1562
A Seminal Vesicle Autoantigen of Mouse Is Able to Suppress Sperm
Capacitation-Related Events Stimulated by Serum Albumin
1Yen-Hua Huang,3Sin-Tak Chu,3and Yee-Hsiung Chen2,3,4
Institute of Biological Chemistry,3Academia Sinica, and Institute of Biochemical Sciences,4College of Science, National Taiwan University, Taipei 106, Taiwan
ABSTRACT
We studied the effect of a mouse seminal vesicle autoantigen (SVA) on BSA-stimulated functions of mouse sperm. Uncapaci-tated, capaciUncapaci-tated, and acrosome-reacted stages of sperm were morphologically scored, and the cellular zinc content was ex-amined cytologically in a modified Tyrode solution at 378C for 80 min. More than 85% of control cells remained uncapacitat-ed. Addition of 0.3% SVA to the cell incubation did not affect the cell status. Approximately 65% of cells were capacitated in the incubation medium containing 0.3% BSA. Only 30% of the cells became capacitated after incubation with 0.3% BSA and 0.3% SVA together. The decapacitation effect by 0.3% SVA could be subdued by more than 3% BSA in the cell incubation. Whereas BSA did, SVA did not cause removal of Zn21 from sperm, but SVA could suppress the BSA effect. The tyrosine phosphorylated proteins in sperm were detected after incuba-tion in a modified HEPES medium containing 0.3% BSA and/or 0.3% SVA at 378C for 90 min. Whereas BSA enhanced greatly, SVA did not cause phosphorylation of proteins in the range of Mr 40 000–120 000. The BSA-stimulated protein tyrosine
phos-phorylation could be suppressed by SVA in the cell incubation. seminal vesicles, sperm motility and transport
INTRODUCTION
Seminal vesicle secretion (SVS) contains a group of pro-teins and constitutes the major portion of seminal plasma on ejaculation. Finding the structure and function of these proteins is a prerequisite to understanding their roles in seminal vesicle physiology and their effects on gamete functions. We have shown that the antiserum obtained from autoimmunization of male mice or isoimmunization of fe-male mice with SVS is immunoreactive to an androgen-stimulated glycoprotein in mouse SVS [1, 2]. This glyco-protein, which we designate tentatively as seminal vesicle autoantigen (SVA), has a core protein consisting of 131 amino acid residues. Among sexual and nonsexual organs, SVA and its RNA message appear only in the seminal ves-icles [1]. However, its primary structure, which shows no significant similarity to protein sequences collected in the databank [1], gives no clues to its function despite our dem-onstration that it can be complexed with Zn21 [3]. In the search of its action target, we found that SVA can bind sperm membrane phospholipids to suppress sperm motility 1Supported in part by three grants (NSC B-001-016,
89-2311-B-002-038, and 89-2311-B-001-064) from National Science Council, Taiwan.
2Correspondence: Yee-Hsiung Chen, Institute of Biochemical Sciences,
College of Science, National Taiwan University, P.O. Box 23-106, Taipei 106, Taiwan. FAX: 886 2 23635038; e-mail: bc304@gate.sinica.edu.tw Received: 20 April 2000.
First decision: 29 May 2000. Accepted: 7 July 2000.
Q 2000 by the Society for the Study of Reproduction, Inc. ISSN: 0006-3363. http://www.biolreprod.org
and BSA-stimulated sperm hyperactivation [4]. These re-sults prompted us to investigate further how SVA affects sperm functions. Here, we present data to demonstrate that SVA acts as a decapacitation factor to inhibit both zinc removal and the protein tyrosine phosphorylation associ-ated with sperm capacitation induced by BSA.
MATERIALS AND METHODS
Materials
The BSA (free from fatty acids), chlortetracycline (CTC), and polyvinyl alcohol were purchased from Sigma Chemical Co. (St. Louis, MO). Antimouse immunoglobulin (Ig) G horseradish peroxidase (HRP) conjugate was pur-chased from Promega (Madison, WI). Antiphosphotyrosine monoclonal antibody (clone 4G10) was from UBI Co. (Lake Placid, NJ). The N-(6-methoxy-8-quinolyl)-p-toluene sulfonamide (TSQ) was purchased from Molecular Probes (Eugene, OR). Enhanced chemiluminescence (ECL) plus was obtained from Amersham Pharmacia Biotech (Buckinghamshire, UK). All chemicals were of reagent grade.
