Al2O3base slurry under load-free and static condition are demonstrated in Fig. 2. In the slurry containing 10 vol % H2O2, as shown in Fig. 2a, the OCP of Cu was 360 mV and remained constant throughout the whole measurement. In this slurry, the initial OCP of Ta was −100 mV but gradually climbed to +65 mV. As clearly seen in Fig. 2a, the potential of Ta was about 300 mV lower than that of Cu, indicating Ta was more active than Cu in this slurry. With the addition of 0.1 M KIO3 into the base slurry, the steady-state potential of Ta was −250 mV while that of Cu was +30 mV. Again, Ta was more active than Cu with a poten- tial difference about 280 mV between them. By comparing Fig. 2a Figure 2. Effects of共a兲 10 vol % H2O2, 共b兲 0.1 M KIO3, and共c兲 0.1 M Fe共NO3兲3
on the OCPs for uncoupled Cu and Ta in 0.01 M Na2SO4+ 1 wt % Al2O3 base slurry under load-free and static condition.
with 2b, it was found that the OCPs for both Cu and Ta measured in the KIO3-containing slurry were lower than those measured in the slurry containing H2O2. This was attributed to the high oxidizing power of H2O2as compared with KIO3present in the slurry.
In the slurry containing 0.1 M Fe共NO3兲3, a dramatic change in polarity was found. As shown in Fig. 2c, a constant OCP of Cu 共about +45 mV兲 was observed. However, the potential of Ta became higher than that of Cu. It was initially at +175 mV and further climbed to a steady-state value of +345 mV. The high OCP of Ta indicated that it was passivated in this slurry and became nobler than Cu. It is known that the redox potential of either Fe3+/Fe2+ or NO3−/N2 is lower than that of H2O2/H2O.11 However, the acidic nature共pH 1.5兲 and high concentration of oxidizing species such as Fe3+and NO3−in the 0.1 M Fe共NO3兲3-containing slurry seemed to have activated the initial dissolution of Ta, which in turn promoted the fast passivation of Ta surface. As a result, the OCP of Ta became higher than that of Cu. Figure 3 shows schematic diagrams illustrat- ing the change of surface Ta5+ion concentration with time in the slurries containing different oxidizers. Though both H2O2and KIO3 could promote the dissolution of Ta, the rate was not fast enough to cause passivation. In the presence of 0.1 M Fe共NO3兲3in the slurry, however, the enhanced dissolution could raise the surface Ta5+ion concentration above the critical concentration for passivation. The transition from active to passive state thus occurred, as commonly found in many passive alloy/environment systems.
By comparing Fig. 2b and c, it was found that the OCPs of Cu in the slurries containing KIO3and Fe共NO3兲3, respectively, were close to each other. This was because the redox potential for IO3−/I2was close to that of NO3−/N2 but much lower than that of H2O2/H2O.
The initial and steady-state OCPs of Cu and Ta measured in the above slurries are summarized in Table I.
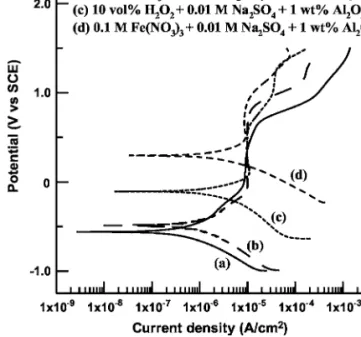
Potentiodynamic polarization curves of Ta determined in 0.01 M Na2SO4+ 1 wt % Al2O3 base slurry and those with different oxi- dizer additions, under static condition, are demonstrated in Fig. 4.
The corrosion potential of Ta was significantly increased when
Fe共NO3兲3 was added into the base slurry. Clearly, Fe共NO3兲3 was even more effective in passivating Ta in the base slurry considered.
Under CMP, the effect of oxidizer on the OCP of uncoupled Cu and Ta is shown in Fig. 5 and in Table I as well. As shown in Fig. 5a and b, the values of OCP for Cu measured in H2O2- and KIO3-containing slurries were still higher than those of Ta. Com- pared with those measured in static condition, the potentials were slightly lowered due to polishing. The contact pressure and hydro- dynamic condition induced by CMP did impose some effect in changing the surface characteristics of both Cu and Ta. Figure 5c shows the potentials measured in the Fe共NO3兲3-containing slurry.
