found that the creation of the charge-compensated oxygen vacancies and the accompanied structural change had a significant influence on the sintering behavior. The direct jumping path through vacant O sites 共path 2 in Fig. 9兲 was further considered as the main reason for the enhancement of the densification rate and slight increase of the activation energy when the Cr2O3content is⬉1 mol % and the decrease of the densification rate but great increase of the activation energy when the Cr2O3 content is⬎1 mol %. Tmax关temperature at the maximum dielectric constant 共max兲兴 decreased with the increase of Cr doping and is essentially determined by the structural change of the NbO6units in SBN. max is suggested to be determined by the competition between the reduction of the polarization due to the substitution of Cr for Nb and the enhancement of the polar moment arising from the weakening of Nb–O bonds due to the creation of the oxygen vacancies. © 2006 American Institute of Physics.关DOI:10.1063/1.2213168兴
I. INTRODUCTION
Sr1−xBaxNb2O6 共SBN兲 exists as a solid solution over a wide composition range共0.25⬉x⬉0.75兲 and belongs to an open structure of tungsten-bronze-type compound.1Also, the structure possesses not only a partially distorted distribution of Sr and Ba cations but also half occupied O共4兲 and O共5兲 sites,2 which probably leads to the important ferroelectric properties and the not well understood relaxor-type phase transition. SBN has been under extensive examination for many applications such as pyroelectric detectors,2 holo-graphic data storage systems,3 or other optical applications.4–11 Recently, the possibility to use a periodi-cally poled SBN for second-harmonic generation,12 a tech-nique which elegantly circumvents the need to satisfy a phase-matching condition, has further stimulated research in this field.
Several dopants were found not only to enlarge pyro-electric, piezopyro-electric, and electro-optic coefficients at room temperature but also to decrease the phase-transition temperature.13–23 Among them, Ce and Cr dopings were more extensively studied. The former was incorporated in Sr sites13,14 and the latter occupied Nb sites,15 therefore, the former acts as the donor and the latter as the acceptor, which seems to indicate that they would have a various influence on the physical properties of SBN. However, both of them were found to enhance the photorefractive properties and decrease the phase-transition temperature.13–15,21 Thus, it would be worth further exploring the mechanism of the defect
struc-tures they create in affecting the physical properties. In the enhancement of the photorefractive properties, the defect was found to be related to the created small Nb4+ polarons upon illumination for both impurities,16 but the defect relat-ing to the decrease of the phase-transition temperature is not known. In the previous work,24 CeO2-doped SBN has been investigated, and it was found that Ce3+ ions per se and its accompanied defect had a profound influence on the sinter-ing and dielectric behaviors.24It is expected that Cr doping would be so, thus, the purpose of this investigation is to realize the sintering behavior, defect structure, and the di-electric properties of Cr2O3-doped SBN50, which could pro-vide deep insight concerning the doping effect in comparison with the results of CeO2-doped SBN.24