Preparation of Spermatozoa
Outbred CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and were maintained and bred in the animal center at the College of Medicine, Na-tional Taiwan University. Animals were treated according to the institutional guidelines for the care and use of ex-perimental animals. The mice were housed under controlled lighting (14L:10D) at 21–228C and were provided with wa-ter and National Institutes of Health 31 laboratory mouse chow ad libitum.
Two culture media were used. One was a modified Ty-rode solution, which consisted of 0.1 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 0.4 mM NaH2PO4,
25 mM NaHCO3, 5.6 mM glucose, 0.5 mM sodium
pyru-vate, 25 mM sodium lactate, 1.0 mg/ml of polyvinyl alco-hol, 100 IU/ml of penicillin, and 100 mg/ml of streptomy-cin. The other was a modified HEPES medium (HM) as described by Lee and Storey [5]. The modified HM con-tained 0.12 mM NaCl, 2 mM KCl, 1.2 mM MgSO4·7H2O,
0.36 mM NaH2PO4, 15 mM NaHCO3, 10 mM HEPES, 5.6
mM glucose, 1.1 mM sodium pyruvate, 1.7 mM CaCl2, 1.0
mg/ml of polyvinyl alcohol, 100 IU/ml of penicillin, and 100 mg/ml of streptomycin. In accordance with a method described elsewhere [6], the pH of the culture medium was adjusted to 7.3–7.4 by aeration with humidified air/CO2
(19:1 v/v) in an incubator at 378C for 48 h before use. Polyvinyl alcohol was added to serve as a sperm protectant [7].
Adult male mice (age5 12–16 wk) were killed by cer-vical dislocation. The epididymis were then removed and immersed in the culture medium in the absence of CaCl2.
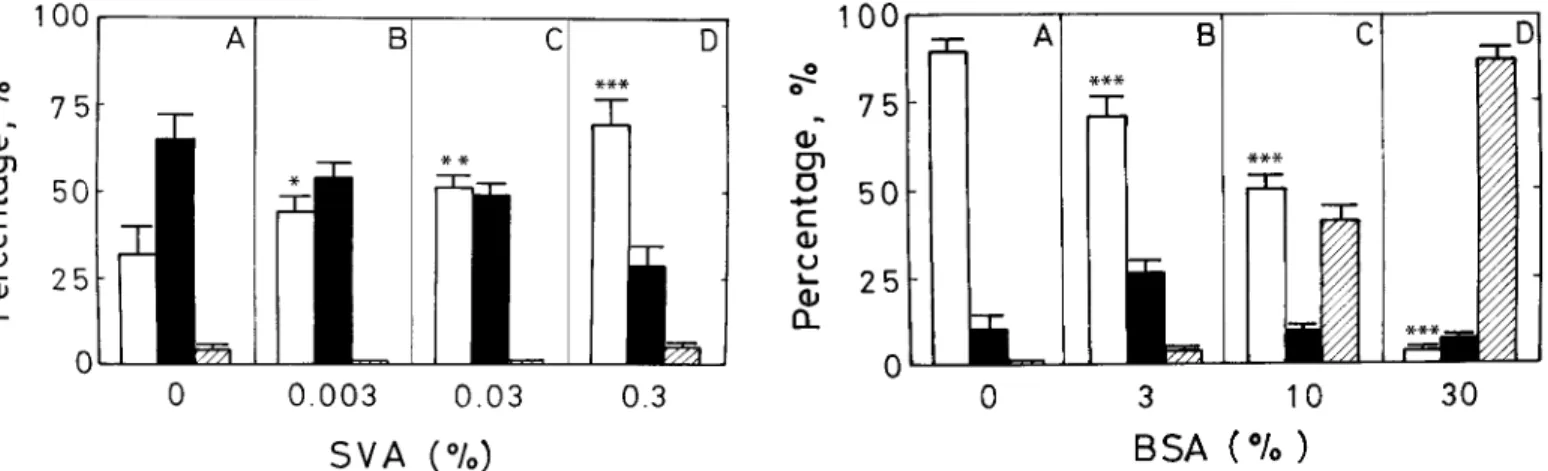
FIG. 1. Suppression of BSA-induced sperm capacitation by SVA. Fresh spermatozoa in modified Tyrode solution (106cells/ml) were incubated
with 0.3% BSA in the presence of SVA at a final concentration of none (A), 0.003% (B), 0.03% (C), or 0.3% (D). Incubation was conducted in 5% CO2in an incubator at 378C for 80 min. The CTC fluorescence
meth-od described in the text was exploited to score the population of unca-pacitation cells (open bars), caunca-pacitation cells (solid bars), and acrosome-reacted cells (hatched bars). Data represent the means of five individual trials counting 200 cells/treatment per trial, and error bars represent the SD. *,P, 0.05; **, P , 0.01; and ***, P , 0.001 in the paired statistical comparison with the corresponding control values being evaluated using one-way ANOVA.
