國 立 交 通 大 學
環境工程研究所
碩士論文
結合部分硝化、厭氧氨氧化及脫硝作用於單一批
次反應槽之發展
Development of simultaneous partial nitrification,
anammox and denitrification (SNAD) process
in a sequential batch reactor
研 究 生:藍茜茹
指導教授:林志高 教授
結合部分硝化、厭氧氨氧化及脫硝作用於單一批次反應槽之
發展
Development of simultaneous partial nitrification, anammox and
denitrification (SNAD) process in a sequential batch reactor
研 究 生:藍茜茹 Student: Chien-Ju Lan
指導教授:林志高 Advisor: Jih-Gaw Lin
國立交通大學
環境工程研究所
碩士論文
A Thesis
Submitted to the Institute of Environmental Engineering
College of Engineering
National Chiao Tung University
in partial Fulfillment of the Requirements
for the Degree of
Master
in
Environmental Engineering
August, 2011
Hsinchu, Taiwan, Republic of China
II
中文摘要
本研究旨在探討單一批次反應槽中 (SBR),結合部分硝化及厭氧氨氮氧化及脫硝 技術 (SNAD) 於不同水力停留時間 (HRT) 對於氨氮去除效率與經濟最適化之 影響。在 SNAD 技術中先進行部分硝化作用,將氨氮硝化成亞硝酸鹽氮,剩餘 氨氮再與亞硝酸鹽氮經由 Anammox 菌作用轉化為氮氣,而同時產生之硝酸鹽 氮,與缺氧性脫硝菌進行脫硝作用,消耗水中有機物質。SNAD 技術的優點為能 在單一反應槽同時去除水中含氮化合物及有機物質,省去以往需經由兩個反應槽 才能達到硝化脫硝之目的。 研究結果顯示當 HRT 從 9 d 降至 3 d 時,氨氮及化學需氧量 (COD) 去除效率 則達極限。另外,隨著 pH、曝氣及溫度低於正常操作範圍時,氨氮及 COD 去 除效率也隨之降低。在 HRT 9 d 時,氨氮及 COD 其去除效率分別為 96% 及 87%,為本研究之最佳操作水力停留時間。最後,本文中也利用化學計量方程式 及模式來推估氨氮去除,在部分硝化、厭氧氨氧化及脫硝之間的比例,結果顯示 有 85-87% 的總氮是經由結合部分硝化及厭氧氨氧化作用所去除,而脫硝作用去 除比例則占 7-9%。反應槽中菌種鑑定則利用分子生物檢驗法:螢光原味雜交法 (FISH) 及定量聚合酵素鏈鎖反應 (qPCR) 分析污泥中菌相。SNAD 系統對基質 的負荷反應及操作條件另可藉由敏感性指標 (sensitive index) 來做評估。研究結 果能作為提供各污水處理廠未來實廠操作改善或增設水處理除氮設施之參考。Abstract
To decrease the cost of nitrogen removal process, anaerobic ammonium oxidation
(anammox) was developed and coupled with partial nitrification. However, significant
quantity of nitrate released from anammox process (10%) is toxic to aquatic
environment. Recently, simultaneous partial nitrification, anammox and
denitrification (SNAD) process was developed in a sequential batch reactor (SBR)
and the influence of hydraulic retention time (HRT) on the SNAD process was
investigated in this study. Around 96% NH4+-N removal and 87% COD removal were observed at 9 d HRT. Marginal decrease in the removal efficiencies were observed
when the HRT was reduced to 3 d or the loading rate was increased by 3 times. On
the other hand, a drastic decrease in NH4+-N and COD removals were observed when the DO, pH and temperature were dropped shockingly. The response of the SNAD
system towards the shock in substrate loading and operating conditions was evaluated
by sensitivity index. Finally, the extent of total nitrogen (TN) removal by partial
nitrification with anammox and denitrification was modeled using stoichiometric
relationship. Modeling results indicated a TN removal of 85-87% by anammox with
partial nitrification and 7-9% by denitrification. The bacterial diversity in the reactor
was also investigated by fluorescence in situ hybridization (FISH) and quantitative
IV
誌謝
誠摯感謝我的指導教授林志高老師, 不論在生活及研究領域上不 厭其煩的諄諄教誨,給予學生最大的幫助 、教導及鼓勵。此外本論 文的完成亦感謝成功大學鄭幸雄教授,美國愛荷華州立大學宋士武 教授,中原大學黃郁慈教授,給予學生寶貴的意見與指導,使得論 文整體架構與內容更加嚴謹 。 在研究生涯兩年的努力中, 特別感謝至誠學長與理安學長不吝指 教,總是耐著性子與我討論研究及生活上的難題 。碩士班學長學姐 紹謙、維芬、依璇、紘瑩、彥均 ,同窗佩芸、怡君、維倫及學弟信 翰與南維,給予我研究上的協助及鼓勵, 我的研究生活有你們增添 了許多色彩;感謝摯友詩珮、佩昕、欣妤等,在研究遇到瓶頸時分 擔壓力。 最後感謝家人長久以來默默的支持與鼓勵,與亡父的庇佑。 讓我 能全心的完成學業,你們是我完成學業的最大動力,僅以此表達 最 真誠的謝意。 茜茹 謹致於 交通大學環境工程研究所 2011 年 8 月
Contents
中文摘要 ... II
Abstract ... III
誌謝 ... IV
Chapter 1 Introduction ... 1
Chapter 2 Literature Review ... 4
2.1 Introduction ... 4
2.2 Nitrogen pollutants - sources and their impact on environment .... 5
2.3 Conventional biological technologies for nitrogen removal ... 6
2.4 Novel biological processes for nitrogen removal ... 8
2.4.1 Anaerobic Ammonium Oxidation (Anammox) ... 8
2.4.2 Single reactor High activity Ammonia Removal over
Nitrite (SHARON)... 12
2.4.4 Completely Autotrophic Nitrogen Removal over Nitrite
(CANON) ... 14
2.4.5 Oxygen-Limited Autotrophic Nitrification–Denitrification
(OLAND) ... 15
2.4.6 Simultaneous partial Nitrification, ANAMMOX and
Denitrification (SNAD) ... 16
2.5 Simultaneous anoxic ammonium removal with sulphidogenesis
... 17
2.6 Comparison of conventional and novel biological nitrogen removal
processes ... 18
Chapter 3 Material and Methods ... 21
3.1 Synthetic wastewater ... 21
VI
3.2 Inoculation sludge ... 21
3.3 Experimental methods and design ... 22
3.3.1 Reactor system and experimental set up ... 22
3.3.2 Analytical methods ... 25
3.3.3 Polymerase chain reaction (PCR) and qPCR ... 25
3.3.4 Fluorescence in situ hybridization (FISH) ... 26
3.3.5 Terminal Restriction Fragment Length Polymorphism
(TRFLP) ... 26
Chapter 4 Result and Discussion ... 28
4.1 Profiles of pH and DO ... 28
4.2 Nitrogen and COD removals under various HRTs ... 29
4.3 Model based evaluation of SNAD ... 36
4.4 Comparison between full-scale SNAD system with lab-scale
SNAD system ... 41
4.5 Diversity of the bacterial community in SNAD system ... 45
Chapter 5 Conclusion ... 51
List of Tables
Table 1. Major anthropogenic sources of nitrogenous pollutants ... 5
Table 2. Doubling time of various acclimation reactors ... 11
Table 3. Comparative performance in biological nitrogen removal ... 19
Table 4. Composition of the synthetic wastewater used in this study. ... 21
Table 5. The ranges of loading rate under different nitrogen removal processes ... 30
Table 6. Characteristics of synthetic wastewater before and after treatment ... 31
Table 7. Free energy of typical organic carbon with different electron donor in denitrificaiton . ... 36
Table 8. Performance of the SBR under various HRTs ... 40
Table 9. Performance of the full-scale SNAD system under different years ... 44
Table 10. Outcomes of Sequence analysis . ... 48
Table 11. Detail outcomes of qPCR. ... 49
Table 12. Relatively quantification of different bacteria to eubacteria ... 49
Table 13. Relatively quantification of different microbial community in SNAD system ... 49
VIII
List of Figurs
Fig. 1. Nitrogen cycle ... 4
Fig. 2. The schematic of conventional nitrification-denitrificaiton process ... 7
Fig. 3. Biochemical pathway of Anammox reaction. ... 9
Fig. 4. Schematic of an SBR for SNAD system ... 23
Fig. 5. Photograph of SBR ... 23
Fig. 6. Time courses of DO and pH during the operation of SNAD process with different HRTs. ... 29
Fig. 7. Performance of the concentration of nitrogen compounds and removal efficiency of ammonium and total nitrogen in the SBR at different HRTs. .. 32
Fig. 8. Performance of the concentration of COD and removal efficiency of COD in the SBR at different HRTs.. ... 33
Fig. 9. Model based evaluation of the SNAD system. ... 38
Fig. 10. Pictures of red granules from aeration tank. ... 46
Fig. 11. Fluorescence micrographs of bacteria granules collected from the aeration tank ... 47
Chapter 1
Introduction
The release of excessive nitrogen into the aquatic systems leads to acidification and
eutrophication problems. At the same time, it can also impair the survival of aquatic
plants and other organisms. Thus, the removal of nitrogenous compounds from
wastewater systems prior to its disposal is an important issue. Nitrogen removal from
wastewaters is usually accomplished through sequential nitrification and
denitrification processes, i.e. conventional nitrification-denitrification process. This is
recognized as the most suitable process for the treatment of wastewater with high C/N
ratio [1]. During the conventional nitrification-denitrification process, NH4+ is oxidized to nitrate (NO3−) followed by NO3- reduction to gaseous nitrogen (N2). However, several novel nitrogen removal processes have been developed to reduce
the energy consumption in the nitrification-denitrification process. These novel
processes include single reactor system for high ammonium removal over nitrite
(SHARON) [2, 3], completely autotrophic nitrogen removal over nitrite (CANON) [4],
oxygen-limited autotrophic nitrification-denitrification (OLAND) [5] and anaerobic
ammonium oxidation (Anammox) [6].