II. EXPERIMENTAL PROCEDURE
High-purity powders of BaCO3 共99.9%兲, SrCO3 共99.9 + %兲 共Aldrich兲, Cr2O3 共99.8%兲 共CERAC兲, and Nb2O5 共99.9%兲 共ALFA-AESAR兲 were wet mixed with ethyl alcohol for 24 h according to the formula Sr0.5Ba0.5Nb2−xCrxO6 共x = 0, 0.005, 0.01, 0.02, 0.03, and 0.04, abbreviated by SBN50, 0.5CrSBN50, 1CrSBN50, 2CrSBN50, 3CrSBN50, and 4CrSBN50, respectively兲. The mixed powders were micro-wave dried, ground in a mortar, and calcined at 1250 ° C for 4 h. The calcined powders were wet-ball milled in ethyl al-cohol for 24 h and dried. The single-phase powders of all compositions were deagglomerated and then uniaxially com-pacted in a 12 mm die at 30 MPa, and thereafter, cold isos-tatically pressed at 200 MPa to form pellets with a green density of about 60% of the theoretical density. The green a兲Electronic mail: ttfang@mail.ncku.edu.tw
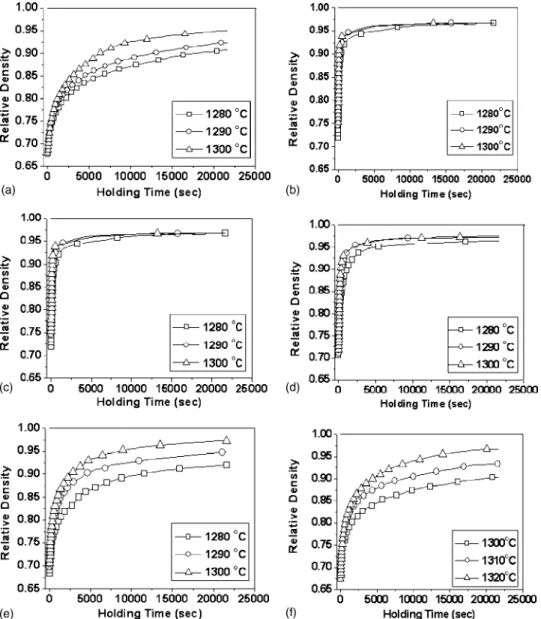
densities were measured by the geometric method. The iso-thermal sintering behaviors of green compacts 共SBN50, 0.5CrSBN50, 1CrSBN50, 2CrSBN50, 3CrSBN50, and 4CrSBN50兲 were performed in a dilatometer using a heating rate of 15 ° C / min to reach the desired temperatures. The isothermal sintering temperatures 1280, 1290, and 1300 ° C were used for the former five samples but higher tempera-tures 1300, 1310, and 1320 ° C were selected for 4CrSBN50 because of its lower densification rate. The sintered densities were measured by the Archimedes’ method. The densifica-tion rate was evaluated by fitting the plot of density versus time共Fig. 1兲 with a polynomial function, and then deriving the densification rate from the slope of this function at a given density. The morphological kinetic equation25 was used to evaluate the activation energies of the samples. It can be expressed as ln共˙T兲 = ln
冋
冉
␥ k冊
D *A iFi共y,z兲冉
p p0冊
mi册
+冋
冉
− Q R冊冉
1 T冊
册
, 共1兲 where ˙ is the densification rate, D* is the diffusion cont-stant,␥is the surface energy, Q is the activation energy, R isthe gas constant, and k is the Boltzmann’s constant; p and p0 represent the porosity and the onset porosity, respectively. The intercept parameter A is a function of the initial condi-tion for each stage, and the morphological terms m and y are related to the pore-size distributions. z represents the fraction of shrinking pores. The subscript i represents the intermedi-ate 共i=1兲 and final 共i=2兲 stages of sintering. In this investi-gation, the difference of the morphological parameters共y, z, and m兲 in the preexponential term on the right-hand side of Eq. 共1兲 is assumed to be small at the same density level for different sintering temperatures; thus, the parameters can be assumed to be constant, especially on a logarithmic scale. X-ray diffractometer was used to measure the lattice param-eters. The temperature-dependent dielectric constant was measured using a precision LCR meter at 1 kHz.
III. RESULTS
Figure 1 shows the isothermal sintering behavior of SBN50 doped with different amounts of Cr2O3. It apparently reveals that Cr2O3has a significant influence on the sintering behavior of SBN50. The densification rates at different tem-peratures derived from the data of Fig. 1 for SBN50 doped FIG. 1. Isothermal sintering behavior of undoped and Cr2O3-doped SBN50 at different temperatures, 共a兲 SBN50, 共b兲 0.5CrSBN50, 共c兲 1CrSBN50, 共d兲 2CrSBN50, 共e兲 3CrSBN50, and 共f兲 4CrSBN50.
