Lancet Oncol 2011; 12: 568–74 Published Online April 15, 2011 DOI:10.1016/S1470- 2045(11)70077-8 See Comment page 517 Genomics Research Centre, Academia Sinica, Taipei, Taiwan (H-I Yang PhD, Prof C-J Chen ScD); Molecular and Genomic Epidemiology Research Centre, China Medical University Hospital, Taichung, Taiwan (H-I Yang); Department of Medicine, University of Hong Kong, Hong Kong (Prof M-F Yuen MD, W-K Seto MBBS); Department of Medicine and Therapeutics and Institute of Digestive Disease, The Chinese University of Hong Kong, Hong Kong (Prof H L-Y Chan MD, V W-S Wong MD); Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Brain Korea 21 Project for Medical Science, Seoul, South Korea (Prof K-H Han MD, D-Y Kim MD, S-H Ahn MD); and Department of Internal Medicine and Hepatitis Research Centre (Prof P-J Chen MD) and Graduate Institute of Epidemiology, School of Public Health (Prof C-J Chen), National Taiwan University, Taipei, Taiwan Correspondence to:
Prof Chien-Jen Chen, Genomics Research Centre, Academia Sinica, Nankang, Taipei 11529, Taiwan cjchen@ntu.edu.tw
Introduction
Chronic hepatitis B is a major cause of cirrhosis and hepatocellular carcinoma (HCC) worldwide, accounting for around 1 million deaths every year.1 The risk of chronic hepatitis B progression to HCC can be reduced by hepatitis B virus (HBV) therapy, as suggested by a meta- analysis.2 The identifi cation and classifi cation of patients who are at high risk of developing HCC is therefore important, and will allow timely intervention in those who will most benefi t.3
Known risk factors for disease progression in chronic hepatitis B can be broadly divided into host, viral, and environmental factors.4 Host factors include age, sex, genetic susceptibility, family history, obesity, and immune status. Findings from a Korean study3 showed that age, male sex, and raised alanine aminotransferase (ALT) concentration were prognostic for HCC development.
Viral factors can include HBeAg status, HBV DNA level, genotype, mutants, and co-infection. HBsAg seropositivity is an important risk factor for HCC, and HBeAg seropositivity has been associated with an increased risk of developing HCC.4,5 Increasing HBV DNA levels have
been associated with a stepwise increase in HCC risk, with high HBV DNA associated with an increased risk of HCC.6–8 Findings from several studies have shown that the risk of liver-related mortality is increased with even slight increases in serum ALT.9,10 Gradual and cumulative liver damage caused by low level viraemia, and indicated by small rises in ALT concentrations, could be the pathway for severe complications and disease progression in Asian patients with chronic hepatitis B.10 HBV genotype is a contentious risk factor for HCC. A previously reported risk between HCC and HBV genotype C11,12 could be attributable to close associations with genotype C and core promoter mutations,7 which are an independent risk factor.13 Furthermore, a meta- analysis14 has suggested that basal core promoter mutants might be an important risk factor. Environmental factors are the most diffi cult to quantify clinically, and include alcohol use, cigarette smoking, chemical carcinogens, and afl atoxin exposure.
The primary aim of therapy for chronic hepatitis B is to prevent disease progression to liver cirrhosis, HCC, and death.15 Although there is general consensus in guidelines
Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score
Hwai-I Yang, Man-Fung Yuen, Henry Lik-Yuen Chan, Kwang-Hyub Han, Pei-Jer Chen, Do-Young Kim, Sang-Hoon Ahn, Chien-Jen Chen, Vincent Wai-Sun Wong, Wai-Kay Seto, for the REACH-B Working Group
Summary
Background Therapy for chronic hepatitis B reduces the risk of progressing to hepatocellular carcinoma (HCC);
however, there is no suitable and accurate means to assess risk. This study aimed to develop and validate a simple scoring system to predict HCC risk in patients with chronic hepatitis B.
Methods The development cohort consisted of 3584 patients without cirrhosis from the community-based Taiwanese REVEAL-HBV study (of whom 131 developed HCC during follow-up), and a validation cohort of 1505 patients from three hospitals in Hong Kong and South Korea (of whom 111 developed HCC during follow-up). We used Cox multivariate proportional hazards model to predict risk of HCC at 3, 5, and 10 years. Variables included in the risk score were sex, age, serum alanine aminotransferase concentration, HBeAg status, and serum HBV DNA level. We calculated the area under receiver operating curve (AUROC) and calibration of predicted and observed HCC risk.