Anammox process is gaining lot of importance for nitrogen removal compared to the
conventional nitrification-denitrification process. Anammox is an autotrophic
oxidation process, which converts NH4+ to N2 using nitrite (NO2-) as the electron acceptor. Since, Anammox process is an anaerobic-autotrophic process it eliminates
the requirements of aeration and exogenous carbon source [7]. However, the
Anammox process depends on the availability of both NH4+ and NO2- in the system (Eq. (1)); therefore, Anammox process was coupled with partial nitrification in a
2
single reactor system. The combination of Anammox and partial nitrification
decreases the overall cost of the nitrogen removal process; however, a significant
quantity of NO3- (10%) is released from the Anammox systems. This could be more than the wastewater disposal standards at times. Combined nitrogen removal process
such as CANON and OLAND process, both of them are autotrophic nitrogen removal
process which operated under oxygen-limited condition, are suitable for the
wastewater with relatively high ammonium concentration but without organic
consumption. Thus, denitrification was added into the CANON process to solve this
problem. On the other hand, combining Anammox and denitrification for complete
nitrogen removal has been reported [8]. The overall equation for this process is as
follows:
NH4+ + 1.32 NO2− + 0.066 HCO3− + 0.13 H+
→ 1.02 N2 + 0.26 NO3− + 0.066 CH2O0.5N0.15 + 2.03 H2O (1)
Recently, simultaneous partial nitrification, Anammox and denitrification (SNAD) [9]
process was developed, which has the potential of treating NH4+ and biodegradable organics from wastewaters. The advantages of this process are the complete nitrogen
removal and a reduction in the portion of chemical oxygen demand (COD). The
granules capable of carrying out the SNAD process were identified in a full-scale
landfill-leachate treatment plant in Taiwan [10]. In the SNAD process, majority of
nitrogen is removed by the Anammox process. However, developing a SNAD process
in the laboratory is highly difficult owing to the requirement of longer start-up time
and slow growth rates of Anammox bacteria (the doubling time was reported to be
approximately 11 days) [11]. In addition, the reactor carrying out Anammox must be
In several studies, the sequential batch reactors (SBRs) have been successfully applied
for the enrichment of very slow-growing microbial community [13-15]. Compare to
other nitrogen removal configurations, SBR provide efficient biomass retention,
leading to a 90% of retention compare to fluidized bed reactor where retention was
only 64%. Also the doubling time was reduced from 30 days to 11 days [11, 16] in
SBR. However, the optimum conditions of SBR for the enrichment of SNAD
organisms (nitrifying, Anammox, denitrifying bacteria) are not well understood.
Therefore, the present study was aimed to (1) develop a SNAD process in a laboratory
scale SBR using synthetic wastewater, (2) investigate the effect of hydraulic retention
time (HRT) on the performance of SNAD and (3) study the bacterial diversity in the
4
Chapter 2
Literature Review
2.1 Introduction
Organisms require nitrogen as nutrients to produce a number of complex organic
molecules like proteins, enzymes, amino acids, nucleic acids and especially DNA.
The nitrogen cycle represents one of the most important nutrient cycles found in
ecosystems. The ultimate store of nitrogen is in the atmosphere, where it exists as
nitrogen gas (N2). This store is about one million times larger than the total nitrogen
contained in living organisms. Other major stores of nitrogen include organic matter
in soil and the oceans (Figure 1).
Ammonia (NH3) Nitrite (NO2-) Nitrate (NO3-) Plant protein Dead organic matter Animal protein Bacterial oxidation (Nitrobacter) Nitrogen (N2) Ammonia as fertilizer Biological fixation (legumes) Denitrification Bacterial oxidation (Nitrosomonas) Consumption of food Death Bacteria decomposition Fertilizer factory Death Fertilizer uptake Nitrous oxide (N2O)
2.2 Nitrogen pollutants - sources and their impact on environment
Increasing human population have altered nitrogen cycle and accelerated the nitrogenpollutants due to the various human activities, such as providing enough food to the
increasing global population. Futhermore, in some parts of the world, nitrogen is
responsible for a prevalence of unhealthy diets, while also contributing to a host of
environmental problems. Nitrogenous pollutants can enter into the ecosystems from
various anthropogenic sources. Some of them are listed in Table 1.
Table 1. Major anthropogenic sources of nitrogenous pollutants [10, 17-20]
Major anthropogenic sources of nitrogenous pollutants
-Alcohol fermentation
- Aquaculture industries (condensates from ferlilizer plants)
- Activities contributing to N mobilization (biomass burning, land clearing and
conversion, and wetland drainage)
- Food processing (fish, shrimps, spawns)
-Leather tanning-Industrial wastewater discharges
-Landfill leachate
-Municipal sewage effluent (including effluent from sweage treatment plants
without tertiary treatments)
-Overflows of combined storms and sanitary sewers
-Runoff and infliltration from waste disposal sites
-Supernatant from anaerobic sludge digesters
-Wastewaters from livestock farming (cattle, pig, chickens)
- Optoelectronics industrial wastewater -Semiconductor industrial wastewater
6
Although, these pollutants can be removed by denitrification process via the
formation of nitrous oxide (N2O), ammonia emissions, and burial of organic matter in sediments [21]. Overall, the impact of nitrogen pollution still remain and has been
pointed out three environmental problems: (1) it can drastically decrease the pH of
freshwater thereby, impaired the ability of aquatic animals to survive, leading to
acidification of water bodies; (2) organic and inorganic nitrogen pollution to
waterbodies can induce adverse effects on human health, including malaria, cholera
and schistosomiasis [21]; and (3) it can harm ecosystems and contribute to global
warming by producing N2O, a major greenhouse gas which has 310 times higher heat trapping effects than carbon dioxide and even higher than methane (23 times than
carbon dioxide) [22].
All these are compelling evidences that human alteration of the nitrogen cycle is
negatively affecting human and ecosystem health. Therefore, it is of great importance
to determine the most appropriate treatment option as well as the optimal operating
conditons to achieve compatibility in combination treatment processes for the
maximum removal of nitrogenous pollutant.
2.3 Conventional biological technologies for nitrogen removal
Conventional biological nitrogen removal process has been widely used by a
combination of two processes, nitrification and denitrification in separate reactors.
Figure 2 shows the schematic representation of conventional nitrification and
denitrification process. In nitrification process, ammonium is oxidized first to nitrite
and then to nitrate by ammonia-oxidizing bacteria (AOB) with molecular oxygen as
electron acceptor. In the subsequent denitrification step, nitrite-oxidizing bacteria
(NOB) oxidize nitrite (NO2
-) to nitrate (NO3 −
nitrogen gas by denitrifying bacteria using NOX- as electron acceptor and using organic matter as carbon and energy source. Nitrification is an oxygen-requiring
process and therefore requires an aerobic environment and most denitrifying bacteria
carry out these reactions only under anaerobic conditions.
Preliminary treatment Inf. Primary clarifier Primary sludge Nitrification Secondary clarifier Nitrified secondary effluent Methanol
Denitrificaiton Clarifier Eff.
