1
國立成功大學「邁向頂尖大學計畫」
延攬優秀人才工作報告表
NCKU’s “Aim for the Top University Project”
Work Report Form for Distinguished Scholars
□續聘
continuation of employment■離職
resignation100 年 7 月 13 日更新 受聘者姓名
Name of the Employee 張雅合
Male Female
聘 期 Period of Employment
from 103 年(y) 4 月(m) 1 日(d) to 103 年(y) 7 月(m) 31 日(d) 研究或教學或科技研發與
管理計畫名稱 The project title of research,
teaching, technology development and management
硫配位基金屬化合物的活性探討 計畫主持人
(申請單位主管)
Project Investigator (Head of Department/Center)
許鏵芬
補助延聘編號
Grant Number
HUA 103 - 3 - 2 - 224
一、
研究、教學、科技研發與管理工作全程經過概述。(由受聘人填寫)
Please summarize the entire research, teaching, or science and technology R&D and management work process (To be completed by the employee)
在生物學與醫藥學方面,金屬與含硫的生物配位基像半胱胺酸和榖胱甘肽之間的作用力是
一門很重要的課題。所以我們致力於了解金屬和官能基類似於半胱胺酸和榖胱甘肽的含硫
配位基之間的基礎化學。而先前已設計和發展一系列新的含硫四牙基 H
2[P2S2]、H
2[P2S2']
及 H
2[P2S2"]配位基,也已成功合成且鑑定了其光譜特性及物理性質,因此本研究繼續將此
系 列 配 位 基 分 別 與 鎘 (Cd) 和 鉛 (Pb) 金 屬 錯 合 獲 得 [Cd(P2S2”)]
2(1) 、 Pb(P2S2”) (2) 和
[Pb(P2S2”)]
2[Pb
2(P2S2”)
2] (3),更進一步利用 X-ray 繞射儀、NMR、UV-vis 等儀器鑑定其物
理及化學性質並探討其配位化學,同時也將此研究成果整理成期刊文獻的型式,於近期發
表此研究成果。
二、
研究或教學或科技研發與管理成效評估(由計畫主持人或單位主管填寫
)Please evaluate the performance of research, teaching or science and technology R&D and management Work: (To be completed by Project Investigator or Head of Department/Center)
(1)是否達到延攬預期目標?
Has the expected goal of recruitment been achieved?
有達到預期目標
(2)研究或教學或科技研發與管理的方法、專業知識及進度如何?
What are the methods, professional knowledge, and progress of the research, teaching, or R&D and management work?
如預期進行
(3)受延攬人之研究或教學或科技研發與管理成果對該計畫(或貴單位)助益如何?
How have the research, teaching, or R&D and management results of the employed person given benefit to the project (or your unit)?
透過受延攬人的合成專長合成金屬硫化合物,並探討其 Cd-S 與 Pb-S 之間的作用關係,有助
於我們未來更進一步了解在生物體中是如何作用而產生金屬毒性的。
(4)受延攬人於補助期間對貴單位或國內相關學術科技領域助益如何?
How has the employed person, during his or her term of employment, benefited your unit or the relevant domestic academic field?
該受攬人於補助期間內,將其研究成果整理成期刊文獻,若日後順利發表可讓大家更了解
Cd-S 與 Pb-S
之間的作用關係,且根據實驗的資料顯示 Pb-S 一旦鍵結是不容易斷裂,可以了解生物體內鉛
金屬與含 S 的生物配位基有高度反應性。
(5)具體工作績效或研究或教學或科技研發與管理成果:
Please describe the specific work performance, or the results of research, teaching, or R&D and management work:
Metalloproteins can be classified into a few categories according to their functions in cell, such as enzymes, transport and storage proteins and signal transduction proteins. Sulfur-dominated coordination sphere plays a key
2
role in the active sites of many metalloenzymes such as nitrogenase, hydrogenase and CO dehydrogenase. This inspires chemists to synthesize low-molecular-weight model complexes with the sulfur-rich ligating environment for the purpose of elucidating the roles of metals in these metalloenzymes. Based on this motivation, we have been studying the chemistry of metal thiolate complexes in our laboratory. At this particular work, we developed new type of tetradentate dithiolato-diphosphine ligands as well as their corresponding metal complexes.