FIG. 2. Removal of the SVA decapacitation effect by BSA. Fresh sper-matozoa in modified Tyrode solution (106cells/ml) were incubated with
0.3% SVA in the presence of BSA at a final concentration of none (A), 3% (B), 10% (C), and 30% (D). Data represent the means of five individual trials counting 200 cells/treatment per trial, and the error bars represent the SD. The cell incubation, cell identification, and statistical tests were performed as described in Figure 1.
After they were carefully dissected away from the connec-tive tissues, spermatozoa were extruded from the distal por-tion of the tissues at 378C for 10 min. The cells were gently filtered through two layers of nylon gauze, washed in three volumes of the same medium (Ca21-free culture medium), and collected by centrifugation at 60 3 g for 10 min at room temperature. The sperm were then resuspended and centrifuged similarly twice more. Cell pellets were resus-pended in a culture medium for further study.
Cytological Observation
The CTC-staining method as described by Ward and Sto-rey [8] was exploited to score the population of mouse sper-matozoa in the uncapacitated, capacitated, and acrosome-reacted stages as seen under a fluorescence microscope (AHBS3; Olympus, Tokyo, Japan).
Spermatozoa were stained with TSQ that can be com-plexed with cellular Zn21to give characteristic fluorescence according to the method described by Andrew et al. [9]. The fluorescence intensity was detected under a fluores-cence microscope.
Detection for Protein Tyrosine Phosphorylation of Spermatozoa
After incubation of spermatozoa in the modified HM un-der specified conditions, the soluble fraction of cell lysate was prepared according to the method described by Vis-conti et al. [10]. Resolution of the protein components in the soluble extract was performed on a 10% polyacryl-amide gel (12.03 10.0 3 0.075 cm) according to the meth-od described by Laemmli [11]. The proteins on the gel were transferred to a filter of nitrocellulose membrane by the electrophoretic method described by Towbin et al [12], con-ducting at 30 V for 6 h at 48C. The protein blots on the filter were immunodetected by Western blot analysis using a monoclonal antibody against phosphotyrosine as the
pri-mary antibody and antimouse IgG conjugated with HRP as the secondary antibody. The enzyme-staining bands were enhanced by chemiluminescence detection using an ECL kit according to the manufacturer’s instructions.
RESULTS
Suppression of BSA-Induced Sperm Capacitation by SVA To determine the effect of SVA on BSA-induced sperm capacitation, sperm (106cells/ml) were suspended in
mod-ified Tyrode solution either alone or in the presence of 0.3% BSA and 0%, 0.003%, 0.03%, or 0.3% SVA. More than 85% of the freshly prepared sperm remained uncapacitated, and nearly no acrosome-reacted cells appeared after incu-bation at 378C for 80 min. (results not shown). The popu-lation of capacitated cells increased remarkably after sim-ilar incubation in Tyrode solution in the presence of 0.3% BSA (Fig. 1A). Approximately 65% of the BSA-treated cells were capacitated then, but acrosome-reacted cells con-stituted less than 5% of the total (Fig. 1A). Addition of SVA to the incubation medium inhibited BSA-induced sperm capacitation. The decapacitation effect of SVA be-came detectable at a concentration greater than 0.003% in the cell incubation medium. The greater the SVA concen-tration in the medium, the greater its inhibition of BSA-induced capacitation (Fig. 1). Approximately 70% of the cells remained in the uncapacitated stage after incubation with 0.3% BSA and 0.3% SVA together (Fig. 1D), sug-gesting that SVA can serve as a decapacitation factor.
To determine whether increasing concentrations of BSA could overcome the inhibitory effect of SVA, sperm (106
cells/ml) were resuspended in modified Tyrode solution containing 0.3% SVA and 0%, 3%, 10%, or 30% BSA for 80 min (Fig. 2). Compared with the stage of sperm incu-bated with SVA alone (Fig. 2A), a remarkable change oc-curred when BSA was added to the cell incubation. The greater the BSA concentration, the more cells became ca-pacitated (Fig. 2, B–D). Almost all cells became caca-pacitated and/or acrosome-reacted at a BSA concentration of 30%, indicating that the decapacitation effect of SVA can be overcome by an excess of BSA in the medium.