Recycle activated sludge
Waste activated sludge Recycle activated sludge
Waste activated sludge
Fig. 2. The schematic of conventional nitrification-denitrification process
However, the limitations of this conventional processes are requirement of high level
of oxygen (4.2 g O2/g NH4 +
-N) for nitrification [23], and sufficient external organic
carbon source (2.86 g chemical oxygen demand (COD)/g NO3
--N) for denitrification
[24]. Therefore, external carbon sources like methanol and acetate are normally addedto complete the denitrification process when treating wastewaters containing high
nitrogen concentration or low C/N ratio, which increases the operational cost.
Moreover, nitrification and denitrification process have to be carried out under
different oxygen required conditions thus should be designed and operated in two
reactors. Consequently, low nitrogen removal efficiency, high oxygen requirement,
long retention time, and requirement of an external carbon source are the driving
8
2.4 Novel biological processes for nitrogen removal
To overcome the limitations of conventional nitrogen removal process, several novel
biological processes are developed. Some of the novel biological nitrogen removal
processes are described below:
2.4.1 Anaerobic Ammonium Oxidation (Anammox)
Anammox is a novel and low cost approach to remove nitrogen from wastewater. In
1995, this process was discovered at Gist-Brocades (Delft, The Netherlands) during
the effluent treatment from methanogenic reactor in a multistage denitrifying fluidized
bed reactor [7, 26, 27]. The researchers found that nitrate and ammonium disappear at
the same time in the reactor. The nitrate was first considered as electron acceptor, but
it was proved that nitrite was more suitable electron acceptor for Anammox process.
In such case, ammonium is oxidized to nitrogen by aerobic AOB with nitrite as the
electron acceptor with the production of nitrogen gas and small amount of nitrate.
This discovery led to the realization that the enormous of nitrogen losses in the marine
environment were due to Anammox process [28].
The discovery of the Anammox bacterium is a revolution in the biological nitrogen
cycle. Anammox is a lithoautotrophic biological conversion process, mediated by a
group of Planctomycete bacteria which named Anammox bacteria. The specific
mechanism of Anammox pathway are quite unique, several researchers used
15
N-labelled compounds including nitrite, nitrate and hydroxylamine to identify the
reaction of Anammox process. Hydrazine and hydroxylamine are both toxic and were
found to be the intermediates of the process [6, 16, 29]. Ammonium combined with
hydroxylamine to produce hydrazine which subsequently oxidized to nitrogen gas.
compartment, anammoxosome, has a large amount of enzyme-liked hydroxylamine
oxidoreductase (HAO), which is responsible for oxidation of hydrazine to nitrogen
gas [30]. Discovery of hydrazine is exciting as it can be used as rocket fuel and play
an important role as electron donor in conversion of nitrite to hydroxylamine. Thus,
hydrazine molecule can be used as an energy source by bacteria showing Anammox is
a distinct process compare to other nitrogen removal process (Figure 3).
Fig.3. Biochemical pathway of Anammox reaction. a, A simplified depiction of the Anammox microbe, showing the anammoxosome. This is the organelle-like structure in which the energy-generating process involving the combination of ammonia with nitrite takes place. b, The anammoxosome membrane, which consists of the ladderane lipid bilayer, and the anammox reaction pathway. Intermediates in the cycle are hydrazine (N2H4) and hydroxylamine (NH2OH), which are highly toxic [25, 31].
The Anammox process removes about 90% of the incoming nitrogen as
ammonium/nitrite and leaves about 10% of nitrogen as nitrate in the effluent (Eq. (1)).
External carbon sources are not needed in Anammox because carbon dioxide serves
10 NH4 + + 1.32NO2 -+ 0.066HCO3 - + 0.13H+ → 1.02N2+ 0.26NO3 - + 0.066CH2O0.5N0.15+ 2.03H2O ∆G0 =-358 Kj (mol NH4 +
)
-1 (1)The first full-scale Anammox reactor was started at the sludge treatment plant in
Rotterdam and the reactor treated up to 750 kg-N/d. The Anammox reactor with
working volume 70 m3, fed with partially nitritated sludge liquor from an adjusted nitritation process. However, the application of Anammox process might be limited by
slow growth rates of Anammox bacteria (the doubling time of Anammox culture was
be reported to be 30 days in a fluidized bed reactor (Table.2)). The critical point of
shorten the start-up time of Anammox is having sufficient biomass retention. Thus in
order to maintain all the biomass, extending the sludge retention time (SRT) of reactor
would help the acclimation of Anammox bacteria. The Anammox processes
acclimation have been successfully used in fluidized bed, moving bed biofilm reactor,
the rotating biofilm reactor and the anaerobic biological filtrated reactor. But it was
very hard to operate in a laboratory-scale reactor owing to its insufficient biomass
Table 2. Doubling time of various acclimation reactors
Doubling time (d) Reactor Reference
30 FBR1 [16]
29 FBR1 [6]
21 SBR2 [32]
18 MBR3 [33]
11 SBR2 [11]
*E-coli has doubling time of 0.02 d, 1 fluidized bed reactor, 2 sequential batch reactor, 3
membrane bioreactor
The sequencing batch reactor (SBR) was considered a powerful reactor for studying
such slow-growth microorganisms due to the four reasons: (1) efficient biomass
retention, leading to a 90% of biomass retention compare to 64% retention in a
fluidized bed, also doubling time was reduced to 11 days, (2) a homogeneous
distribution of substrates and aggregates, with 1 mm effective aggregate diameter,
50% of the biomass was active, (3) reliable operation for more than one year, and (4)
stable conditions for the first time mass balance under defined conditions [11].
Compared with conventional biological nitrogen removal processes, Anammox
process has two major advantages. First, Anammox is carried out by autotrophic
bacteria under anoxic condition, there is no need for aeration and organic carbon
sources, which saves operation costs. Second, the biomass yield during Anammox
process is very low (0.11 g VSS/g NH4 +
-N, VSS—volatile suspended solids), which
12
2.4.2 Single reactor High activity Ammonia Removal over Nitrite
(SHARON)
In Anammox process, ammonium has to be partly oxidized to nitrite before feeding to
the reactor. Thus, the SHARON process was developed in Delft University of
Technology for treating recycled water from the sludge digesting unit [2]. In this
process, 53% of ammonium was oxidized to nitrite at 1.2 kg N load per m3 per day, without any need of pH control.
Compare with Anammox process where removal of 1 mole of ammonium consumed
1.32 mole nitrite which needs extra nitrite to complete the reaction, SHARON process
offers a good nitrite source without increasing operation cost by purchasing chemical.
It’s feasible for substantial ammonium reduction in a wastewater with relatively high ammonium content and with an elevated temperature. This process takes advantage of
high temperature, enabling high specific growth rates, so that no sludge retention is
required and SRT is controlled by HRT. Also, by carefully selecting the HRT, nitrite
oxidizers can be washed out, while ammonium oxidizers are retained in the reactor.
This process is most suited to treat high ammonium concentration (>500mg-N/L),
where the effluent quality is not critical because it can be sensitively influenced by
changing the reactor pH between 6.5 and 7.5.
The SHARON process is a partial nitrification process which contains fast growing
ammonium oxidizers and this is one of the best suited processes to treat wastewater
with a high ammonium concentration. Thus, the SHARON reactor where only 50%
ammonium is converted to nitrite (Eq. (2)) can be used to provide the feed for the
NH4 + + HCO3 – + 0.75 O2→0.5 NH4 + + 0.5 NO2 – + CO2 + 1.5 H2O (2)
This stoichiometric reaction shows that no base is needed, since anaerobic digestion
will contain enough alkalinity to compensate for the acid production. By using
combined SHARON-Anammox process [3], the oxygen requirement for nitrogen
removal will be reduced to 60% and no longer require the input of COD. The system
can thus be operated independently. The combination of the Anammox process and a
partial nitrification (SHARON) process has been tested using sludge digester effluent,
successfully (Figure 3). These two new concepts for the removal of nitrogen from
wastewater have been developed in which a substantial reduction in the energy and
chemical use is achieved.
Fig 3. Implementation of the SHARON-Anammox process at the WWTP,
14
2.4.4 Completely Autotrophic Nitrogen Removal over Nitrite
(CANON)
No matter how Anammox process combined with different novel biological nitrogen
removal process, Anammox process has to be operated under anoxic condition. The
oxygen-limited condition below 0.5% air saturate provide an adequate environment
for Anammox bacteria [12]. Consequently, the CANON process has been discovered
where high ammonia and low organics loadings in wastewater treatment plant under
oxygen limited condition results in a complete conversion of ammonium to nitrogen
gas in a single autotrophic reactor [34, 35]. Subsequently, Sliekers et al. (2002)
developed a laboratory-scale reactor to have substantial nitrogen losses with a low
dissolved oxygen concentration and with small amounts of COD present in the
wastewater [4].