The structure of complex 1 [Cd(P2S2”)]2 was resolved from X-ray crystallography and its ORTEP diagram is shown in Figure 1. Complex 1 contains two five-coordinate cadmium(II) centers by binding to two tetradentate P2S2” ligand. Two S atoms and two P atoms of each P2S2” ligand bridge two Cd(II) center. The geometry of each metal center is considered as a distorted trigonal-bipyramid with P2 and P1 atoms in the axial position and S1, S2’, S2 in the equatorial plane. The Cd-S bond distances of 2.496 Å, 2.573 Å and 2.650 Å found in 1, are comparable to those found in cadmium thiolate complexes (2.453~2.86). The electronic spectrum of 1 measured in CH2Cl2 exhibits two bands at 232 nm(ε = 6.6×103 M-1cm-1) and 274 nm(ε = 3.3×103 M-1cm-1) which are most likely attribute to the charge transfer band between metal and ligand.
Figure 1. ORTEP diagram of [Cd(P2S2”)]2 (1) with 35% probability thermal ellipsoids. H atoms are omitted for clarity.
The 1H NMR spectrum of 1 taken in CD2Cl2 shown the signal at 0.14 ppm which is assign to the protons of trimethylsilyl group. The ethylene group splitting into four peaks at 1.9~2.7 ppm is caused by the connection to two non-equivalent phosphours atoms chelated by metal. The protons of the phenyl ring display characteristic peaks at region 7.0-8.0 ppm. The 31P NMR spectrum shows two sets of resonances at δ = -26.42 and -40.54 ppm with the ratio of 1:1 indicating two chemical environment of phosphorus atom present in 1. Each phosphrous atom couples with 113Cd and 111Cd splitting into doublet at -24.68 ppm, -28.15 ppm (P2, 1JCd-P2 = 568 Hz) and -39.79 ppm, -41.27 ppm (P1, 1JCd-P1 = 236 Hz). The ratio of the amount of doublet to singlet is 2 : 6 (~30%), which is nearly the sum of nature abundance of 113Cd and 111Cd (113Cd = 12.26% and 111Cd = 12.75%, compare to
1H). The 113Cd NMR spectrum of 1 shows single chemical environment of cadmium which splitting into doublet of doublet at δ = 590.5, 587.8, 585.3 and 582.7 ppm due to couple with two non-equivalent P atoms, and the coupling constant of 113Cd NMR is in agreement with the 31P NMR spectrum. Compare to the literature 113Cd NMR, the chemical shift more downfield while the S atom increase due to the electron withdrawing by the S atoms.
Complex 2 Pb(P2S2”) contains a four-coordinate lead(II) center by binding to a tetradentate P2S2” ligand (Figure 2). The geometry is considered as hemi-direct distorted square-pyramid with P1, P2, S1 and S2 atoms in the basal plane. The angles of S1-Pb1-S2, S2-Pb1-P2, P2-Pb1-P1, P1-Pb1-S1 and S1-Pb1-P2 are 81.68 ゚, 70.98 ゚,
3
69.66 ゚, 65.40 ゚ and 84.25 ゚. The bond lengths of Pb1-S1 and Pb1-S2 are 2.733Å and 2.738 Å, respectively, it is within the range of Pb-S bond lengths in literatures (2.69-3.01 Å). There are intermolecular interaction between two Pb(P2S2”) units. The active lone-pair of Pb(II) ion of complex 2 further interacts weakly with a thiolate group and the phenyl ring of the neighboring molecule. The distance Pb(II) ion and thiolate S atom of the neighboring molecule is 3.3644 Å, within the range of the sum of Pb and S van der waals radii (3.82Å). The distances of Pb(II) ion and phenyl C atoms of the neighboring molecule (Pb1-C44 =3.449 and Pb1-C47 = 3.694 Å) are also within the range of the sum of Pb and C van der waals radii (3.72Å). Thus, considering intermolecular interaction through the active lone pair of Pb(II) ion, two complexes form a dimeric-like structure in the solid state. The geometry of the Pb(II) center can be viewed as a square-pyramid with P2 atom in the vertex position and P1, S1, S2, S4 (neighboring molecule) forming a basal plane (the mean plane deviation is 12.7199 Å). The Pb ion is on the bottom of the plane with the distance of 0.7604 Å away from the plane. The two thiolate groups of one P2S2” ligand (S1 and S2) are in cis-position of the basal plane.