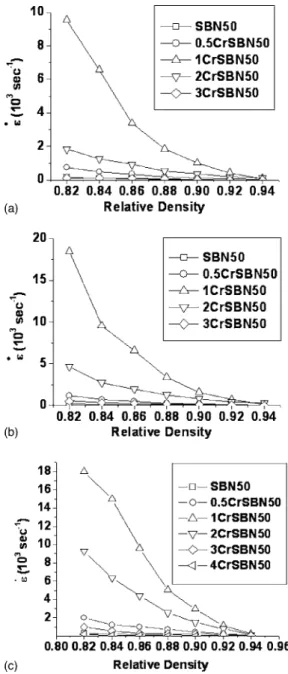
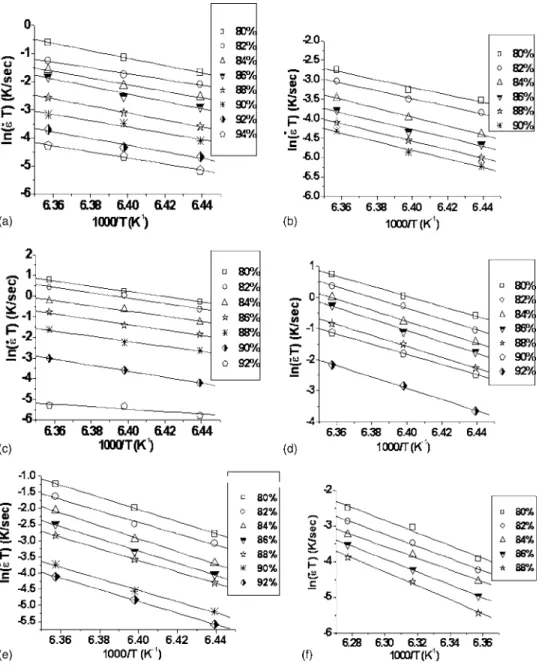
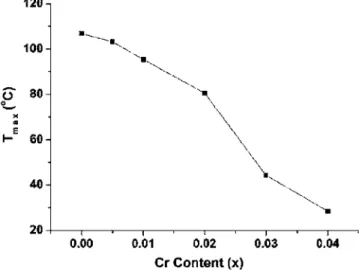
with different amounts of Cr2O3were shown in Fig. 2. They revealed that at a given density, they increased initially as the increase of the Cr2O3 content but become decreased when the Cr2O3 content was共⬎1 mol %兲. Furthermore, based on Eq. 共1兲 and the data of Fig. 2, the activation energies could be evaluated by the plot of ln共˙T兲 vs 共1/T兲 at each density level, shown in Fig. 3. The evaluated values of the activation energy were listed in Table I. Figure 4 shows the variations of the lattice parameters of SBN50 with different amounts of Cr2O3. It shows that the lattice parameters remain almost unchanged initially as the increase of the Cr2O3 content but become greatly changed revealing the increase in a axis and the decrease in c axis when the Cr2O3content is⬎1 mol %. The temperature-dependent dielectric constants of SBN50 doped with different concentrations of Cr2O3were shown in Fig. 5. As observed, the values of Tmax 关temperature at the maximum dielectric constant 共max兲兴 decreased with the in-crease of the Cr2O3 content shown in Fig. 6, and the result
quite close to that of Nb 共0.078 nm兲. Therefore, the Nb site would be the most probable one for Cr ion to occupy, which was indeed justified.15 And, there are two possible charge-compensated defects for such a substitution, which would affect the diffusion, i.e., oxygen vacancies and Cr intersti-tials. However, on the basis of the energy consideration, namely, higher energy for Cr ions in the interstitial sites due to the high charge and the large ionic radius, oxygen vacan-cies are assumed to be the main compensating defect. Con-sequently, as the Cr2O3 content increases, the concentration of the oxygen vacancies would increase, which in turn would enhance the densification rate if the oxygen ions act as the rate-limiting species. However, this viewpoint is not consis-tent with the result of the variation of the densification rate with the Cr2O3content in SBN50 shown in Fig. 2, indicating that the oxygen ions should not be the rate-limiting species. Nevertheless, they still have a profound influence on the structure, sintering behaviors, and dielectric responses of SBN50, which could be rationalized by the following model. Based on the structural analysis of SBN,1there are five kinds of the oxygen-ion sites, labeled with 1–5. It is proposed that the variation of the lattice parameters is assumed to be domi-nated by the structural change of NbO6 octahedral units through the interactions of the Nb and O ions. Because NbO6 octahedral units are linked with the corners, the removal of the oxygen at O共4,5兲 sites would enlarge the c axis due to the repulsion of Nb ions but decrease the a axis due to the stron-ger attraction between Nb and O共1,2,3兲 ions, and vice versa at O共1,2,3兲 sites, which was schematically shown in Fig. 8. When the doping concentration of Cr2O3 is ⬉1 mol %, the probabilities of the creation of the oxygen vacancies at O共4,5兲 and O共1,2,3兲 sites are almost equal, therefore, the variations of lattice parameters of a and c axes would bal-ance and remain unchanged. However, it has been reported1 that only half of the O共4兲 and O共5兲 sites of the B1 framework in the SBN structure were occupied. Thus, the continuous removal of the oxygen ions at O共4,5兲 sites would become unfavorable because the structure would be unstable. Hence, when the Cr2O3content is⬎1 mol %, the generation of oxy-gen vacancies would be mainly at O共1,2,3兲, leading to the significant increase but decease in a and c axes, respectively. Furthermore, based on the defect distribution and the struc-tural variations, the densification and the activation energies of sintering could be explained. It has been suggested24,27,28 that Nb ions via lattice path be the rate-limiting species in sintering SBN, thus, intuitively, the created oxygen vacancies FIG. 2. Densification rate vs relative density of undoped and Cr2O3-doped