In this process ammonia would be converted partly to nitrite (Eq. (3)) by
oxygen-limited AOB (Nitrosomonas-like aerobic bacteria) and subsequently,
anaerobic ammonium oxidizers (Planctomycete-like anaerobic bacteria) would
convert ammonia with nitrite to dinitrogen gas (Eq. (4)). The combination of Eq.(3)
and (4) results in the following overall nitrogen removal reaction in Eq. (5)[36]:
1NH3+1.5O2→NO2 -+ H2O +H + (3) 1NH3+1.32NO2 -+H+→1.02N2+0.26NO3 -+2H2O (4) 1NH3+0.85O2→0.11NO3 -+0.44N2+0.14H + +1.43H2O (5)
There are few key factors for operating CANON process, including ammonia
concentration, dissolved oxygen concentration and an AOB population. Especially,
oxygen levels (> 0.5 mg/L) has inhibition on anaerobic AOB with extra nitrite
in CANON process[37]. The microbial interaction between aerobic AOB and
anaerobic AOB affects this process as aerobic AOB utilize ammonia and oxygen as
substrates while anaerobic AOB utilize ammonia and nitrite as substrate. In the
presence of nitrite oxidizing bacteria (NOB) which utilize oxygen and nitrite as
substrates, the CANON process is disrupted because NOB competes with aerobic
AOB for oxygen and with anaerobic AOB for nitrite.
However, no extra carbon source is required because it is completely autotrophic.
This can be achieved in one single reactor, at oxygen limited conditions, without the
production of N2O or NO. Also, CANON consumes 63% less oxygen than conventional nitrogen removal processes [4]. Altogether, CANON process has a high
potential for application in treating low C/N wastewater.
2.4.5 Oxygen-Limited Autotrophic Nitrification–Denitrification
(OLAND)
The OLAND process is discovered in a nitrifying rotating contactor treating
ammonium-rich leachate without consumption of organic carbon under
oxygen-limited condition that can remove the extensive nitrogen by converting NH4
+
to N2 [5]. The operative microorganisms were assumed to be autotrophic populations which could denitrify under low dissolved-oxygen (DO) conditions. Therefore,
oxygen concentration is critical for OLAND because the population of aerobic AOB
drastically decreases at low oxygen concentration. The operative microorganisms
were assumed to be autotrophic populations which could denitrify under low
dissolved-oxygen conditions. There is no big difference between OLAND and
CANON and they differ only on the microbial diversity of these two process, whereas
16
AOB and anaerobic AOB.OLAND is supposed to take place via two steps (Eq. (6)
and (7)). Combining these two steps, it can get an overall reaction in Eq (8): [38]
NH4 + +1.5O2→NO2 -+H2O+2H + (6) NH4 + +NO2 -→N2+2H2 (7) 2NH4 + +1.5O2→N2+3H2O+2H + (8)
The major advantage of this system is that the inoculums can readily be grown in
large quantities which favor the applicability of the OLAND system for practical
purposes.Moreover, operation of this system has no requirement for an NO2
supply.
An ammonium-rich wastewater can be fed directly at a suitable loading rate.
Although the process requires limited oxygen conditions, it does not require strictly
anaerobic conditions. Therefore, inhibition by trace oxygen exposure is not a serious
problem of concern in practice.
2.4.6
Simultaneous
partial
Nitrification,
ANAMMOX
and
Denitrification (SNAD)
CANON and OLAND process, both of them are autotrophic nitrogen removal process
which operated under oxygen-limited condition without organic consumption. These
two treatments are suitable for the wastewater with relatively high ammonium
concentration but no COD. Thus, a novel non-woven rotating biological contactor
reactor was applied for the SNAD process [9]. It allows microorganisms to adhere and
colonize throughout the material, making a very well layer of microorganisms. This
SNAD process is for the simultaneous nitrogen and COD removal for the
high-strength ammonium, with low-carbon wastewater. This is in accord with the
ammonia, whereas denitrification and Anammox occurs under anoxic condition in the
presence of electron donors [12]. Ammonium is oxidized to nitrite by AOB,
subsequently, nitrite can be used by Anammox bacteria which finally convert nitrite
to nitrogen gas with small amounts of nitrate under oxygen-limiting conditions.
Afterwards, the carbon source may be required since the organic carbon demand for
the denitrification reaction is directly consumed from the wastewater COD (as
electron donor could deoxidize nitrate to nitrogen gas through denitrifying process
(Eq.(9), (10) and (11) ), so that the purpose of removing nitrogen and COD can be
achieved simultaneously and the total operation cost will be reduces.
2NH4 + +3O2→2NO2 -+4H++2H2O (9) NH4 + +1.32NO2 -+0.066HCO3 -+0.13H+ →1.02N2+0.26NO3 -+0.066CH2O0.5N0.15+2.03H2O (10) NO3 -+ 1.08CH3OH+ 0.24 H2CO3 →0.056C5H7O2N+0.47N2+1.68 H2O+HCO3 - (11)
The idea of coupling the partial nitrification process with Anammox and
denitrification process has been deemed to one of the most economical process and
can be used extensively in the ammonium rich wastewater.
2.5 Simultaneous anoxic ammonium removal with sulphidogenesis
As alternatives for oxygen, nitrate and nitrite can be used to control sulfide generationduring treatment of S-containing wastewaters. However, sulfate is also likely to be a
suitable selection for its strong oxidation capacity. The simultaneous ammonium and
sulfate was discovered in an Anammox reactor. The dissimilatory sulfate reducing
18
Few researchers postulated that nitrite formation and subsequent Anammox were
responsible for following equation:
SO42-+2NH4+→S+N+H2O (12) The new postulated anaerobic process of nitrogen and sulfate removal seems to
convert into the nitrogen gas and sulfur. The free energy of this reaction is 47 KJ/mol.,
which make simultaneous anoxic ammonium removal with sulphidogenesis possible.
2.6 Comparison of conventional and novel biological nitrogen
removal processes
Table 3 represents the comparative performance of various biological nitrogen
removal processes. Combined novel technologies possess advantages in terms of less
energy consumption, saving configuration and no need for organic carbon sources.