Figure 2. ORTEP diagram of Pb(P2S2”) (2) with 35% probability thermal ellipsoids. H atoms and solvated THF are omitted for clarity.
The electronic spectrum of 2 measured in CH2Cl2 exhibits one broad band at 300 nm (ε= 2.02×104 M-1cm-1). This peak of complex 2 is most likely attribute to the charge transfer band between metal and ligand.
The 1H NMR spectrum of 2 taken in CD2Cl2 shown the signal at 0.4 ppm is assign to the protons of trimethylsilyl group. The protons of ethylene group are separate into two set of signals at 2.55 and 2.90 ppm cause by chelation with metal. The protons of the phenyl ring display characteristic peaks at region 6.7-7.7 ppm. The 31P NMR spectrum of complex 2 displays one set of doublet signal at δ = 3.61, -2.36 ppm and singlet signal at δ = 0.61 ppm with the intensity ratio of 1: 8.4:1. The doublet peak can be assigned to the coupling effect between Pb ion and binding P atom of P2S2” ligand, with the coupling constant 1JPb-P = 970 Hz. The singlet peak indicates that all the P atoms of complex 2 have same chemical environment. The ratio of the amount of doublet to singlet is 2:8.4 (~23%), which is nearly the nature abundance of 207Pb (22.6% compare to 1H). The 207Pb NMR spectrum of complex 2 displays one set of doublet signal at δ = 1535 and 1526 ppm with coupling constant 965 Hz. This coupling constant is consist with the 31P NMR result (1JPb-P = 970 Hz) as mentioned above, and can be assigned to the coupling effect between Pb(II) ion and binding P atom of P2S2” ligand.
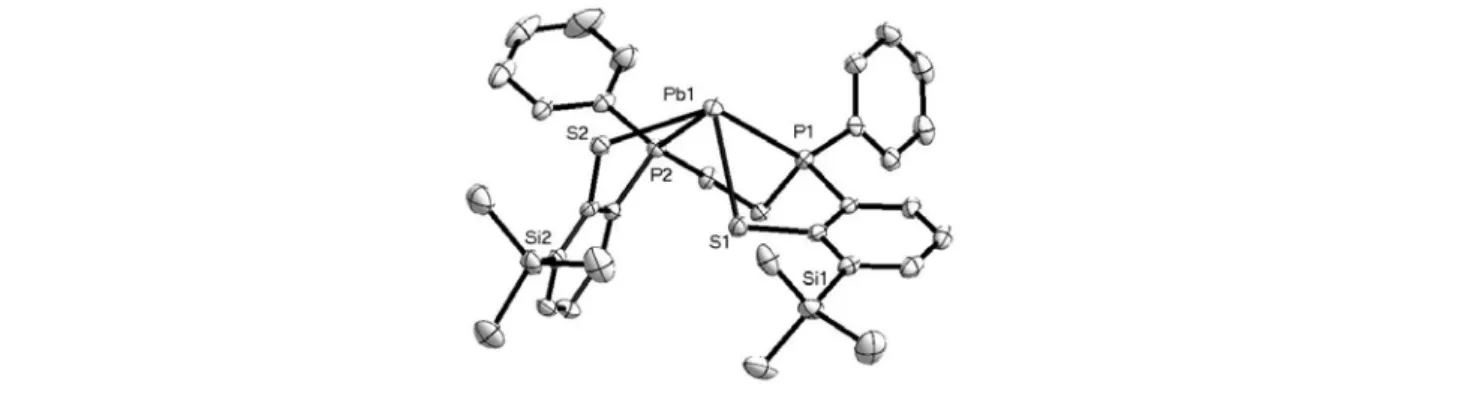
The geometry of this monolead(II) unit of complex 3 is similar as that of complex 2. However, the lone-pair of Pb(II) does not have weak interaction with other neighboring donors, different from the finding in complex 2.