seem not to play a role in affecting the sintering behavior. However, they actually would have an influence on the fol-lowing properties, namely, the bonding energy and the diffu-sion path of Nb ions and the structural change of the NbO6 octahedra. The former two would enhance the densification rate, and the latter would affect the jumping distance due to the change of the Nb–O bond length.
Regarding the ceramic structures, they can be considered
as constructed by the linking of octahedral or tetrahedral units of oxygen ions and the cations are inserted in the units. Three possible types of linking are corners, edges, and faces, and the diffusion pathway for each linking would be differ-FIG. 3. Kinetic field responses of un-doped and Cr2O3-doped SBN50, 共a兲 SBN50, 共b兲 0.5CrSBN50, 共c兲 1CrSBN50, 共d兲 2CrSBN50, 共e兲 3CrSBN50, and共f兲 4CrSBN50.
TABLE I. Activation energies of sintering of undoped and Cr2O3-doped SBN50. Sr0.5Ba0.5Nb2−xCrxO6 Activation energy 共eV兲 x = 0 870 x = 0.005 988 x = 0.01 1059 x = 0.02 1446 x = 0.03 1511
x = 0.04 1517 FIG. 4. Variations of the lattice parameters in a and c axes of SBN50 with
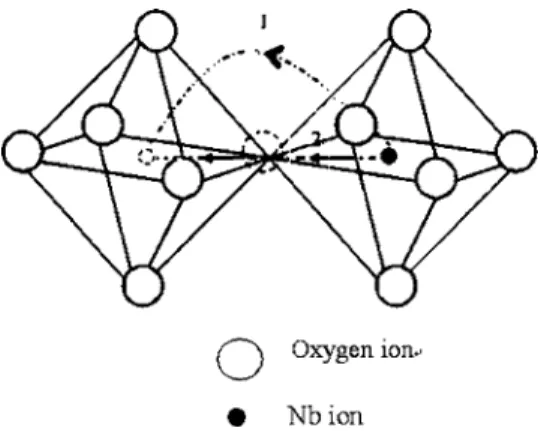
ent. Because the NbO6units in SBN are linked with corners, the corner linking is only considered. For the corner linking, the possible diffusion paths are schematically plotted in Fig. 9. Path 1 represents the indirect jump and the Nb ions jump through the edges of the octahedra. Another diffusion path, namely, path 2, represents the direct jump via the neighbor-ing vacant sites of oxygen if they are available. It should be noted that the jumping distance of direct jump is always shorter than that of the indirect jump even the lattice param-eters are changed. Though the former has a shorter jumping distance, the energy would be higher for Nb ions to jump because the self-diffusivity of Nb ions would also depend on the concentration of the oxygen vacancy, and its formation energy should be involved. If the Schottky defects are as-sumed to be dominant, the Nb vacancies would decrease as the oxygen vacancies increase. Therefore, as the Cr2O3 con-tent increases, the diffusivity of Nb ions would be lowered, which in turn would reduce the sintering rate in view of the fact that Nb ions are the rate-limiting species of sintering.24,27,28However, in Fig. 2, the results showed that at a given density, the densification rate increased initially as the increase of the Cr2O3 content but become decreased when the Cr2O3 content was ⬎1 mol %. Thus, the compli-cated effect of Cr2O3 on the sintering behavior of SBN50
needs to be further clarified. According to Eq.共1兲, the den-sification rate and the activation energy are determined by the self-diffusivity of the rate-limiting species. There are two factors in determining the value of the diffusivity, namely, the jumping distance and the activation energy. The former is essentially determined by the jumping paths shown in Fig. 9. Compared to undoped SBN50, the increase of the densifica-tion rate of 0.5CrSBN50 or 1CrSBN50 is attributed to the higher probability of the direct jump mechanism, which brings large diffusion coefficient resulting from the fast jump. However, the densification rate becomes declined when the amount of Cr2O3 is ⬎1 mol %, which actually is closely related to the change of the lattice parameters shown in Fig. 4 and could be interpreted as