However, each process has its own advantages and potential problems. Many
challenges still remain for the optimization and application of Anammox and its
combination process either in pilot or full scale treatment plant. The Anammox
process can operate under high nitrogen loading and possess distinct advantages of
saving aeration costs and carbon source, but the long start-up time for Anammox
bacteria still remain as a significant obstacle. On the other hand, CANON and
OLAND processes considered using a compact reactor configuration with good
biomass retention, nevertheless the enrichment of anaerobic microorganism capable
of oxidizing ammonia with nitrite as electron acceptor. The SHARON process is
commonly used because it can be operated without any biomass retention, but the
Table 3. Comparative performance in biological nitrogen removal
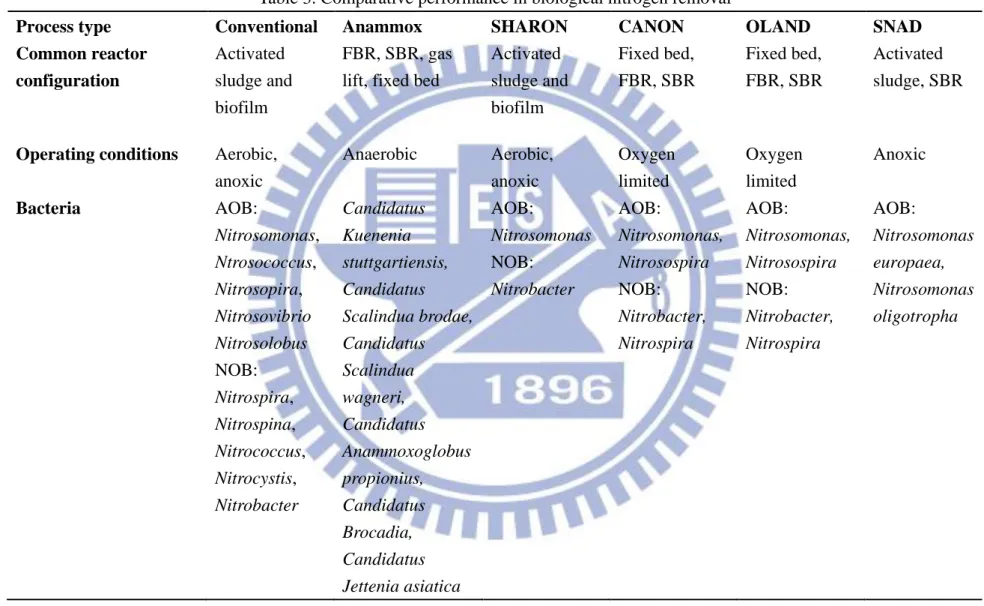
Process type Conventional Anammox SHARON CANON OLAND SNAD
Common reactor configuration Activated sludge and biofilm FBR, SBR, gas lift, fixed bed
Activated sludge and biofilm Fixed bed, FBR, SBR Fixed bed, FBR, SBR Activated sludge, SBR
Operating conditions Aerobic,
anoxic Anaerobic Aerobic, anoxic Oxygen limited Oxygen limited Anoxic Bacteria AOB: Nitrosomonas, Ntrosococcus, Nitrosopira, Nitrosovibrio Nitrosolobus NOB: Nitrospira, Nitrospina, Nitrococcus, Nitrocystis, Nitrobacter Candidatus Kuenenia stuttgartiensis, Candidatus Scalindua brodae, Candidatus Scalindua wagneri, Candidatus Anammoxoglobus propionius, Candidatus Brocadia, Candidatus Jettenia asiatica AOB: Nitrosomonas NOB: Nitrobacter AOB: Nitrosomonas, Nitrosospira NOB: Nitrobacter, Nitrospira AOB: Nitrosomonas, Nitrosospira NOB: Nitrobacter, Nitrospira AOB: Nitrosomonas europaea, Nitrosomonas oligotropha
20
Optimum pH 6.5-8.5 6.7-9.5 7-8 7.8 7-7.2 7.4-8.2
Optimum DO (mg/L) 4-8 <0.2 1-1.5 0.5 <0.1 0.5-0.7
Optimum temperature 12-35 30-40 >25 30-40 30-40 30-40
Oxygen requirement High None Low Low Low Low
COD requirement Yes No No No No No
Substrate Municipal wastewater Synthetic wastewater, Anaerobic digester effluent, Piggery waste Anaerobic digester supernatant/liq uor Synthetic wastewater, Anaerobic liquor Synthetic wastewater Synthetic wastewater, Leachate
Sludge production High Low Low Low Low Low
Max N loading
(Kg N m-3 reactor d-1)
2-8 10-20.5 0.5-1.5 2-3 0.1 0.67-0.022
Total nitrogen removal 95% 87% 90% 75% 85% 97%
Chapter 3
Material and Methods
3.1 Synthetic wastewater
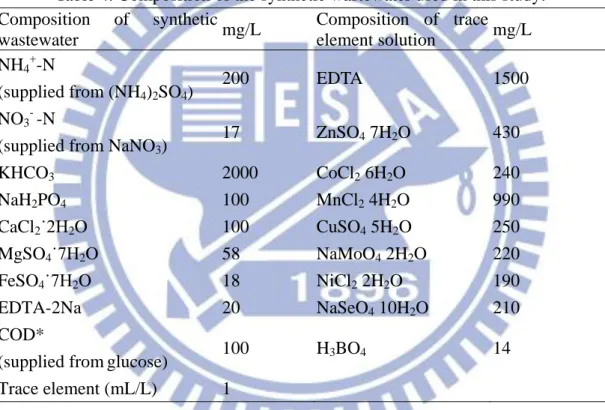
The SBR was fed with a synthetic wastewater (mineral medium). The composition of
the mineral medium used for enrichment of Anammox bacteria is shown in Table 4.
Table 4. Composition of the synthetic wastewater used in this study. Composition of synthetic wastewater mg/L Composition of trace element solution mg/L NH4+-N (supplied from (NH4)2SO4) 200 EDTA 1500 NO3- -N
(supplied from NaNO3)
17 ZnSO4 7H2O 430 KHCO3 2000 CoCl2 6H2O 240 NaH2PO4 100 MnCl2 4H2O 990 CaCl2˙2H2O 100 CuSO4 5H2O 250 MgSO4˙7H2O 58 NaMoO4 2H2O 220 FeSO4˙7H2O 18 NiCl2 2H2O 190 EDTA-2Na 20 NaSeO4 10H2O 210 COD*
(supplied fromglucose) 100 H3BO4 14
Trace element (mL/L) 1
*COD is supplied as glucose (C6H12O6), and 1 g of glucose produces 1.06 g of COD
3.2 Inoculation sludge
The SNAD seed sludge used for inoculating the synthetic wastewater in SBR was
collected from a biological treatment unit (aeration tank) of the full-scale
landfill-leachate treatment plant in Taiwan. The operating conditions established the
22
1.26 d, and the sludge retention time (SRT) ~ 12 to 18 d. The concentrations of mixed
liquor suspended solids (MLSS) and mixed liquor volatile suspended solids (MLVSS)
in the SBR were 1676 and 1140 mg/L, respectively. The fluorescence in-situ
hybridization (FISH) and polymerase chain reaction (PCR) techniques were applied to
verify the presence of Anammox bacteria in the SNAD seed sludge [10]. In addition
to Anammox bacteria the seed sludge also consists of nitrosomonas-like aerobic
microorganisms and denitrifiers. The preliminary investigation of the SNAD seed
sludge revealed that the seed sludge has very high affinity for NH4+ and NO2-. The activity of the SNAD sludge in the present study corresponds to a total nitrogen
removal of 320 mg/g of VSS, which is several times higher than the activity (a total
nitrogen removal of 48 mg/g of VSS) reported by Chen et al. (2009).
3.3 Experimental methods and design
3.3.1 Reactor system and experimental set up
A SBR with a working volume of 18 L was used for the establishment of the SNAD
process. The schematic diagram and photograph of the SBR is shown in Figure 4 and
Fig. 4. Schematic of an SBR for SNAD system (1) mechanical stirrer, (2) influent, (3) effluent, (4) DO measurement, (5) thermostat, (6) controller for mechanical stirrer,
and (7) thermostatic water jacket
24
As a precursor, the acclimation process was started using 2 L of the SNAD seed
sludge. The synthetic wastewater was used as a feed; the composition of the feed
wastewater is shown in Table 4. The acclimation of the SNAD sludge was started
immediately after the inoculation of the seed sludge. The temperature of the SBR was
always controlled at 35C by using a thermostatic water jacket, and the pH was maintained in a range of 7 to 8. The air flow into the reactor was controlled using a
pneumatic valve. The dissolved oxygen (DO) concentration in the sample was
measured outside the SBR using a DO meter. The DO concentration in the reactor
was maintained around 0.3-0.4 mg/L and the alkalinity was maintained in a range of
250-300 mg CaCO3 /L. At the same time, NH4+ oxidation to NO2- (partial nitrification) was controlled by adjusting the DO concentration in the reactor. At any stage, the
NO2--N concentration was not allowed to exceed 100 mg NO2--N /L beyond which Anammox process could be inhibited [7]. By controlling the NO2--N concentration in the SBR, the Anammox reaction was initiated with a proper stoichimetric requirement
of NH4+ and NO2-. A complete mixing inside the SBR was ensured by mixing the reactor contents via a 3-bladed mechanical stirrer at a rate of 100 rpm. After the
acclimation process, the performance of the SBR for treating synthetic wastewater
with ammonium (200 mg/L) and COD (100 mg/L) was investigated under four
different hydraulic retention times (HRTs), i.e. 9 , 4.5, 3 and 6 d. For acclimation as
well as studying the effect of HRT, the SBR was operated in cycles of 24 h and each
cycle consists of feeding and reaction (23.4 h), settling (0.35 h) and decanting the
3.3.2 Analytical methods
The concentrations of nitrogen compounds, suspended solids (SS), volatile suspended
solids (VSS), mixed-liquor suspended solids (MLSS), mixed-liquor volatile
suspended solids (MLVSS) and alkalinity were measured according to the Standard
Methods (APHA, 1998). The NH4+–N, NO2- –N, NO3- –N and SO42- concentrations in influent and effluent were determined spectrophotometrically by using standard
methods (APHA, 1998), and the organic matter content in the synthetic wastewater
was expressed as COD. The pH was determined potentiometrically with a digital pH
meter (SUNTEX SP-701, Taiwan) and the DO was monitored outside of the reactor
with a digital DO meter (YSI 5100, Taiwan).