Findings A 17-point risk score was developed, with HCC risk ranging from 0·0% to 23·6% at 3 years, 0·0% to 47·4%
at 5 years, and 0·0% to 81·6% at 10 years for patients with the lowest and highest HCC risk, respectively. AUROCs to predict risk were 0·811 (95% CI 0·790–0·831) at 3 years, 0·796 (0·775–0·816) at 5 years, and 0·769 (0·747–0·790) at 10 years in the validation cohort, and 0·902 (0·884–0·918), 0·783 (0·759–0·806), and 0·806 (0·783–0·828), respectively, after exclusion of 277 patients in the validation cohort with cirrhosis. Predicted risk was well calibrated with Kaplan-Meier observed HCC risk.
Interpretation A simple-to-use risk score that uses baseline clinical variables was developed and validated. The score accurately estimates the risk of developing HCC at 3, 5, and 10 years in patients with chronic hepatitis B. Clinicians can use this score to assess risk of HCC in patients with chronic hepatitis B and subsequently make evidence-based decisions about their clinical management.
Funding The Academia Sinica; the National Health Research Institute, Taiwan; and Bristol-Myers Squibb.
about when and how to treat this disease and who should be treated, several other areas of management are controversial.16 Defi nitions for normal ALT, appropriate serum HBV DNA cutoff points, and the suitability of liver biopsy diff er between guidelines.15,17–19 There is no standardised and accurate guidance about assessment of HCC risk in chronic hepatitis B. Several HBV risk scores have been published; however, most are limited by population size.3,7,20,21 A published nomogram had high potential to be adapted into a simplifi ed clinical instrument, but it has not been externally validated.20
The successful development and use of risk calculators in cardiovascular disease have shown the benefi ts of such instruments to both patients and physicians.22,23 All reliable, easily accessible, and accurate clinical factors predicting complications from chronic hepatitis B need to be identifi ed and consolidated. This process could facilitate the timely identifi cation of high- risk patients for whom treatment is still viable. The REACH-B (Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B) study aimed to develop and validate a simple, clinically useful long- term prediction score, to identify the risk of progression to HCC in patients with chronic hepatitis B by use of a snapshot risk profi le consisting of currently used non- invasive parameters.
Methods
Study populations for model derivation
The patient population used to develop the risk score (development cohort) consisted of patients from the population-based prospective REVEAL-HBV database, which has been described previously.8 Briefl y, 3584 patients aged 30–65 years who were HBsAg seropositive, were anti-HCV seronegative, did not have cirrhosis, and had serum HBV DNA measured at study entry were used for this study. Patients did not receive antiviral treatment during the median follow-up of 12·0 years (IQR 11·5–12·4), during which time 131 developed HCC.
The population used to validate the risk score (validation cohort) was a hospital-based composite international cohort. This cohort consisted of 1505 patients from three independent university hospital databases, with a mean follow-up of 7·3 years. Patients from the University of Hong Kong (UHK; n=820) were aged 14–83 years and were followed up for a mean of 6·3 years; those from the Chinese University of Hong Kong (CUHK; n=426) were aged 12–80 years and followed up for a mean of 9·4 years; and those from Yonsei University Hospital (YUH; n=259) were aged 24–70 years and followed up for a median of 7·0 years (IQR 5·0–10·3). 111 patients from the validation cohort developed HCC during follow-up (46 at CUHK; 40 at UHK; 25 at YUH). No patients received antiviral therapy throughout the follow-up period for this study.
All validation centres used ultrasonographic evidence plus other clinical and serological data including ascites, varices, hypersplenism, hypoalbuminaemia, and the ratio of aspartate aminotransferase to platelet count to defi ne patients with cirrhosis. HCC was identifi ed in the development cohort by histopathological confi rmation;
detection of a positive lesion with at least two imaging techniques (abdominal ultrasound, angiogram, or CT);
or detection with one imaging technique coupled with an α-fetoprotein concentration greater than 400 μg/L.
Identifi cation of HCC cases in centres comprising the validation cohort was by positive histology or increased α-fetoprotein (>50 μg/L or a rising trend >20 μg/L), combined with identifi cation of HCC features by CT, MRI, or hepatic angiogram.