FIG. 3. Distribution of TSQ-Zn21fluorescence in spermatozoa after
sev-eral treatments. Spermatozoa in modified Tyrode solution (106cells/ml)
were incubated alone (A) or in the presence of 0.3% BSA (B), 0.3% SVA (C), or a combination of 0.3% SVA and 0.3% BSA (D) at 378C for 80 min. The cells were stained with TSQ and photographed with a fluorescence microscope. The fluorescence intensity represented the cellular Zn21
con-tent and could be compared qualitatively among the four micrographs. Bar5 10 mm.
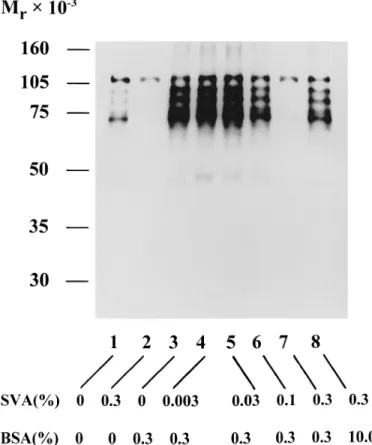
FIG. 4. Appearance of phosphotyrosine-containing proteins in caudal epididymal mouse sperm incubated in several conditions. The cells (53 106cells/ml) in modified HM were incubated in the presence of BSA and/
or SVA at 378C for 90 min (as listed at the bottom). The soluble fraction of the cell lysate was resolved by SDS-PAGE on a 10% gel slab. The phosphotyrosine of a protein on the gel was immunodetected by a West-ern blot procedure as described in the text.
Effect of SVA on BSA-Stimulated Sperm Functions
Figure 3 displays the distribution of fluorescence arising from TSQ-Zn21 complex in the epididymal sperm after in-cubation with 0.3% BSA and/or 0.3% SVA in the modified Tyrode solution at 378C for 80 min. The TSQ-Zn21 fluo-rescence extended over the head, midpiece, and tail of each control cell (Fig. 3A), whereas in the BSA-treated cells, almost no fluorescence was evident in the head and only very weak fluorescence over the midpiece and tail (Fig. 3, A and B), indicating that BSA-induced sperm capacitation was accompanied by removal of Zn21 from the cell. The pattern of TSQ-Zn21 fluorescence in the cells exposed to SVA alone or to SVA and BSA together remained virtually the same as that of the control cells (Fig. 3, A, C, and D). Apparently, inhibition of Zn21removal from sperm by SVA correlates with its suppression of sperm motility, as shown previously [4].
Figure 4 displays the protein tyrosine phosphorylation patterns of epididymal spermatozoa after incubation with 0.3% BSA or/and 0%–0.3% SVA in the modified HM at 378C for 90 min, unless otherwise stated. In the control cells, phosphorylation was mainly restricted to a protein band with a molecular mass of approximately 120 kDa, which according to Kalab et al. [13] is the p95/106 hexo-kinase (Fig. 4, lane 1). In addition, several weak phos-phorylated bands appeared in a group of proteins in the range of Mr40 000–100 000. Except for the p95/106
hexo-kinase, no phosphorylated proteins appeared in the SVA-treated cells (Fig. 4, lanes 1 and 2). Relative to the protein tyrosine phosphorylation of the control cells, phosphory-lation of proteins in the range of Mr 40 000–120 000 was
greatly enhanced in the BSA-treated cells (Fig. 4, lane 3). This was similar to the report of Visconti et al. [10]. The extent of BSA-induced protein tyrosine phosphorylation was gradually suppressed as a function of the concentration of SVA (Fig. 4, lanes 4–7). Only the p95/106 hexokinase was phosphorylated in the cells incubated with 0.3% BSA
and 0.3% SVA together (Fig. 4, lane 7). Apparently, the protein tyrosine phosphorylation associated with BSA-in-duced sperm capacitation could be prevented by the pres-ence of SVA. Reducing the decapacitation effect of SVA by an increased concentration of BSA overcame this effect and was correlated with capacitation-associated protein ty-rosine phosphorylation (Fig. 4, lanes 7 and 8).