3.3.3 Polymerase chain reaction (PCR) and qPCR
The total genomic DNA present in the samples was extracted using the UltraClean
Microbial DNA isolation Kit (MO BIO Laboratories, USA). The 16S rDNA
sequences were amplified from the genomic DNA by PCR using 11f
(5’-GTTTGATCCTGGCTCAG-3’) and 1512r (5’-GGYTACCTTGTTACGACTT-3’)
oligonucleotide primers [39]. The thermal cycling consisted of 10 min at 94 oC followed by 35 cycles each of 90 sec at 94oC, 45 sec at 52oC, 120 sec at 72oC and ended by additional 10 min at 72oC. The nucleotide sequence of PCR products were determined using the BigDye terminator cycle sequencing kit (Applied Biosystems,
USA). The resulting sequences were used to do nucleotide-nucleotide blast search
through National Center for Biotechnology Information (NCBI). To amplify 16S
rDNA of Anammox bacteria, PCR was performed using an oligonucleotide primer
pair, 16S-1 (5’-AGTGGCGAAAGGGTGAGTAA-3’) and 16S-2
26
of 10 min at 94oC followed by 40 cycles each of 15 sec at 94oC, 2 sec at 50oC, 60 sec at 68oC and ended by additional 10 min at 72oC.
3.3.4 Fluorescence in situ hybridization (FISH)
The 16S rRNA-targeted oligonucleotide probe used in this study was Amx820 [41]
for Anammox bacteria. The probe was synthesized and directly labeled with
fluorescein isothiocyanate (FITC) at the 5’ end. In situ hybridization was performed
according to the procedure described by Amann et al. [42]. A100X objective Olympus
BX51 microscope (Olympus Optical Co., Japan) fitted with a mercury bulb and blue,
green and red filter sets were used for viewing and observing the slides. The
photomicrograph was made using an Olympus U-CMAD 3 camera (Olympus Optical
Co., Japan) with exposure times of 0.05 s for DAPI and 0.5 s for Amx820.
3.3.5 Terminal Restriction Fragment Length Polymorphism
(TRFLP)
TRFLP is based on PCR amplification of a target gene. In the case of TRFLP, the
amplification is performed with one or both the primers having their 5’ end labeled with a fluorescent molecule. Add 0.5 μl of restriction endonuclease enzyme, Hhal,
and 2 μl of complimentary buffer into 15μl sample of positive PCR product. The
restriction enzyme and complimentary buffer, Buffer C (R003 A), are Catalog No.
R6441 System Lot No. 221280 produced by Promega Corporation. The cut sites of
the enzyme are 5’GCG^C3’ and 3’C^GCG5’. Then put it into thermocycler at 37℃
for two hours. The above procedures are called as digestion reaction. The labeled
Synthesis Core Laboratory to analyze with ABI PRISM3100 Genetic
28
Chapter 4
Result and Discussion
4.1 Profiles of pH and DO
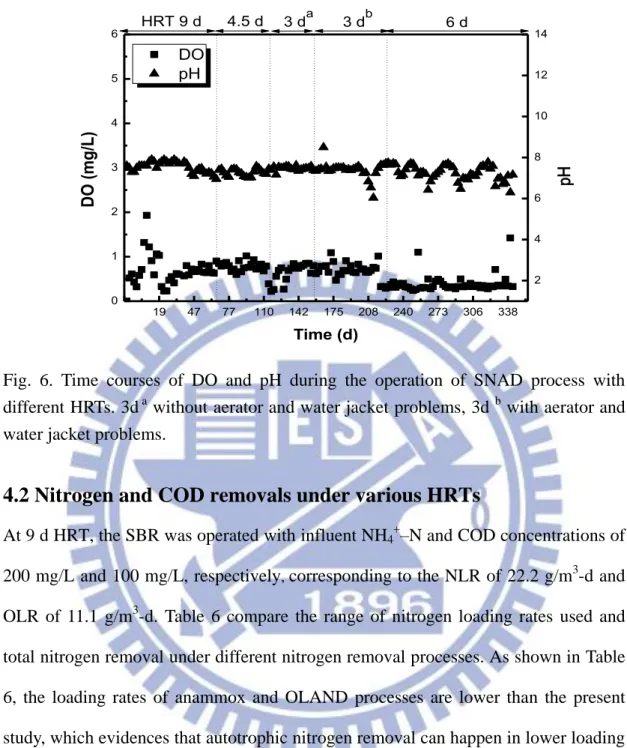
Fig. 4 shows the profiles of pH and DO concentration in the SBR under various HRTs
investigated. The pH profile was fairly constant over the HRTs, except the final 6 d of
HRT, owing to the malfunction of the aerators on 3 d of HRT. The decrease in pH at
any point of time was compensated by the addition of alkalinity to the reactor. If
ammonium concentration increased in the effluent, the DO valve was adjusted in such
a way that the excess ammonium undergoes partial nitrification. The DO
concentration in the reactor was varying a lot in the initial days of operation, i.e. 9 d
HRT. The activity of anammox bacteria and denitrifiers in the SNAD system relies on
partial nitrification becuase the later supplies NO2--N to anammox and denitrification. Moreover, anammox bacteria and denitrifiers prefer anoxic/anaerobic environment.
Therefore, there was some difficulty in controlling the air flow rate to the system in
the initial days of SBR operation (0-19 d, Fig. 4). After this stage, the airflow was
adjusted in such a way to maintain the DO of the reactor at a constant level. To
measure the DO concentration precisely in the reactor DO was measured using the
BOD bottle at the end of 3 d HRT. The DO concentration at HRT 6 d was close to
19 47 77 110 142 175 208 240 273 306 338 0 1 2 3 4 5 6 DO pH Time (d) DO (mg/L) 2 4 6 8 10 12 14 3 db 3 da HRT 9 d 4.5 d pH 6 d
Fig. 6. Time courses of DO and pH during the operation of SNAD process with different HRTs. 3d a without aerator and water jacket problems, 3d b with aerator and water jacket problems.
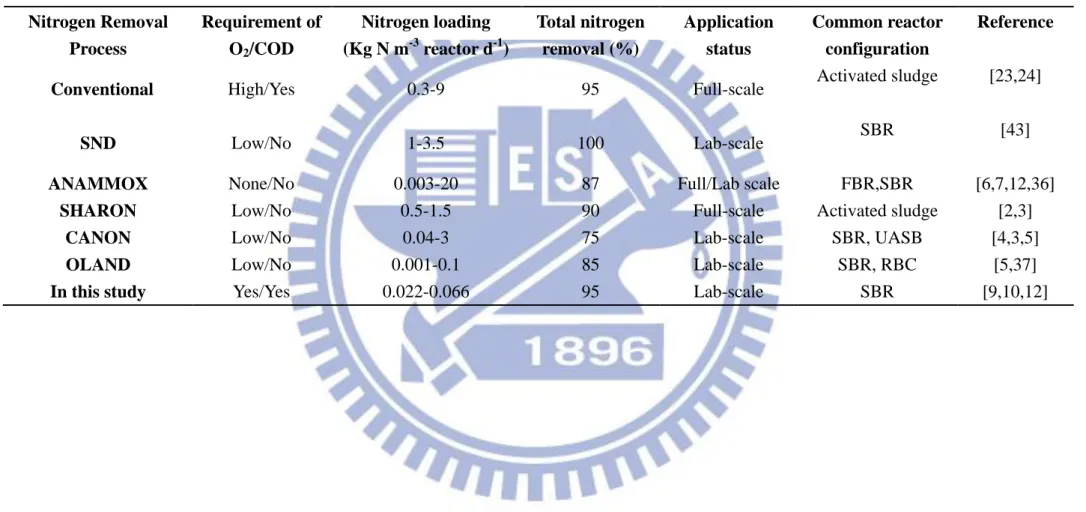
4.2 Nitrogen and COD removals under various HRTs
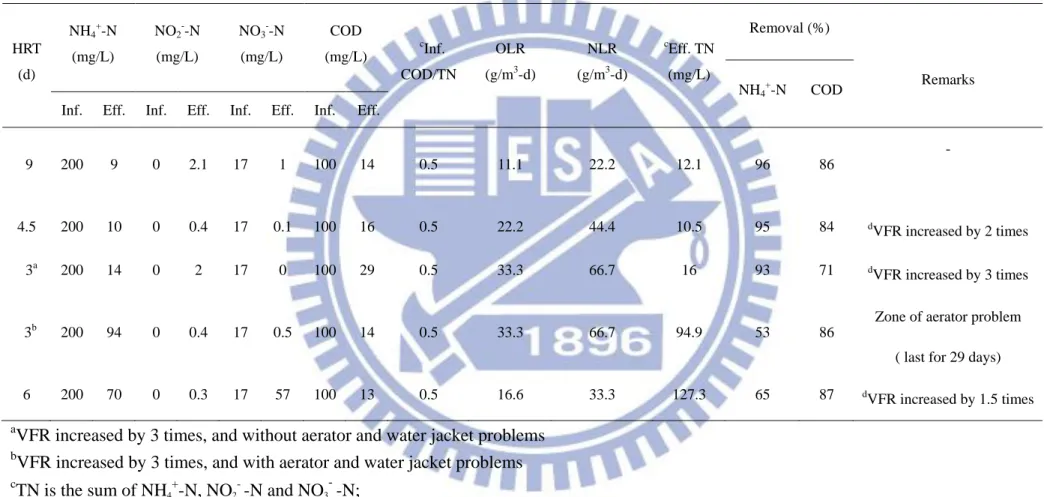
At 9 d HRT, the SBR was operated with influent NH4+–N and COD concentrations of 200 mg/L and 100 mg/L, respectively, corresponding to the NLR of 22.2 g/m3-d and OLR of 11.1 g/m3-d. Table 6 compare the range of nitrogen loading rates used and total nitrogen removal under different nitrogen removal processes. As shown in Table
6, the loading rates of anammox and OLAND processes are lower than the present
study, which evidences that autotrophic nitrogen removal can happen in lower loading
rates also. Moreover, this is the first stage of SNAD seed sludge acclimation in the
SBR; thus, the reactor was operated in moderate loading conditions to avoid substrate
inhibition. The operating conditions of the SBR under various HRTs are shown in
Table 6. The organic loading rate (OLR) and nitrogen loading rate (NLR) to the SBR
under various HRTs were worked out, and are also shown in Table 5. However, the
NH4+-N and COD concentrations were kept constant under all HRTs and the ratio of influent COD/TN was maintained at a constant level (0.5).