The potential of Cu remained almost constant at about +85 mV, which was slightly higher than that in static condition. For un- coupled Ta, the initial potential in Fe共NO3兲3-containing slurry was
−50 mV, more negative than Cu. However, as shown in Fig. 5c, the potential of Ta increased and eventually became higher than that of Cu as CMP continued. The change of polarity was attributed to the combined effects of accelerating dissolution and mass transfer of Ta ions away from the surface, as illustrated in Fig. 6. With the pres- ence of either H2O2or KIO3, the dissolution rate was not so fast and the flowing slurry could carry Ta5+ions away from the surface, as demonstrated in Fig. 6a. Thus, the surface Ta5+ion concentration could not build up substantially to favor passivation. However, as Fe共NO3兲3was added, the hydrodynamic condition was not in a con- dition fast enough to remove Ta5+ions away from the surface. As a result, as depicted in Fig. 6b, the precipitation of Ta2O5 occurred and passivation was attained. The transition of OCP of Ta and the Figure 3. Schematic diagram showing the effect of different oxidizer on
passivation of Ta in CMP slurry.
Table I. OCPs of Cu and Ta in 0.01 M Na2SO4+ 1 wt % Al2O3base slurries with addition of different oxidizers, measured in static or under CMP condition.
Potential measured at static
condition共mVSCE兲 Potential measured under CMP
共mVSCE兲
Cu Ta Cu Ta
Oxidizer added Initial
Steady
state Initial
Steady
state Initial
Steady
state Initial
Steady state
10 vol % H2O2 +360 +360 −100 +65 +300 +340 −250 −130
0.1 M KIO3 −10 +30 −280 −250 −50 −50 −445 −340
0.1 M Fe共NO3兲3 +45 +45 +175 +345 +85 +85 −50 +140
Figure 4. Potentiodynamic polarization curves of Ta in 0.01 M Na2SO4 + 1 wt % Al2O3base slurries with various oxidizers, under static condition.
B195 Journal of The Electrochemical Society, 153共6兲 B193-B198 共2006兲 B195
reversion of its polarity with respect to Cu were thus explained. All these results indicated that Fe共NO3兲3 at a concentration of 0.1 M was a stronger oxidizer for Ta. As noted in Table I, the difference between the OCPs of uncoupled Cu and Ta was reduced 共about 55 mV兲 under CMP as compared with that in static condition 共about 300 mV兲.
Effect of oxidizer on the galvanic current of coupled Cu/Ta.—
The notable potential difference between the uncoupled Cu and Ta suggests that a significant galvanic current can occur when they are coupled to each other. The theoretical considerations of galvanic corrosion have been discussed by some researchers.12-15The gal- vanic current through the anode is equal to the difference between the anodic and cathodic currents for the anode at galvanic potential 共Eg兲
Ig= IaA共Eg兲 − Ic A共Eg兲
where IaAand IcAare the anodic and cathodic current for the anode.
Under cathodic diffusion control condition, the galvanic current is equal to the cathodic current at Eg,14,15namely
Ig= IaA共Eg兲 − Ic A共Eg兲 = Ic
C共Eg兲
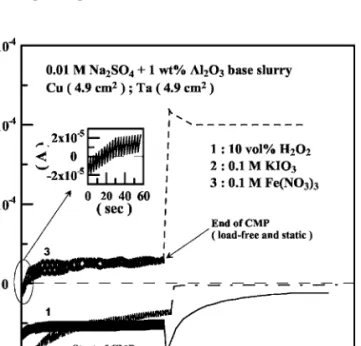
where IcCis the cathodic current at cathode. In this investigation, the galvanic currents for the Cu/Ta couples measured in the base slurry with different oxidizer additions are depicted in Fig. 7. Curves 1 and 2 in Fig. 7 demonstrated the galvanic currents for Cu/Ta couple
共both with an exposed surface area of 4.9 cm2兲 measured under CMP in the slurries containing 10 vol % H2O2 and 0.1 M KIO3, respectively. The negative currents measured indicated that Ta was the anode with respect to Cu cathode in these two different slurries.
More specifically, accelerated dissolution of Ta occurred when Ta was coupled to Cu. The results obtained were consistent with the OCP measurements for the uncoupled Cu and Ta as described above.
Curves 1 and 2 also demonstrated the variation of galvanic currents with time of the Cu/Ta couples when CMP was terminated at 600 s and thereafter. The negative sign of galvanic current measured still indicated that Ta was the anode in both slurries. The magnitude of galvanic current decreased under static condition, suggesting that the polarity difference diminished perhaps due to the increase of surface Ta5+ion concentration. However, the increase in Ta5+ion concentration was not high enough to ensure the passivation of Ta.
