Systemic dextromethorphan and dextrorphan are less toxic than bupivacaine at equianesthetic doses in the rat
Yu-Wen Chen
1,2, PhD, Jhi-Joung Wang
2, MD, PhD, Tzu-Ying Liu
3, MS, Yu-Chung Chen
4, MS, Ching-Hsia Hung
3,*, PhD
1 Department of Physical Therapy, China Medical University, Taichung, Taiwan 2 Department of Medical Research, Chi-Mei Medical Center, Tainan, Taiwan 3 Institute & Department of Physical Therapy, National Cheng Kung University,
Tainan, Taiwan
4 Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan
Y.W. Chen and Y.C. Chen equally contributed to this study.
Funding: The financial support was provided by the Cheng Hsin General Hospital (99-35) and the China Medical University (CMU96-099) of Taiwan.
Conflicts of interest: There is no conflict of interests for all authors.
Short heading (40 characters or less); Dextromethorphan or dextrorphan is less toxic.
Summary: Dextromethorphan and dextrorphan produce cutaneous anesthesia.
Dextromethorphan and dextrorphan were less likely to induce systemic toxicity when
compared to bupivacaine. There is a trend in slower decrease of such parameters
(mean arterial blood pressure, heart rate, cardiac output, and stroke volume) in the
dextromethorphan and dextrorphan groups.
*Address correspondence and reprint requests to: Ching-Hsia Hung, PhD, Department of Physical Therapy, National Cheng Kung University, No.1 Ta-Hsueh Road, Tainan, Taiwan
Tel: 886-6-2353535 ext 5939 Fax: 886-6-2370411
E-mail: chhung@mail.ncku.edu.tw
Abstract
Purpose Dextrorphan, a major metabolite of dextromethorphan, produces the
duration of spinal and cutaneous anesthesia similar to bupivacaine, and the suitability
of dextrorphan for clinical use is worth further investigation. The purpose of this
study was to test the central nervous system and cardiovascular toxicity of
bupivacaine, dextromethorphan, and dextrorphan.
Methods First, equipotent doses were determined for cutaneous analgesia on the rat
back by determination of dose–response curves for dextromethorphan, dextrorphan
and bupivacaine (n = 8 rats at each testing point). Then, during continuous
intravenous infusion of equipotent doses of bupivacaine, dextromethorphan,
dextrorphan and saline (n = 8 rats in each group except saline group, n =7 rats), we
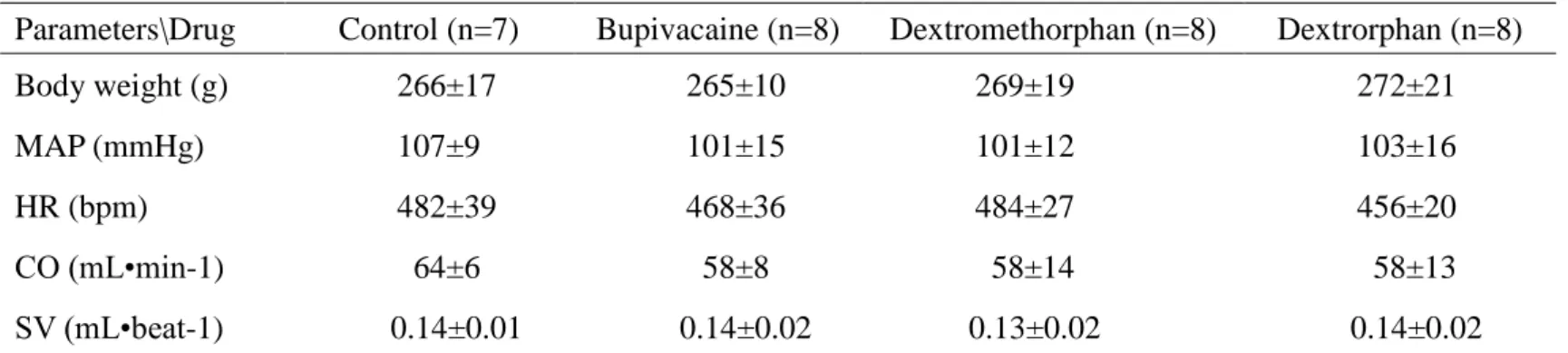
observed the time to seizure, apnea and complete cardiac arrest. Mean arterial blood
pressure (MAP), heart rate (HR), stroke volume (SV), and cardiac output (CO) were
also monitored.