30
Table 5. The ranges of loading rate under different nitrogen removal processes
Nitrogen Removal Process Requirement of O2/COD Nitrogen loading (Kg N m-3 reactor d-1) Total nitrogen removal (%) Application status Common reactor configuration Reference
Conventional High/Yes 0.3-9 95 Full-scale Activated sludge [23,24]
SND Low/No 1-3.5 100 Lab-scale SBR [43]
ANAMMOX None/No 0.003-20 87 Full/Lab scale FBR,SBR [6,7,12,36]
SHARON Low/No 0.5-1.5 90 Full-scale Activated sludge [2,3]
CANON Low/No 0.04-3 75 Lab-scale SBR, UASB [4,3,5]
OLAND Low/No 0.001-0.1 85 Lab-scale SBR, RBC [5,37]
Table 6. Characteristics of synthetic wastewater before and after treatment HRT (d) NH4+-N (mg/L) NO2--N (mg/L) NO3--N (mg/L) COD (mg/L) c Inf. COD/TN OLR (g/m3-d) NLR (g/m3-d) c Eff. TN (mg/L) Removal (%) Remarks NH4+-N COD
Inf. Eff. Inf. Eff. Inf. Eff. Inf. Eff.
9 200 9 0 2.1 17 1 100 14 0.5 11.1 22.2 12.1 96 86 - 4.5 200 10 0 0.4 17 0.1 100 16 0.5 22.2 44.4 10.5 95 84 d VFR increased by 2 times 3a 200 14 0 2 17 0 100 29 0.5 33.3 66.7 16 93 71 d VFR increased by 3 times 3b 200 94 0 0.4 17 0.5 100 14 0.5 33.3 66.7 94.9 53 86
Zone of aerator problem ( last for 29 days)
6 200 70 0 0.3 17 57 100 13 0.5 16.6 33.3 127.3 65 87 d
VFR increased by 1.5 times a
VFR increased by 3 times, and without aerator and water jacket problems b
VFR increased by 3 times, and with aerator and water jacket problems c TN is the sum of NH4 + -N, NO2 - -N and NO3 - -N; d
32
The influent and effluent profiles of nitrogenous matter and organics are shown in Fig.
4 and 5, respectively. In the first 40 d of operation, a consistent NH4+–N removal (more than 90%) was observed and small quantities of NO2--N and NO3--N accumulation were found in the SBR. However, SBR displayed a very poor COD
removal efficiency (less than 65%) during this period. In the subsequent days (40-65
d), the removal efficiencies increased gradually and have shown a stable NH4+–N and COD removal efficiencies of 96% and 87%, respectively.
0 30 60 90 120 150 180 210 240 270 300 330 0 25 50 75 100 125 150 175 200 225 Time (d) Co n ce n tr at ion ( mg-N/L ) Eff. NH4+-N Eff. NO2--N Eff. NO3--N Inf. NH4+-N Inf. NO2--N Inf. NO3--N TN removal efficiency NH4+-N removal efficiency HRT 9 d 4.5 d 3 da 3 db 6 d 0 25 50 75 100 Removal effic iency ( % )
Fig.7. Performance of the concentration of nitrogen compounds and removal
efficiency of ammonium and total nitrogen in the SBR at different HRTs. 3d a without aerator and water jacket problems, 3db with aerator and water jacket problems.
0 30 60 90 120 150 180 210 240 270 300 330 0 25 50 75 100 125 150 HRT 9 d Inf. COD Eff. COD
COD removal efficiency
Time (d) Co n ce n tr at ion ( mg/L ) 0 25 50 75 100 6 d 3 db 3 da 4.5 d CO D r emo va l ef ficie n cy ( % )
Fig. 8. Performance of the concentration of COD and removal efficiency of COD in the SBR at different HRTs. 3d a without aerator and water jacket problems, 3db with aerator and water jacket problems.
In order to find the effect of loading rate on the SNAD process, the NLR and OLR
were progressively increased by decreasing the HRT from 9 d to 4.5 d, and operated
for 47 d (Table 5). Despite the higher influent NLR and OLR, a stable conversion of
NH4+–N, without accumulation of NO2--N/NO3--N was observed in the SBR. The increased NLR (44 g/m3-d) and OLR (22 g/m3-d), decreased the COD removal efficiency of the SBR from 87% to 78%, whereas the NH4+–N removal efficiency was maintained in the same level, i.e. 95%. This reveals that the increase NLR and OLR
have no significant effect on the SNAD system. Table 5 shows the steady-state
concentrations of NH4+–N, NO2--N, NO3--N and COD under various HRTs. Following to the steady-state condition at 4.5 d HRT, the reactor NLR and OLR were
further increased to 66 g/m3-d and 33 g/m3-d, respectively, also the HRT was decreased to 3 d. The decrease in the HRT to 3 d has decreased the NH4+–N and COD
34
removals in the system. An increasing trend in the effluent NH4+–N concentration can be noticed in Fig. 5. This indicates that the increases in NLR and OLR (at 3 d HRT)
have produced slight inhibition/toxicity to the partial nitrifiers; as a result, insufficient
NO2--N was produced in the system. Therefore, the performance of Anammox and denitrification were deprived and an overall decrease in the TN and COD removal
efficiencies of the system were observed. However, an improvement in the COD
removal was observed in the subsequent period (120-150 d) and reached a stable
COD removal of 72%.
Unexpectedly, aerator and water jacket were went out-of-order under this recovery
stage, which drastically decreased the reactor performance. During this stage, the DO
in the SBR has went down to below 0.2 mg/L, pH drop down to less than 6 and the
temperature decreased by 5 to 8C. It can be noticed in Table 5 that only 52% of the NH4+–N was removed in the reactor, and interestingly, around 86% of the COD was removed in the reactor. Under this situation, it is hypothesized that Anammox bacteria
might be inactive and the NO2--N produced as a result of partial nitrification could have been utilized only by denitrifiers. In order not to increase further loading under
these circumstances, the reactor NLR and OLR were decreased to 33 g/m3-d and 16 g/m3-d. The reactor started to recover when the HRT was increased from 3 to 6 days. The effluent concentration of NH4+-N was decreased from 94 mg/L to 25 mg/L, also by slightly adjusting the DO and pH value back to optimal condition, the removal
efficiencies of NH4+–N and TN has come back to 75% and 67%, respectively. These observations and hypothesis indicate that high DO concentrations (>2 mg/L) could
result complete nitrification in the SNAD system, whereas low DO concentration
(<0.5 mg/L) could reduce the rate of nitrification and overall performance of the
shock loading compared to sudden change in aeration rate and temperature.