follows. When the Cr2O3 content is ⬎1 mol %, the probability for Nb ions to jump through vacant O共1,2,3兲 sites become higher, due to the higher concentration of vacancies at O共1,2,3兲, as mentioned above. And because a axis was greatly lengthened, the jump-ing distance become enhanced, leadjump-ing to the decrease of the densification rate. As for the activation energy of the self-diffusivity of Nb ions, it is dependent on the diffusion paths. For Cr2O3-doped SBN50, the probability via direct jump in-creases, leading to the increase of the activation energy of sintering because the direct jump共path 2兲 needs more energy than the indirect jump 共path 1兲, as mentioned above. When the amount of Cr2O3 is ⬎1 mol %, both the jumping dis-tance and the jumping energy were greatly increased, leading to the higher activation energy of sintering. While the results
FIG. 6. Tmaxas a function of the Cr2O3content for SBN50.
FIG. 8. The effect of the occupied sites of the created oxygen vacancies共the dashed circles兲 on the lattice parameters: 共a兲 at O共1,2,3兲, lengthening a axis but shortening the c axis and共b兲 at O共4,5兲, lengthening c axis but shortening the a axis for NbO6octahedra in SBN50.
FIG. 5. Temperature dependence of dielectric constant of undoped and Cr2O3-doped SBN50.
FIG. 7. Comparison between Tmaxand Cr2O3content as a function of c / a ratio for SBN50.
of the sintering behavior of the Cr2O3-doped SBN could be rationalized by the above discussion, more works have been undertaken to justify the detailed mechanism.
Figure 5 shows the temperature dependence of the di-electric constant of undoped and Cr2O3-doped SBN and re-veals a behavior of diffuse phase transition. SBN has been considered as a uniaxial random-field dominated relaxor.29 The explanation of such a phase-transition picture is usually based on the common feature of the presence of electric di-poles with random sites and orientations in the host crystal lattice.30However,maxand Tmaxwere found to be dependent on the lattice parameter c and c / a ratio, respectively.31,32The latter was further shown to have a linear relationship. How-ever, as observed in Fig. 7, they do not reveal such a linear relationship but show an inverse behavior in comparison with the variation of the Cr2O3content with the c / a ratio. It seems to indicate that there is a direct relation between Tmax and the Cr2O3 content, whereas, in Fig. 6, no such direct linear relationship between them was observed. In the light of this fact and the result of Fig. 7, it is suggested that Tmax is essentially determined by the structural change of the NbO6units in SBN because it is the origin of the variation of
c / a ratio with the Cr2O3 content, as discussed above. Re-garding the variations of max with the Cr2O3content, there are two factors influencing them, namely, the reduction of the polarization leading to low dielectric constant and the enhancement of the polar moment increasing the dielectric constant. The former is due to the substitution of Cr for Nb and the latter arises from the weakening of Nb–O bonds due to the creation of the oxygen vacancies. As the Cr2O3content increases, the effect of the polarization gets weak and the effect of the enhancement of the polar moment gets strong because the defect concentration of the latter is double than the former. Consequently, max reduces first and enlarges later, compared to that of the undoped SBN50. The decrease of the 4CrSBN50 can be attributed to the lower density 共 ⬍90% of the theoretical density兲 compared to other samples with different compositions 共⬎95% of the theoretical den-sity兲 under the same preparation conditions, i.e., sintering temperature at 1300 ° C and time for 6 h.