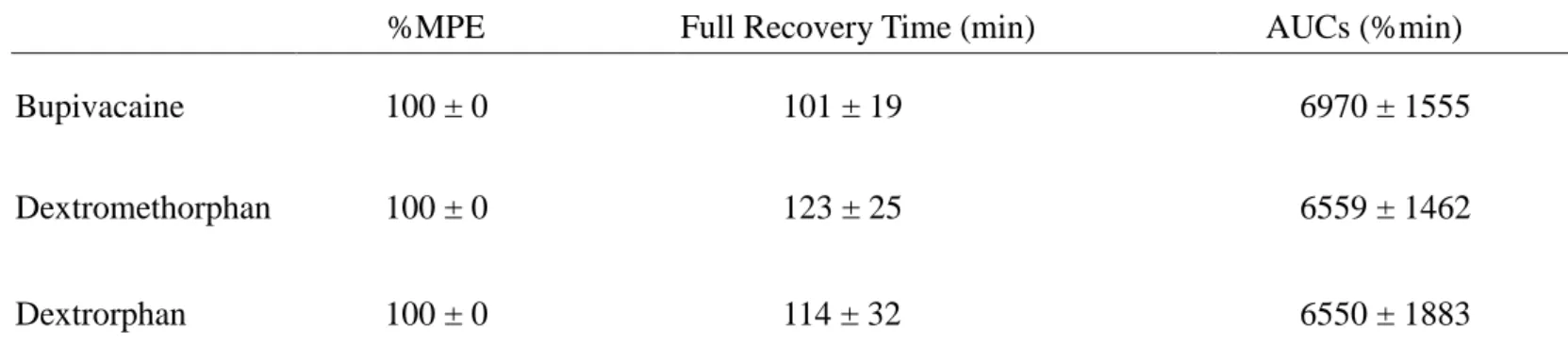
Results Bupivacaine, dextromethorphan, and dextrorphan produced
dose–dependent cutaneous anesthesia. A longer infusion of equipotent infusion doses
was required to produce seizures in the dextrometorphan group (10.6±1.3 min) than in
the bupivacaine group (7.6±2.1 min) (P = 0.005). Dextrorphan did not produce any
seizures. Time to apnea and complete cardiac arrest was shorter in the bupivacaine
group than in the dextrorphan (P < 0.001 between bupivacaine and dextrorphan) and
dextrometorphan groups (P = 0.001 between bupivacaine and dextrometorphan). The
decline curve in MAP, HR, CO, and SV was slower in the dextromethorphan and
dextrorphan groups compared with the bupivacaine group (P < 0.001 between
bupivacaine and dextromethorphan or dextrorphan).
Conclusions Dextromethrophan and dextrorphan were similar to bupivacaine at
producing durations of cutaneous anesthesia but were less likely than bupivacaine to
induce central nervous system and cardiovascular toxicity.
Key Words: cutaneous anesthesia, systemic toxicity, dextromethorphan, dextrorphan,
bupivacaine
Introduction
Dextromethorphan, an antitussive drug, has been used for more than 50 yr
clinically, and is primarily metabolized by O-demethylation to dextrorphan in human
liver.
1Recently, an experiment demonstrated that dextromethorphan and dextrorphan
are sodium channel blockers
2that produce dose-related local anesthetic effects on
spinal and sciatic nerves, causing decreased motor function, proprioception and
nociception in rats.
3, 4Moreover, dextromethorphan and dextrorphan are potent local
anesthetics with 2.4- and 1.9-folds higher systemic safety indices (50% lethal
doses/50% effective doses) than lidocaine on infiltrative cutaneous anesthesia.