In Fig. 7, curve 3 represents the galvanic current measured in Fe共NO3兲3-containing slurry. A negative current was determined ini- tially under CMP, indicating Ta was the anode. However, the nega- tive galvanic current rapidly decreased to zero and became positive for a very short time. The switch of polarity was clearly due to the passivation of Ta in such slurry, in agreement with the potential measurement shown in Fig. 5c. This result again indicated that the addition of Fe共NO3兲3 could favor and was more effective for the passivation of Ta, even in acidic slurry. When CMP stopped, the galvanic current was further increased due to the increase in the potential difference between Cu and Ta in static condition. The Figure 5. Effects of共a兲 10 vol % H2O2, 共b兲 0.1 M KIO3, and共c兲 0.1 M Fe共NO3兲3
on the OCPs for uncoupled Cu and Ta in 0.01 M Na2SO4+ 1 wt % Al2O3 base slurry under CMP condition.
change of polarity has also been found for the Cu/W coupling in the slurries with different oxidizers. Ernur et al.9indicated that W was the anode in H2O2-containing slurry, yet Cu was the anode in HNO3 slurry. These results pointed out that the magnitude and polarity of galvanic current strongly depend on material characteristics and slurry composition.
Effect of Cu/Ta area ratio on galvanic current.— It is known that the dissolution rate of the anode, as well as the galvanic current density 共the galvanic current divided by the anode area兲, in a galvanic couple depends on the cathode-to-anode area ratio
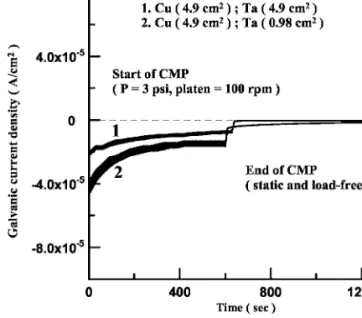
共AC/AA兲.14,15 The galvanic current densities between Cu/Ta with area ratios of 1:1共4.9 cm2/4.9 cm2兲 and 5:1 共4.9 cm2/0.98 cm2兲, in the Fe共NO3兲3-containing slurry, were compared as shown in Fig. 8.
In the initial stage of CMP, Ta was the anode in the Cu/Ta couple with an area ratio of 1:1共curve 1兲 or 5:1 共curve 2兲. By reducing the area of Ta from 4.9 to 0.98 cm2, the galvanic current density was increased from about −4.1⫻ 10−6to −7.7⫻ 10−5A/cm2. The in- creasing anodic dissolution rate of Ta with increasing Cu/Ta area ratio had also been found in NH4OH base slurry.10In this investi- gation, it was found that the galvanic current density共with a Cu/Ta area ratio of 5:1兲 decreased as the time of CMP was increased and Figure 6. Schematic diagrams showing the combined effects of anodic dissolution and mass transfer of Ta5+ions on the sur- face with共a兲 H2O2or KIO3addition and 共b兲 Fe共NO3兲3addition.
Figure 7. Effects of共1兲 10 vol % H2O2,共2兲 0.1 M KIO3, and 共3兲 0.1 M Fe共NO3兲3 on the galvanic currents of the Cu/Ta coupling in 0.01 M Na2SO4+ 1 wt % Al2O3base slurry during CMP and in static condition.
Figure 8. Effect of Cu/Ta area ratio on the galvanic current density of the Cu/Ta coupling in 0.1 M Fe共NO3兲3+ 0.01 M Na2SO4+ 1 wt % Al2O3 slurry during CMP and in static condition.
B197 Journal of The Electrochemical Society, 153共6兲 B193-B198 共2006兲 B197
reached a steady current of about −5.1⫻ 10−5A/cm2. However, Ta remained as the anode during the whole period of measurement, dissimilar to that with an area ratio of 1:1. This result indicated that Ta was not passivated during CMP if the Cu/Ta area ratio was reduced to 5:1. The absence of Ta passivation of the Cu/Ta couple was probably due to the insufficient Ta5+concentration on the sur- face. Though the dissolution of the anode was dependent on the reduction reaction on cathode, the increase in Cu/Ta area ratio seemed not sufficient to raise the Ta5+concentration above the criti- cal value for passivation. However, as soon as CMP was terminated, Ta became the cathode and the reverse of polarity was observed, as demonstrated by curve 2 in Fig. 8.