This study was approved by the institutional review board of the College of Public Health, National Taiwan
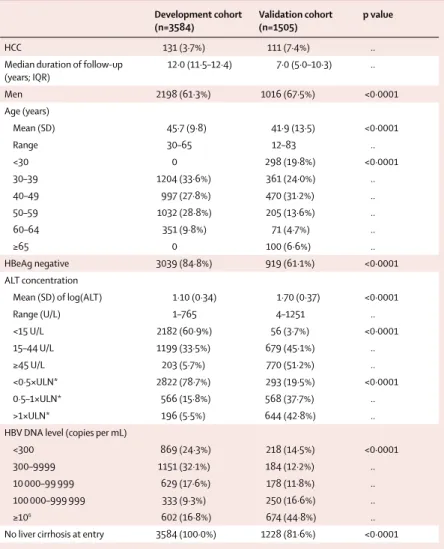
Development cohort (n=3584)
Validation cohort (n=1505)
p value
HCC 131 (3·7%) 111 (7·4%) ..
Median duration of follow-up (years; IQR)
12·0 (11·5–12·4) 7·0 (5·0–10·3) ..
Men 2198 (61·3%) 1016 (67·5%) <0·0001
Age (years)
Mean (SD) 45·7 (9·8) 41·9 (13·5) <0·0001
Range 30–65 12–83 ..
<30 0 298 (19·8%) <0·0001
30–39 1204 (33·6%) 361 (24·0%) ..
40–49 997 (27·8%) 470 (31·2%) ..
50–59 1032 (28·8%) 205 (13·6%) ..
60–64 351 (9·8%) 71 (4·7%) ..
≥65 0 100 (6·6%) ..
HBeAg negative 3039 (84·8%) 919 (61·1%) <0·0001
ALT concentration
Mean (SD) of log(ALT) 1·10 (0·34) 1·70 (0·37) <0·0001
Range (U/L) 1–765 4–1251 ..
<15 U/L 2182 (60·9%) 56 (3·7%) <0·0001
15–44 U/L 1199 (33·5%) 679 (45·1%) ..
≥45 U/L 203 (5·7%) 770 (51·2%) ..
<0·5×ULN* 2822 (78·7%) 293 (19·5%) <0·0001
0·5–1×ULN* 566 (15·8%) 568 (37·7%) ..
>1×ULN* 196 (5·5%) 644 (42·8%) ..
HBV DNA level (copies per mL)
<300 869 (24·3%) 218 (14·5%) <0·0001
300–9999 1151 (32·1%) 184 (12·2%) ..
10 000–99 999 629 (17·6%) 178 (11·8%) ..
100 000–999 999 333 (9·3%) 250 (16·6%) ..
≥106 602 (16·8%) 674 (44·8%) ..
No liver cirrhosis at entry 3584 (100·0%) 1228 (81·6%) <0·0001
Data are number (%) unless otherwise indicated. HCC=hepatocellular carcinoma. ALT=alanine aminotransferase.
ULN=upper limit of normal. HBV=hepatitis B virus. *ULN=45 U/L in datasets from REVEAL, 58 U/L in datasets from Chinese University of Hong Kong, 45 U/L in datasets from Yonsei University Hospital, and 58 U/L (men) and 36 U/L (women) in datasets from the University of Hong Kong.
Table 1: Baseline characteristics of development and validation cohorts
University in Taipei, Taiwan. All patients provided written informed consent.
Rationale for risk factor inclusion
Risk factors identifi ed for inclusion were those previously shown to be contributing factors and important long- term risk predictors in the development of HCC.8 Factors chosen were those easily measured by widely available, non-invasive clinical tests. Risk factors that met these criteria and were common to all databases comprising the development and validation cohorts were sex, age, serum ALT concentration, HBeAg status, and serum HBV DNA level (by PCR assay).
Statistical analyses
All statistical analyses were done with SAS (version 9.1).
Development of the risk score had three steps. First, a Cox proportional hazards model was used to estimate the β regression coeffi cient, p value, and hazard ratio and its 95% CI for each of the selected risk predictors. We also estimated baseline disease-free probabilities at 3, 5, and 10 years. Second, the regression coeffi cient in the Cox
proportional hazards model of each risk predictor was divided by the regression coeffi cient for 5-year increase of age, and was rounded into an integer value to generate the risk score. Third, the projected HCC risk was estimated with the equation:
where P0 is the baseline disease-free probability; βage is the β regression coeffi cient for 5-year increment of age; score is the cumulative risk score to be projected; βi is the β regression coeffi cient for the ith covariate; and Mi is the mean level of the ith covariate. Projected HCC risk was calculated for three timepoints (3, 5, and 10 years) for all cumulative risk scores possible with this method (range 0–17).