DISCUSSION
Mammalian sperm capacitation involves multiple steps, and its molecular mechanism is far from being understood. Identifying the capacitation-associated molecular events be-comes a prerequisite to unraveling this puzzle. Here, we attempted to induce sperm capacitation in vitro, during which biochemical changes in the cell were assayed. In this regard, BSA-induced sperm capacitation has been correlat-ed with many events that include an efflux of cholesterol and phospholipid [14–16], a redistribution of surface and intramembranes components and loss of absorbed compo-nents originating from seminal plasma [17], an increase in Ca21 influx [18], stimulation of protein tyrosine phosphor-ylation [10, 19], removal of Zn21 from cells [9], and the hyperactivation of cell motility that constitutes the last step of capacitation [20]. Subsequently, studying modification of the BSA-stimulated sperm functions by materials in the lu-men of the reproductive tract becomes important to assess the physiological significance of the capacitation-associated molecular events studied in vitro. Relatively less progress
has been made in studies of the inhibition of sperm capac-itation. Results of the present work, together with those of our previous report [4], shed some light onto how SVA plays a role in this regard. The suppression of sperm mo-tility correlated with the inhibition of protein tyrosine phos-phorylation as a result of SVA-sperm binding implicates the involvement of phosphorylated proteins in the range of
Mr40 000–100 000 in the mechanism responsible for sperm motion. In addition, SVA can suppress Zn21 removal from sperm. The generation of ATP required for protein tyrosine phosphorylation might be reduced in the SVA-treated cells, because Zn21 is an uncoupler that inhibits oxidative phos-phorylation of respiratory chain in mitochondria [21–25]. Conversely, BSA can stimulate the two molecular events inhibited by SVA, indicating that protein tyrosine kinase activity is enhanced in those sperm induced to hyperacti-vation by BSA. Taken together, the inhibitory effect of SVA on the two molecular events mentioned earlier may play a role in the suppression of hyperactivated motility, and this may partially account for the counteraction of SVA to the BSA effect in the suppression of sperm hyperactivation [4]. Regarding the interaction between SVA and BSA in their effects on sperm functions, comparing the lipid-bind-ing characteristics of SVA and BSA may provide some educated guesses. Both BSA and SVA are lipid-binding proteins, but they bind to different moieties of phospholip-ids. Serum albumin is well known as a carrier for the trans-portation of fatty acid in the blood circulation, and BSA shows hydrophobic interaction with the acyl chain of phos-phatidylcholine [26]. Thus, the lipid-bilayer structure around the BSA-phospholipid binding region in the plasma membrane of sperm may be perturbed to induce the bio-chemical changes needed for capacitation. On the other hand, the sperm surface has SVA-binding sites that cover the entire cell surface, and SVA shows specific binding to the choline-containing phospholipids such as phosphatidyl-choline and sphingomyelin of sperm membrane [4], with both being predominantly distributed in the outer leaflet of a membrane and the phosphocholine moiety facing the cell’s exterior. Therefore, BSA binding to sperm may hinder the attachment of SVA on the cell surface, reducing the effect of SVA on sperm function, and the mask of SVA on the sperm surface may protect the plasma membrane lipids from the attack of BSA to prevent its stimulation of sperm functions. Contrary to the decapacitation effect of SVA, bovine seminal plasma proteins, which are the choline phospholipid-binding proteins secreted from bovine semi-nal vesicle, can induce bovine sperm capacitation [27–29]. We are unable to explain this discrepancy right now.
The fertile condition of spermatozoa is not a terminal condition but, rather, a transient one [30]. Mammalian sperm display an intriguing sense of timing during their transit in the reproductive tract. They must spend a definite period of time in the female tract to undergo some modi-fication before they acquire the ability to fertilize the egg. However, certain cellular modifications occurring far from the oviduct might cause sperm to become infertile before encountering an egg. On ejaculation, the SVA-sperm bind-ing that occurs must prevent sperm capacitation until SVA is removed from sperm in the uterus or oviduct. This is in accord with our results on the interaction of BSA and SVA regarding capacitation in vitro. More studies are required to determine whether this work is relevant to most mam-mals or only to mice.
REFERENCES
1. Yu LC, Chen JL, Tsai WB, Chen YH. Primary structure and charac-terization of an androgen-stimulated autoantigen purified from mouse seminal-vesicle secretion. Biochem J 1993; 296:571–576.
2. Yu LC, Hsiao YL, Yang YH, Lin M, Chen YH. The genomic structure of a mouse seminal autoantigen. Biochem Biophys Res Commun 1997; 231:106–110.