Thus, monolead(II) unit has slightly difference in bond distances and angles from those in complex 2 due to the intermolecular interaction in the latter. Dilead(II) unit contains two five-coordinate lead(II) center by binding to two tetradentate P2S2” ligand(Figure 3). Two S atoms of each P2S2” ligand bridge two Pb(II) center. The geometry is considered as hemi-direct distorted square-pyramid with P1 atom in the vertex position and S1, S1’,
4
S2, and P2 in the basal plane. The two thiolato groups of one P2S2” ligand (S1 and S2) are in trans-position of the basal plane. The angles of S1’-Pb1-P1, S1-Pb1-P1, P2-Pb1-P1 and S2-Pb1-P1 are 80.750 ゚, 69.678 ゚, 71.004 ゚ and 81.73 ゚. The bond lengths of Pb1-S1, Pb1-S1’ and Pb1-S2 are 2.998 Å, 2.970 Å and 2.767 Å, respectively, it is within the range of Pb-S bond lengths in literature. The electronic spectrum of 3 measured in CH2Cl2 exhibit two band at 305 nm (ε= 5.08×103 M-1cm-1) and 368 nm (ε= 1.5×103 M-1cm-1). By comparing with the spectrum of complex 2, the band of 305nm is attribute to the monomeric species of 3. Therefore, the other band is caused by the dimeric species. The 1H NMR spectrum of 3 taken in CDCl3 displays signals from the mixture of dilead(II) and monolead(II) units. Peaks can be assigned by comparing with the 1H NMR spectrum of complex 2. The signal at 0.4 ppm is assigned to the protons of trimethylsilyl group. The protons of ethylene group are separate into two set of signals at 2.5 and 2.90 ppm cause by chelation with metal. The protons of the phenyl ring display characteristic peaks at region at 6.75~7.46 ppm. The 31P NMR spectrum of complex 3 displays two sets of peaks at δ = 0.61 and 54.99 ppm. The set centered at δ = 0.61 ppm can be confirmed to the monolead unit by comparing with 31P NMR spectrum of complex 2, and therefore the set centered at δ = 54.99 ppm can be assigned to the dilead unit. The dilead unit gives one set of doublet signal at δ = 62.66, 47.44 ppm and one singlet signal at δ = 54.99 ppm with the intensity ratio of 1: 8:1. The singlet peak implies that all the P atoms of dilead unit have the same chemical environment. The doublet peak is contributed to the coupling effect between 207Pb Pb ion and binding 31P atoms of P2S2” ligand, with the coupling constant 1JPb-P = 2440 Hz.
The ratio of intensity (1:8:1) for this three peaks, in agreement with the nature abundance of 207Pb (22.6%).
However, according to the solvent-variable and temperture-variable spectroscopic studies, we observed the dimeric and monomeric species did not exchangeable in the solution state. This implies that V-S band was rigid if Pb-S bond was binding to each other.
Figure 3.ORTEP diagram of dilead unit in [Pb(P2S2”)]2[Pb2(P2S2”)2] (3) with 35% probability thermal Ellipsoids. H atoms and solvated CH2Cl2 are omitted for clarity.
In this work, we have synthesized a dicadmium(II) species [Cd(P2S2”)]2 (1), a monolead(II) complex Pb(P2S2”) (2) and a dilead(II) unit and two monolead(II) unit co-crystallized in the crystalline lattice [Pb(P2S2”)]2[Pb2(P2S2”)2] (3). At the same time, we observed the dilead and monolea species did not exchangeable in the solution state.
(6)
是否續聘受聘人?
Will you continue hiring the employed person?□續聘
Yes■不續聘
No※ 此報告表篇幅以三~四頁為原則。This report form should be limited to 3-4 pages in principle.
※ 此表格可上延攬優秀人才成果報告繳交說明網頁下載。
This report form can be downloaded in http://scholar.lib.ncku.edu.tw/explain/
![Figure 1. ORTEP diagram of [Cd(P2S2”)] 2 (1) with 35% probability thermal ellipsoids](https://thumb-ap.123doks.com/thumbv2/9libinfo/9038660.324518/2.892.80.822.408.667/figure-ortep-diagram-cd-p-probability-thermal-ellipsoids.webp)
![Figure 3.ORTEP diagram of dilead unit in [Pb(P2S2”)] 2 [Pb 2 (P2S2”) 2 ] (3) with 35% probability thermal Ellipsoids](https://thumb-ap.123doks.com/thumbv2/9libinfo/9038660.324518/4.892.98.813.644.866/figure-ortep-diagram-dilead-unit-probability-thermal-ellipsoids.webp)