Model validation had two steps: discrimination and calibration. Discrimination was assessed with the receiver operating characteristic (ROC) curve, area under ROC (AUROC) curves, sensitivity, and specifi city. A cumulative risk score was calculated for every patient in the validation cohort. ROC curves were plotted with 1–specifi city and sensitivity measured along the horizontal and vertical axes, respectively, with all possible cumulative risk scores in the validation cohort as cutoff points for the prediction of HCC events within 3, 5, and 10 years of follow-up. For the assessment of calibration, we calculated observed HCC risk with the Kaplan-Meier method for patients in the validation cohort with the same cumulative risk scores. When the number of HCC cases in a group with the same cumulative risk score was sparse, this group was combined with the neighbouring group of cumulative risk score to secure a stable estimate of HCC risk observed by the Kaplan- Meier method. Kaplan-Meier estimates were plotted against the mean predicted risk in the group to form a calibration chart.
Role of the funding source
The sponsors of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had fi nal responsibility for the decision to submit for publication.
Results
Table 1 shows the baseline characteristics of the development and validation cohorts. In both cohorts, most patients were men and HBeAg negative. As per the entry criteria, all patients in the development cohort did not have cirrhosis at baseline, compared with 82% (1228 of 1505) of patients in the hospital-based validation cohort. Patients in the development cohort diff ered from those in the validation cohort in terms of the distribution of sex, age, viral load, HBeAg status, and ALT and HBV DNA levels (table 1).
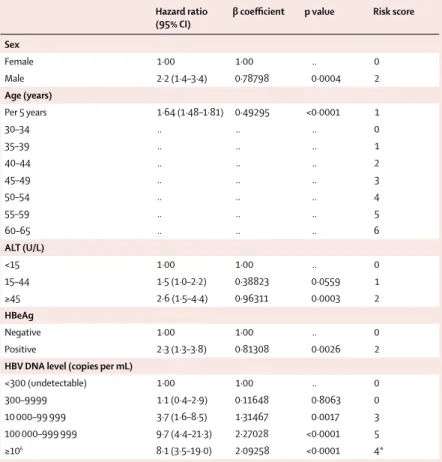
Hazard ratio (95% CI)
β coeffi cient p value Risk score
Sex
Female 1·00 1·00 .. 0
Male 2·2 (1·4–3·4) 0·78798 0·0004 2
Age (years)
Per 5 years 1·64 (1·48–1·81) 0·49295 <0·0001 1
30–34 .. .. .. 0
35–39 .. .. .. 1
40–44 .. .. .. 2
45–49 .. .. .. 3
50–54 .. .. .. 4
55–59 .. .. .. 5
60–65 .. .. .. 6
ALT (U/L)
<15 1·00 1·00 .. 0
15–44 1·5 (1·0–2·2) 0·38823 0·0559 1
≥45 2·6 (1·5–4·4) 0·96311 0·0003 2
HBeAg
Negative 1·00 1·00 .. 0
Positive 2·3 (1·3–3·8) 0·81308 0·0026 2
HBV DNA level (copies per mL)
<300 (undetectable) 1·00 1·00 .. 0
300–9999 1·1 (0·4–2·9) 0·11648 0·8063 0
10 000–99 999 3·7 (1·6–8·5) 1·31467 0·0017 3
100 000–999 999 9·7 (4·4–21·3) 2·27028 <0·0001 5
≥106 8·1 (3·5–19·0) 2·09258 <0·0001 4*
ALT=alanine aminotransferase. HBV=hepatitis B virus. *The risk score attributed to HBV DNA ≥106 copies per mL was less than that for HBV DNA of 100 000–999 999 copies per mL because most patients with HBV DNA ≥106 copies per mL were also HBeAg positive, thus sharing the associated higher score for this category.
Table 2: β coeffi cient and hazard ratio estimation from development cohort with multivariate Cox proportional hazards model and corresponding risk score
1–P0exp(Σβage × score–Σβi × Mi)
Table 2 shows β regression coeffi cient estimation with the multivariate Cox proportional hazards model. Table 3 shows the cumulative risk score and associated 3-year, 5-year, and 10-year risk of developing HCC; for example, a male patient (risk score=2), aged 59 years (5), who was HBeAg positive (2), with ALT concentration of 47 IU/L (2), and HBV DNA viral load of 10 000–99 999 copies per mL (3) would have a cumulative risk score of 14, with a projected HCC risk of 6% at 3 years, 14% at 5 years, and 32% at 10 years of follow-up. The webappendix shows the risk function used to estimate the risk of developing HCC.