5, 6The
local anesthetic durations of dextromethorphan and dextrorphan on cutaneous
anesthesia
5, 6and sciatic nerve blockade
4are longer than that of lidocaine, but the
spinal blockade caused by dextrorphan was similar in duration to bupivacaine,
3a
long-acting local anesthetic. Thus, the suitability of these drugs as clinical local
anesthetics is worth further evaluation.
Despite physical or chemical differences, local anesthetics all have central
nervous system (CNS) toxicity and cardiovascular (CV) toxicity.
7-12Although some
may have less toxicity to the CNS or CV system, however, the differences are minor.
This may be explained by their similar structures.
8Before dextromethorphan and
dextrorphan, two potentially novel local anesthetics, are used in clinical practice, the
toxicity of these drugs should be tested. There are no studies evaluating the systemic
toxicity of dextromethorphan and dextrorphan; it is known that bupivacaine carries
significant CV toxicity.
13, 14The purpose of the study is to compare the CNS and CV toxicity of
dextromethorphan, dextrorphan and bupivacaine given as intravenous infusions, when
given in equianalgesic doses. A model of infiltrative cutaneous anesthesia was used to
determine the equivalent potencies of the drugs in non-anesthetized, spontaneously
breathing rats.
Materials and methods
Animals
Male Sprague-Dawley rats (260-310 g) were used to test cutaneous anesthesia
and systemic toxicity. They were obtained from the Animal Center of National Cheng
Kung University Medical College (Tainan, Taiwan) and housed in a climate
controlled room maintained at 21 degree C with 50% relative humidity. Lighting was
on a 12-hr light/dark cycle (light on at 6:00 AM), with food and water available ad
libitum up to time of testing. The experimental protocols were approved by the animal
investigation committee of National Cheng Kung University Medical College, Tainan,
Taiwan and conformed to the recommendations and policies of the International
Association for the Study of Pain.
Drugs
Bupivacaine HCl, dextromethorphan hydrobromide monohydrate, dextrorphan
tartrate, and sodium chloride were purchased from Sigma Chemical Co. (St. Louis,
MO). Drugs were dissolved in normal saline (0.9% NaCl).
Experimental protocol
The protocol was divided into two parts. In Part I, the effect of different doses
of bupivacaine (8.0, 6.7, 2.0, 1.25 μmol · kg
-1), dextromethorphan (20.0, 13.3, 5.3, 2.7
μmol · kg
-1), dextrorphan (40.0, 26.7, 13.1, 6.7 μmol · kg
-1), and saline on cutaneous
anesthesia was evaluated ((n = 8 rats for each dose of each drug) to determine the
equivalent potencies of the drugs. In Part II, time to cause toxicity (seizures, apnea
and cardiac arrest), mean arterial blood pressure (MAP), heart rate (HR), stroke
volume (SV), and cardiac output (CO) were evaluated after equipotent doses of the
drugs (bupivacaine, dextromethorphan, and dextrorphan) were infused into the rat ((n
= 8 rats for each dose of each drug). Saline group (n = 7 rats) was used as a control.
Part I - Infiltrative cutaneous anesthesia
Before subcutaneous injections, the hair on the rats' dorsal surface of the
thoracolumbar region (6×10 cm
2) was mechanically shaved. Subcutaneous injections
of drugs were performed as reported previously.
5, 6, 15In brief, the drugs, dissolved in
0.6 mL normal saline, were injected subcutaneously using a 30-gauge needle in
unanesthetized rats on the dorsal surface of the thoracolumbar region. The back of the
rat was further divided into left and right parts, either of which received one drug
injection with a washout period of 1 wk. After subcutaneous injection, a circular
elevation of the skin, a wheal, approximately 2 cm in diameter occurred. The wheal
was marked with ink within 30 seconds after injection. The cutaneous anesthetic
effects of drugs was evaluated using the cutaneous trunci muscle reflex (CTMR),
characterized by the reflex movement of the skin over the back produced by twitches
of the lateral thoracispinal muscle in response to local dorsal cutaneous stimulation.
5, 6,15