Figure 9 and 10 show the effect of Cu/Ta area ratio on the galvanic currents measured under CMP in the slurries with 10 vol % H2O2and 0.1 M KIO3additions, respectively. In both cases, Ta was the anode regardless of the Cu/Ta area ratio. It was noted that the galvanic current density with a Cu/Ta area ratio of 5:1 was higher than that of 1:1. The results indicated that accelerated dissolution on anode occurred if the area of cathode was increased.
Conclusions
The electrochemical behavior the Cu/Ta galvanic couple in 0.01 M Na2SO4+ 1 wt % Al2O3base slurry with the presence of different oxidizers, in static or under CMP, was investigated. In static condition and under CMP, the OCP of Cu was higher than that of Ta in 0.01 M Na2SO4+ 1 wt % Al2O3base slurry with the addi- tion of either 10 vol% H2O2or 0.1 M KIO3. However, the addition of 0.1 M Fe共NO3兲3into the 0.01 M Na2SO4+ 1 wt % Al2O3base slurry made the OCP of Ta nobler than Cu in static condition. Under CMP, the potential of Ta was initially lower than that of Cu but became higher at a latter stage. The presence of Fe共NO3兲3promoted the passivation of Ta. The results of galvanic current measurements showed that the direction of current flow was consistent with the results obtained in OCP measurement. The magnitude and polarity of the galvanic current also depended on the Cu/Ta area ratio. In the
Fe共NO3兲3-containing slurry, Ta was the anode with a Cu/Ta area ratio of 5:1, while a switch of polarity from anode to cathode was observed if the area ratio was 1:1. The dependences of galvanic behavior on the type of oxidizer, polishing condition, and area ratio were clearly demonstrated.
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China for financially supporting this research under contract no. NSC 91-2216-E-006-036.
National Cheng Kung University assisted in meeting the publication costs of this article.
References
1. J. M. Steigerwald, S. P. Murarka, and R. J. Gutmann, Chemical Mechanical Pla- narization of Microelectronic Materials, John Wiley & Sons, New York共1997兲.
2. S. Kondo, N. Sakuma, Y. Homma, and N. Ohashi, Jpn. J. Appl. Phys., Part 1, 39, 6216共2000兲.
3. Y. Fang and S. Raghavan, J. Electrochem. Soc., 151, G878共2004兲.
4. K. Holloway, P. M. Fryer, C. Cabral, Jr., J. M. E. Harper, P. J. Bailey, and K. H.
Kelleher, J. Appl. Phys., 71, 5433共1992兲.
5. K. H. Min, K. C. Chun, and K. B. Kim, J. Vac. Sci. Technol. B, 14, 3263共1996兲.
6. J. M. Steigerwald, R. Zirpoli, S. P. Murarka, D. Price, and R. J. Gutmann, J.
Electrochem. Soc., 141, 2842共1994兲.
7. D. Zeidler, Z. Stavreva, M. Plötner, and K. Drescher, Microelectron. Eng., 33, 259 共1997兲.
8. D. Zeidler, Z. Stavreva, M. Plötner, and K. Drescher, Microelectron. Eng., 37/38, 237共1997兲.
9. D. Ernur, S. Kondo, D. Shamiryan, and K. Maex, Microelectron. Eng., 64, 117 共2002兲.
10. W. H. Huang, S. Raghavan, Y. Fang, and L. Zhang, Mater. Res. Soc. Symp. Proc., 566, 161共2000兲.
11. M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions, NACE, Houston, Texas共1975兲.
12. F. Mansfeld, Corrosion (Houston), 27, 436共1971兲.
13. F. Mansfeld, Corrosion (Houston), 29, 403共1973兲.
14. F. Mansfeld and J. V. Kenkel, in Galvanic and Pitting Corrosion – Field and Laboratory Studies, ASTM STP 576, p. 20, American Society for Testing and Materials, West Conshohocken, PA共1976兲.
15. S. Fangteng and E. A. Charles, Corros. Sci., 28, 649共1988兲.
Figure 9. Effect of Cu/Ta area ratio on the galvanic current density of the Cu/Ta coupling in 10 vol % H2O2+ 0.01 M Na2SO4+ 1 wt % Al2O3 slurry during CMP and in static condition.
Figure 10. Effect of Cu/Ta area ratio on the galvanic current density of the Cu/Ta coupling in 0.1 M KIO3+ 0.01 M Na2SO4+ 1 wt % Al2O3 slurry during CMP and in static condition.