3. Huang YH, Luo CW, Yu LC, Chu ST, Chen YH. The protein confor-mation and a zinc-binding domain of an autoantigen from mouse sem-inal vesicle. Biophys J 1995; 69:2084–2089.
4. Huang YH, Chu ST, Chen YH. Seminal vesicle autoantigen (SVA), a novel phospholipid-binding protein secreted from luminal epithelium of mouse seminal vesicle, exhibits the ability to suppress mouse sperm motility. Biochem J 1999; 343:241–248.
5. Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs; mouse sperm require it to undergo the acrosome reaction. Biol Reprod 1986; 34:349–356.
6. Bellve´ AR, Zheng W, Martinova YS. Recovery, capacitation, acro-some reaction, and fraction of sperm. Methods Enzymol 1993; 225: 113–137.
7. Bavister BD. Substitution of a synthetic polymer for protein in a mam-malian gamete culture system. J Exp Zool 1981; 217:45–51. 8. Ward CR, Storey BT. Determination of the time course of capacitation
in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol 1984; 104:287–296.
9. Andrew JC, Nolan JP, Hammerstedt RH, Bavister BD. Role of zinc during hamster sperm capacitation. Biol Reprod 1994; 51:1238–1247. 10. Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I: correlation between the capac-itation state and protein tyrosine phosphorylation. Development 1995; 121:1129–1137.
11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680–685.
12. Towbin H, Staehelin TH, Gordan J. Electrophoretic transfer of pro-teins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc Natl Acad Sci USA 1979; 76:4350–4354. 13. Kalab P, Visconti PE, Leclerc P, Kopf GS. P95, the major
phospho-tyrosine-containing protein in mouse spermatozoa, is a hexokinase with unique properties. J Biol Chem 1994; 269:3810–3817. 14. Davis BK. Timing of fertilization in mammals: sperm cholesterol/
phospholipid ratio as a determinant of the capacitation interval. Proc Natl Acad Sci USA 1981; 78:7560–7564.
15. Davis BK, Byren R, Bedigian K. Studies on the mechanism of ca-pacitation: albumin-mediated changes in plasma membrane lipids dur-ing in vitro incubation of rat sperm cells. Proc Natl Acad Sci USA 1980; 77:1546–1550.
16. Go KJ, Wolf DP. Albumin-mediated changes in sperm sterol content during capacitation. Biol Reprod 1985; 32:145–153.
17. Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (eds.), The Physiology of Reproduction, 2nd ed. New York: Raven Press; 1994: 189–317.
18. Fraser LR. Ca21is required for mouse sperm capacitation and
fertil-ization in vitro. J Androl 1982; 3:412–419.
19. Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59:1–6.
20. Wolf DE, Hagopian SS, Ishijima S. Changes in sperm plasma mem-brane lipid diffusibility after hyperactivation during in vitro capaci-tation in the mouse. J Cell Biol 1986; 102:1372–1377.
21. Huacuja L, Sosa A, Delgado N, Rosado A. A kinetic study of the participation of zinc in human spermatozoa metabolism. Life Sci 1973; 13:1383–1394.
22. Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci 1973; 13:1041– 1049.
23. Hidiroglou M, Knipfe JE. Zinc in mammalian sperm: a review. J Dairy Sci 1984; 67:1147–1156.
24. Riffo M, Leiva S, Astudillo J. Effect of zinc on human sperm motility and the acrosome reaction. Int J Androl 1992; 15:229–273. 25. Henkel R, Bittner J, Weber R, Huther F, Miska W. Relevance of zinc
in human sperm flagella and its relation to motility. Fertil Steril 1999; 71:1138–1143.
26. Jonas A. Interaction of phosphatidylcholine with bovine serum albu-min. Specificity and properties of the complex. Biochim Biophys Acta 1976; 427:325–336.
27. The´rien I, Bleau G, Manjunath P. Phosphatidylcholine-binding pro-teins of bovine seminal plasma modulate capacitation of spermatozoa by heparin. Biol Reprod 1995; 52:1372–1379.
28. The´rien I, Soubeyrand S, Manjunath P. Major proteins of bovine
sem-inal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod 1997; 57:1080–1088.
29. The´rien I, Moreau R, Manjunath P. Bovine seminal plasma phospho-lipid-binding proteins stimulate phospholipid efflux from epididymal sperm. Biol Reprod 1999; 61:590–598.
30. Barros C. The fertile mammalian spermatozoa. Rev Micros Electron 1977; 4:107–113.