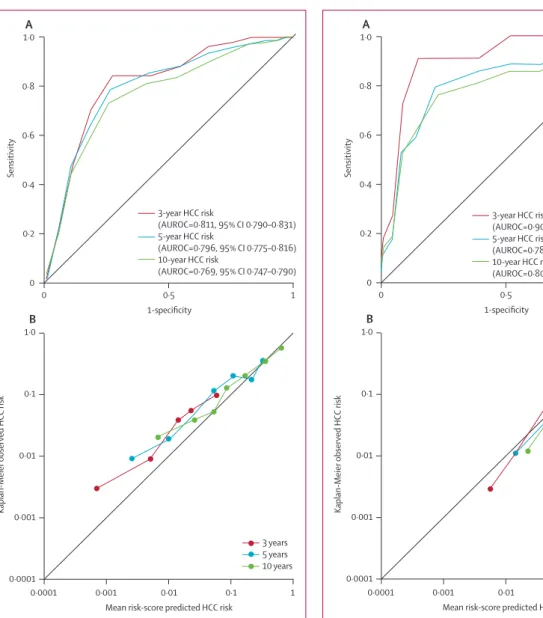
We plotted ROC curves for 3-year, 5-year, and 10-year risk with the 1505 patients from the validation cohort.
We also plotted a calibration chart for predicted and observed risk (fi gure 1). The overall model showed a fairly good discrimination capability, with AUROC of 0·811 (95% CI 0·790–0·831) for risk at 3 years, 0·796 (0·775–0·816) at 5 years, and 0·769 (0·747–0·790) at 10 years (fi gure 1). The predicted HCC risk calibrated well with the observed risk, with a correlation coeffi cient of 0·973 for 3-year risk, 0·942 for 5-year risk, and 0·994 for 10-year risk (fi gure 1). In an analysis stratifi ed by age, the AUROC for patients aged 30–65 years was 0·782 (0·756–0·806) at 3 years, 0·771 (0·746–0·796) at 5 years, and 0·751 (0·725–0·776) at 10 years. For patients younger than 30 years or older than 65 years the AUROC was 0·922 (0·891–0·946) at 3 years, 0·904 (0·871–0·931) at 5 years, and 0·854 (0·816–0·888) at 10 years. In patients from each of the three validation datasets, the AUROCs for risk at 3, 5, and 10 years were: 0·857 (0·821–0·889), 0·832 (0·793–0·866), and 0·785 (0·743–0·824), respectively, in the CUHK cohort; 0·881 (0·857–0·902), 0·859 (0·833–0·882), and 0·838 (0·811–0·862), respectively, in the UHK cohort; and 0·703 (0·644–0·758), 0·707 (0·648–0·762), and 0·717 (0·657–0·771), respectively, in the YUH cohort.
We also plotted ROC curves for risk at 3, 5, and 10 years in 1228 patients from the validation cohort who did not have cirrhosis at study entry. The non-cirrhotic model showed good sensitivity and specifi city, with AUROC of 0·902 (0·884–0·918) for risk at 3 years, 0·783 (0·759–0·806) at 5 years, and 0·806 (0·783–0·828) at 10 years in patients without cirrhosis (fi gure 2), compared with 0·671 (0·612–0·726), 0·698 (0·640–0·752), and 0·647 (0·588–0·704), respectively, in patients with cirrhosis. We also plotted a calibration chart for predicted HCC risk and observed risk, showing a good correlation in the non-cirrhotic model (fi gure 2).
The correlation coeffi cient was 0·975 for HCC risk at 3 years, 0·991 at 5 years, and 0·999 at 10 years.
Discussion
This study was undertaken to develop a clinically useful HCC risk-prediction score in patients with chronic hepatitis B, with use of a community-based natural history cohort from Taiwan. The resulting predictive risk score was validated in a hospital-based composite cohort
from several centres in Hong Kong and South Korea. To maximise clinical use, this risk calculator was designed to include simple, non-invasive, and routinely measured factors. Factors predictive of HCC in this study—sex, age, serum ALT concentration, HBeAg status, and serum HBV DNA level—were consistent with those identifi ed in previous studies.3–5,8,9 A 17-point risk-score was developed and validated with these clinical factors. The resulting risk score was accurate and reliable, with predicted risk correlating well with observed risk.