V. CONCLUSIONS
The lattice parameters remain almost unchanged initially as the increase of Cr2O3content but become greatly changed revealing the increase in a axis and the decrease in c axis when the amount of Cr2O3 is ⬎1 mol %. The significant influence of Cr2O3 doping on the lattice parameters of SBN50 is attributed to the concentration of the created charge-compensated oxygen vacancies located at O共1,2,3兲 and O共4,5兲.
When the amount of Cr2O3 was⬉1 mol %, the densifi-cation rate increased and the activation energy of sintering increased slightly but when the Cr2O3 content was ⬎1 mol %, the densification rate decreased and the activa-tion energy of sintering increased greatly. The main reason is due to the creation of the charge-compensated oxygen vacan-cies, inducing the high probability of direct jumping and the accompanied structural change.
Tmaxdecreased with the increase of the content of Cr2O3, which is essentially determined by the structural change of the NbO6units in SBN.
max would be determined by the competition between the reduction of the polarization due to the substitution of Cr for Nb and the enhancement of the polar moment arising from the weakening of Nb–O bonds due to the creation of the oxygen vacancies.
ACKNOWLEDGMENT
The authors appreciate the financial support from the National Science Council of Taiwan under the Contract No. NSC-92-2216-E-006-010.
1P. B. Jamieson, S. C. Abrahams, and J. L. Bernstein, J. Chem. Phys. 48, 5048共1968兲.
2A. M. Glass, J. Appl. Phys. 40, 4699共1969兲.
3K. Megumi, H. Kozuka, M. Kobayashi, and Y. Furuhata, Appl. Phys. Lett.
30, 631共1977兲.
4E. L. Venturini, E. G. Spencer, P. V. Lenzo, and A. A. Ballman, J. Appl. Phys. 39, 343共1968兲.
5P. V. Lenzo, E. G. Spencer, and A. A. Ballman, Appl. Phys. Lett. 11, 23 共1967兲.
6C. R. Giuliano, Phys. Today 34共4兲, 27 共1981兲. 7J. Feinberg, Phys. Today 41共10兲, 46 共1988兲.
8M. D. Ewbank, R. R. Neugaonkar, W. K. Cory, and J. Feinberg, J. Appl. Phys. 62, 374共1987兲.
9N. V. Bogodaev, V. V. Eliseev, L. I. Ivleva, A. S. Korshunov, S. S. Orlov, N. M. Polozkov, and A. A. Zozulya, J. Opt. Soc. Am. B 9, 1493共1992兲. 10F. Kahmann, R. Pankrath, and R. A. Rupp, Opt. Commun. 107, 6共1994兲. 11K. Buse, R. Pankrath, and E. Kratzig, Opt. Lett. 19, 260共1994兲. 12J. Dec, W. Kleemann, S. Miga, C. Filipic, A. Levstik, R. Pirc, T. Granzow,
and R. Pankrath, Phys. Rev. B 68, 092105共2003兲.
13Th. Woike, G. Weckwerth, H. Palme, and R. Pankrath, Solid State Commun. 102, 743共1997兲.
14J. Wingbermühle, M. Meyer, O. F. Schirmer, R. Pankrath, and P. K. Kre-mer, J. Phys.: Condens. Matter 12, 4277共2000兲.
15T. Woike et al., Appl. Phys. B: Lasers Opt. 72, 661共2001兲.
16M. Gao, R. Pankrath, S. Kapphan, and V. Vikhnin, Appl. Phys. B: Lasers Opt. 68, 849共1999兲.
17M. Gao, S. Kapphan, and R. Pankrath, J. Phys. Chem. Solids 61, 1959 共2000兲.
18S. Sayano, A. Yariv, and R. R. Neurgaonkar, Appl. Phys. Lett. 55, 328 共1989兲.
19R. Vazquez, M. Ewbank, and R. R. Neurgaonkar, Opt. Commun. 80, 253 共1991兲.
20R. Vazquez, R. R. Neurgaonkar, and M. Ewbank, J. Opt. Soc. Am. B 9, 1416共1992兲.
FIG. 9. The jumping paths of NbO6octahedra linked with corner in SBN50: path 1 represents the indirect jump via the edges and path 2 represents the direct jump through the oxygen vacancy. The dashed circles represent vacancies.