The risk score developed in this study can provide guidance about treatment by focusing on the long-term outcomes in chronic hepatitis B, which are not covered in treatment recommendations. This risk score is of particular benefi t for patients who do not meet existing treatment initiation recommendations—a group who are not addressed in current treatment guidelines.
Another conceivably useful application would be to assess the change in risk after initiation of therapy;
however, further verifi cation would be needed before recommendation of the risk score for this purpose. This risk-prediction instrument could be used by a wide range of health-care professionals, from general practitioners to experienced hepatologists. It might also provide valuable insights for health authorities needing information for long-term resource planning. Another opportunity for this risk calculator is in communication of risk to patients, which could lead to better treatment acceptance and compliance in patients needing therapy.
For patients assessed as having a high risk score, several management strategies might be considered, including more intensive follow-up, more frequent HCC
3 years 5 years 10 years
0 0·0% 0·0% 0·0%
1 0·0% 0·0% 0·1%
2 0·0% 0·0% 0·1%
3 0·0% 0·1% 0·2%
4 0·0% 0·1% 0·3%
5 0·1% 0·2% 0·5%
6 0·1% 0·3% 0·7%
7 0·2% 0·5% 1·2%
8 0·3% 0·8% 2·0%
9 0·5% 1·2% 3·2%
10 0·9% 2·0% 5·2%
11 1·4% 3·3% 8·4%
12 2·3% 5·3% 13·4%
13 3·7% 8·5% 21·0%
14 6·0% 13·6% 32·0%
15 9·6% 21·3% 46·8%
16 15·2% 32·4% 64·4%
17 23·6% 47·4% 81·6%
Table 3: Cumulative risk score and associated 3-year, 5-year, and 10-year risk of developing hepatocellular carcinoma in patients with chronic hepatitis B
See Online for webappendix
surveillance with use of sensitive imaging techniques such as CT and MRI, and the initiation of antiviral therapy when appropriate. By contrast, patients with low risk scores might be managed with less vigorous follow- up or surveillance, and delay treatment until more eff ective antiviral therapies are available.
This study used the largest cohort of patients so far to develop a risk score predictive of HCC in chronic hepatitis B. The datasets used provide not only tangible data, but also allow the determination of risk at diff erent timepoints. This risk score also takes into account factors such as age and sex, which are known to be important but not addressed in present guidelines.4 Selection bias in the development cohort was minimised
because the cohort was taken from a population-based natural history study. The validation cohort was large and diverse and, importantly, independent from the development cohort.
The risk factors included in this study are not exhaustive, and we omitted several known and suggested risk factors to instead use widely available and easily measureable data. We did not include α-fetoprotein because testing has variable sensitivity and specifi city,24 which is poor for prediction of HCC occurrence in the long term. The absence of a standardised defi nition of heavy alcohol use between countries means that appropriate cutoff s remain contentious, and such data are diffi cult to collect. Although genotype and core promoter or precore mutations might
Sensitivity
1·0
0·8
0·6
0·4
0·2
0
0 0·5 1
1-specificity
A
Kaplan-Meier observed HCC risk
1·0
0·1
0·01
0·001
0·0001
0·0001 0·01 1
Mean risk-score predicted HCC risk
B
0·1 0·001
3-year HCC risk
(AUROC=0·811, 95% CI 0·790–0·831) 5-year HCC risk
(AUROC=0·796, 95% CI 0·775–0·816) 10-year HCC risk
(AUROC=0·769, 95% CI 0·747–0·790)
3 years 5 years 10 years
Sensitivity
1·0
0·8
0·6
0·4
0·2
0
0 0·5 1
1-specificity
A
Kaplan-Meier observed HCC risk
1·0
0·1
0·01
0·001
0·0001
0·0001 0·01 1
Mean risk-score predicted HCC risk
B
0·1 0·001
3-year HCC risk
(AUROC=0·902, 95% CI 0·884–0·918) 5-year HCC risk
(AUROC=0·783, 95% CI 0·759–0·806) 10-year HCC risk
(AUROC=0·806, 95% CI 0·783–0·828)
3 years 5 years 10 years
Figure 1: ROC curves for 3-year, 5-year, and 10-year risk of developing HCC (A), and calibration chart for predicted versus observed risk in the overall validation cohort (including patients with cirrhosis at study entry; B) ROC=receiver operating characteristics. HCC=hepatocellular carcinoma.
AUROC=area under receiver operating characteristic curve.
Figure 2: ROC curves for 3-year, 5-year, and 10-year risk of developing HCC (A), and calibration chart for predicted versus observed risk in the non- cirrhotic validation cohort (patients without cirrhosis at study entry; B) ROC=receiver operating characteristics. HCC=hepatocellular carcinoma.
AUROC=area under receiver operating characteristic curve.
be important risk factors, they are not widely available.
The potential for underestimation of HCC cases in the cohorts might be a weakness of this study. Although we excluded patients with overt cirrhosis, the proportion of patients with subclinical cirrhosis was assumed to be similar between datasets. This issue could be addressed by non-invasive measurement of liver stiff ness in clinical practice. Some existing clinical scores are associated with fi brosis stages, but these scores are not widely validated.
Therefore, clinicians have to judge further risk in patients whom they suspect have cirrhosis.
This risk score was developed and validated in Asian patients. Because the clinical characteristics included are common to clinical guidelines prepared by the Asia- Pacifi c Association for the Study of the Liver, the American Association for the Study of Liver Diseases, and the European Association for the Study of Liver, this risk score might be applicable to non-Asian patients. However, further validation is needed for patients of other ethnic origins, and might need to take into account diversities in age at infection (perinatal vs adulthood), genetic background, HBV genotype or species, and exposure to environmental factors such as afl atoxin and alcohol. Although these additional characteristics have not been included in our risk score, it can realistically be modifi ed and validated with use of long-term follow-up data from non-Asian patients with chronic hepatitis B.
Further caution in use of this risk calculator should be given for patients who are co-infected with HIV or hepatitis C virus, or those with ALT fl ares that could be indicative of several factors. Calculation of risk in immune-tolerant patients might be inappropriate, because antiviral treatment is not indicated for these patients. To include immune-stable patients only, an age cutoff of 30 years was used for this risk score. Similarly, patients with evidence of cirrhosis need immediate antiviral therapy, so this risk calculator would not apply to this patient group. Treatment with antiviral agents, resulting in a lowering of HBV DNA viral load and ALT concentrations and accelerating of HBeAg seroclearance and seroconversion, has the potential to substantially change the risk profi le of an individual patient. However, because responses are not durable in many patients, the risk profi le could be highly dynamic both during and after treatment. Further analysis is recommended, with application of the current risk score in a treated cohort to establish whether the treatment-modifi ed risk profi le actually corresponds to reduced HCC risk.
The results of this study represent a simple-to-use risk score combining widely available clinical variables for the estimation of HCC risk within a specifi c timeframe for Asian patients with chronic hepatitis B. This risk score needs to be validated in patients of other ethnic origins. Further investigation and validation of risk calculation and the change of risk in patients undergoing therapy would also be benefi cial. International consensus
on what constitutes high risk of developing HCC in chronic hepatitis B is also needed. Although further validation of this risk score with use of data from a prospective study is desirable, it is unrealistic, because a large cohort of patients with untreated disease is unlikely to be recruited. The developed risk score has the potential to be incorporated into a clinical risk-prediction instrument that could improve patient management through appropriate and timely intervention (panel).
Contributors
Datasets used in this report were collected and analysed by M-FY and W-KS of the University of Hong Kong on behalf of their respective study groups; HL-YC and VW-SW of the Chinese University of Hong Kong on
Panel: Research in context Systematic review
We searched PubMed for relevant articles up to June 30, 2010, with the search terms “risk prediction AND hepatocellular carcinoma AND hepatitis B”. The resulting references were reviewed, and only English language articles associated with prospective estimation of the risk for developing
hepatocellular carcinoma (HCC) with risk functions were considered. To the best of our knowledge, four HCC risk prediction models have been published.3,7,20,21 Most of them were hospital-based studies with small sample sizes for model derivation and validation. The studies included a diverse set of risk predictors, some of which were diffi cult to standardise because of varied defi nitions (eg, cirrhosis and alcohol use). Most importantly, these studies were validated with samples drawn from the same cohort as the derivation sets, or had similar characteristics to the derivation sets. Therefore external validation was urgently needed for HCC prediction models in chronic hepatitis B, because the most stringent test of a risk prediction score is applying it to populations with very diff erent characteristics from those from which it was derived.
Interpretation
Previously published studies established that long-term HCC risk is predictable with clinical parameters, but they were limited by inadequate external validation. The risk score presented here was developed with data from a
population-based natural history cohort and validated in a large, independent multicentre cohort. The fi ndings of our study are much less aff ected by selection bias than the previous studies, and the robustness of the method for HCC prediction was much improved. Our study provides a useful and accurate instrument for prediction of long-term HCC risk in patients with chronic hepatitis B on the basis of their age, sex, serum alanine aminotransferase concentrations, hepatitis B virus (HBV) DNA levels, and HBeAg serostatus.
Clinicians could use this score to assess the risk of progressing to HCC in patients with chronic hepatitis B, and subsequently make evidence-based decisions about the clinical
management of choice for these patients.
behalf of their respective study groups; D-YK, S-HA, and K-HH of Yonsei University College of Medicine on behalf of their respective study groups;
and C-JC on behalf of the REVEAL-HBV Study Group. H-IY did statistical analyses. All authors contributed equally to the content of this report.
Confl icts of interest
H-IY and C-JC have received speakers’ honoraria and travel expenses from Bristol-Myers Squibb in relation to the REACH-B working group meeting. C-JC has received research grant support from the Department of Health; Academia Sinica and National Health Research Institute, Taiwan; and Bristol-Myers Squibb. M-FY’s institution has received a grant from Research Grants Council and Research Fund for Clinical Infectious Diseases, Hong Kong. M-FY has received payment for lectures from GlaxoSmithKline and Bristol-Myers Squibb for work unrelated to this study. HL-YC has received paid consultancy from Bristol-Myers Squibb, Norvatis, Roche, and Merck. P-JC’s institution has received a grant for the HCC BRIDGE study. P-JC has received speakers’
honoraria, travel expenses, and payment for development of educational presentation from Bristol-Myers Squibb. VW-SW is a paid advisory board member of Norvatis, Roche, and Gilead; and has received lecture fees from Norvatis, Abbott, and Echosens. K-HH, D-YK, S-HA, and W-KS declare that they have no confl icts of interest.
Acknowledgments
The REACH-B Working Group meeting was supported through an educational grant from Bristol-Myers Squibb (Singapore). Writing support was provided by Jesse Quigley Jones (MediTech Media Asia Pacifi c) and funded by Bristol-Myers Squibb. The REVEAL-HBV study was supported by research grants from the Department of Health, Executive Yuan, Taipei, Taiwan; Academia Sinica, Taipei, Taiwan;
National Health Research Institute, Chunan, Taiwan; and Bristol-Myers Squibb (Wallingford, CT, USA) to undertake laboratory tests.
References
1 Lee WM. Hepatitis B virus infection. N Engl J Med 1997;
337: 1733–45.
2 Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis:
treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2008; 28: 1067–77.
3 Han KH, Ahn SH. How to predict HCC development in patients with chronic B viral liver disease? Intervirology 2005; 48: 23–28.
4 Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol 1997;
12: S294–308.
5 Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002; 347: 168–74.
6 Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA.
Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study.
Am J Gastroenterol 2006; 101: 1797–803.
7 Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009; 50: 80–88.
8 Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level.
JAMA 2006; 295: 65–73.
9 Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328: 983.
10 Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005;
54: 1610–14.
11 Chan HL, Hui AY, Wong ML, et al. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 2004; 53: 1494–98.
12 Chan HL, Tse CH, Mo F, et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol 2008; 26: 177–82.
13 Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst 2008; 100: 1134–43.
14 Liu S, Zhang H, Gu C, et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis.
J Natl Cancer Inst 2009; 101: 1066–82.
15 European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B.
J Hepatol 2009; 50: 227–42.
16 Ahn SH, Chan HL, Chen PJ, et al. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int 2010; 4: 386–95.
17 Lok AS, McMahon BJ. Chronic hepatitis B: update 2009.
Hepatology 2009; 50: 661–62.
18 Liaw YF, Leung N, Kao JH, et al. Asian-Pacifi c consensus statement on the management of chronic hepatitis B: a 2008 update.
Hepatol Int 2008; 2: 263–83.
19 Bedossa P, Dargere D, Paradis V. Sampling variability of liver fi brosis in chronic hepatitis C. Hepatology 2003; 38: 1449–57.
20 Yang HI, Sherman M, Su J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis b virus infection. J Clin Oncol 2010; 28: 2437–44.
21 Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers.
J Clin Oncol 2010; 28: 1660–65.
22 D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General
cardiovascular risk profi le for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–53.
23 Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–47.
24 Mok TS, Yeo W, Yu S, et al. An intensive surveillance program detected a high incidence of hepatocellular carcinoma among hepatitis B virus carriers with abnormal alpha-fetoprotein levels or abdominal ultrasonography results. J Clin Oncol 2005;
23: 8041–47.