Despite of stable conversion of ammonium, nitrate accumulation was detected in
effluent from the end of HRT 3 d. Nitrate production was related to a possible
response of different electron acceptors such as sulfate which supplied from
(NH4)2SO4 in the medium instead of nitrite in SNAD process. In many researches, except for nitrite, nitrate and propionate, there might be some other electron acceptors
for ammonium oxidation and sulfate is considered to be a suitable selection for its
strong oxidization capacity. Polanco et al. (2001) showed the possibility of removing
ammonium and sulfate simultaneously. They postulated that the nitrite formation and
subsequent Anammox process were responsible for nitrogen removal according to the
following equations (Eq. (13), (14), (15) and (16))[44]:
3SO42-+4NH4+→3S2-+4NO2-+4H2O+8H+ (13) 3S2-+2NO2-+8H+→N2+3S+4H2O (14) 2NO2-+2NH4+→2N2+4H2O (15)
SO42-+2NH4+→N2+S+4H2O (16)
After disturbance during HRT 3 d, the reactor was in unsteady state, the end product
of combining sulfate with ammonium might also produce nitrate as well. On the other
hand, the long period acclimation of SNAD system may result to accumulation of
sulfide which can be toxic to microorganisms. The sludge might be covered by sulfur
which could limit the sufficient contact among reactants. The Anammox activity
36
nitrate accumulation. With decreasing Anammox activity, further works need to focus
on reduction of the released sulfureted hydrogen and collection of sulfur from
reaction.
4.3 Model based evaluation of SNAD
The consumption of nitrogen compounds in partial nitrification, Anammox and
denitrification are modeled using the stoichiometric equations and the experimental
data. Generally, the presence of organic carbon is inhibitory to anammox bacteria. For
example, the presence of methanol is found to have irreversible inhibition at
concentration as low as 0.5 mM. However, a recent study indicated that anammox
bacteria were successful in the oxidation of propionate, and the presence of glucose,
acetate, formate and alanine had no effect on the anammox process[45]. The free
energy of denitrification using typical organic carbon is shown in Table 7 Moreover,
anammox bacteria can be competitive with heterotrophic denitrifiers for the utilization
of organic matter, i.e. propionate. But, the rate of propionate utilization by anammox
bacteria was 0.6 mM/mg of protein/d, which is far less than the utilization rate by
denitrifiers in real-time wastewater systems.
Table 7. Free energy of typical organic carbon with different electron donor in denitrificaiton [46-48].
Denitrification
(organic carbon)
Stoichiometric equation Free energy
(kJ/mol)
Acetate with nitrite NO2-+0.375CH3COO-+H+
0.5N2+0.375CO2+0.375HCO3-+0.875H2O
Methanol NO2-+0.5CH3OH+H+ 0.5N2+0.5CO2+1.5H2O -388 Glucose NO2-+0.125C6H12O6+H+ 0.5N2+0.75CO2+1.25H2O -402
Acetate with nitrate NO3-+0.625CH3COO-+H+
0.5N2+0.625CO2+0.625HCO3-+1.125H2O -498 Methanol NO3-+0.83CH3OH+H+ 0.5N2+0.83CO2+2.17H2O -545 Glucose NO3-+0.208C6H12O6+H+ 0.5N2+1.25CO2+1.75H2O -568
The following stoichiometic relationships are used for modeling: (i) the molar ratio of
NH4+-N: NO2--N in partial nitrification is 1:1, (ii) the stoichiometric consumption (molar ratio) of NH4+-N: NO2--N in Anammox process is 1:1.32, and produces 0.26 mole of NO3--N, subsequently that can be utilized in denitrification, (iii) 1 mg/L of NO3--N is used for consuming 1.74 mg/L COD in denitrification. The TN removal in partial nitrification with Anammox and denitrification under all the HRTs based on
the stoichiometric modeling are shown in Table 8. Moreover, the detailed modeling
concept and the outcomes for 3 d HRT based on the average influent and effluent data
38
Table 8 indicates that around 85-87% of the TN removal is by the combination of
Anammox and partial nitrification. The NO3--N produced in Anammox process is utilized in denitrification along with COD, which is responsible for a TN removal of
7-9%. These observations indicate that under steady-state condition all three
processes in the SBR, i.e. partial nitrification, Anammox and denitrification,
synchronize each other and establish a firm relationship within the reactor irrespective
of the NLR and OLR. However, the shock in the operating DO, pH and temperature
of the SNAD system greatly affected the relationship of these processes. This can be
evidenced from the poor NH4+-N removal efficiency of the system (52%). However, the overall TN removal efficiency of the SNAD system was maintained around 50.7%
owing to the consumption of NO2--N and/or NO3--N in denitrification. The stoichiometric modeling results also indicate that the decrease in the HRT of the
system (from 9 to 3 d) could facilitate the increase in the production of Anammox
bacteria (from 0.067 to 0.357 g/d). This approach could be useful to enrich the slow
growing Anammox bacteria in the real-time conditions. However, a very high
40
Table 8. Performance of the SBR under various HRTs
HRT (d)
TN removal (%) Biomass
produced (g/d)
Sensitivity Index (SI)c*
Partial nitrification + anammox denitrification NH4 NO2 - NO3- COD 9 85.7% 8.7% 0.067 - {9} - {2.1} - {1} - {14} 4.5 87.3% 7.8% 0.259 0.4(13) 0.1(2.3) 0.8(1.8) 0.9(27) 3a 85.5% 7.3% 0.357 1.3(21) 0.7(3.6) 2.6(3.6) 1.8(39) 3b 41.9% 8.7% 0.305 14(135) 0.1(2.2) 0.6(1.6) 2.6(50) 6 32.2% 8.8% 0.197 14(133) 2.5(7.5) 91(92) 1.5(35) a
VFR increased by 3 times, and without aerator and water jacket problems b
VFR increased by 3 times, and with aerator and water jacket problems c
Sensitivity index based on the species concentration at 9 d HRT
Alternatively, the sensitivity of the SNAD system to the change in VFR was evaluated
based on sensitivity index (SI) as shown in Eq. (17) [49].
(17)
where, Omax is the maximum concentration of substrate in the effluent at 4.5, 3 and 6 d HRTs (mg/L), and Os is the average concentration of substrate in the effluent at 9 d HRT (mg/L). The values of SI for all nitrogen species and COD are shown in Table 8.
The SI values indicate that the SNAD process is not greatly affected by the change in
VFR of the system compared to the shock in the operating DO, pH and temperature
conditions. Under the shocking DO, pH and temperature conditions, the SI values
increased by 14 and 2.6 times for NH4+-N and COD, respectively. As indicated before, the Anammox bacteria might be inactive under the shocking condition and the NO2--N produced as a result of partial nitrification could have been utilized only by denitrifiers.
This reveals that the SNAD system has the capability of acting as shortcut
nitrification-denitrification (SND), i.e. NH4+-N is oxidized to NO2--N in nitritation, and subsequently, the NO2--N is reduced to N2 gas. However, the removal efficiency of the SND system (under shocking condition) is far less than the efficiency observed in the
SNAD system.
4.4 Comparison between full-scale SNAD system with lab-scale SNAD
system
Landfill is the most common methods of organized waste disposal and remained so in
many places around the world. A large number of adverse impacts may occur from
42
organic and inorganic matters characterized by high concentration of nitrogen
compounds generated during decomposition of waste in the landfill. Leachate has the
specific meaning of having dissolved or entrained environmentally harmful substances
which may then enter the environment. In older landfills and those with no membrane
between the waste and the underlying, leachate is free to egress the waste directly into
the groundwater. The most common method of handling collected leachate is on-site
treatment.
The full scale SNAD system is applied in landfill leachate treatment plant [10]. The
aeration tank is treating an average leachate flow of 304m3 d−1 with a sludge retention time between 12 and 18 d. Similarly, the full-scale SNAD system was evaluated by the
model and sensitivity index describe in previous section. Table 9 shows the result of
full-scale SNAD system, it indicated that the nitrogen removal mainly by partial
nitrification and Anammox. In 2010, annual precipitation amounts vary from less than 332 mm/month to more than 479 mm/month. This makes the influent concentration varies a lot and the nitrogen removal percentage of partial nitrification and Anammox below than 50%. Moreover, the sensitivity index of ammonium in 2010 has significant effect on the performance of SNAD system.
The comparisons between full-scale and lab-scale SNAD system: (1) Despite of heavy rain in 2010, the full-scale SNAD system demonstrated a stable and high treatment performance for nitrogen removal from actual landfill leachate. Due to the small volume of lab-scale reactor, the buffer capacity of lab-scale SNAD system is way more sensitive than full-scale system, and (2) The operation of lab-scale SNAD system can be more precise on controlling different parameters, such as pH value and DO. pH value in the optimal range to maintain the concentration of free ammonia between 3.5
to 10 mg NH3-N/L, which made sure the nitrification process stop at ammonium
oxidation step and DO concentration in a range of 0.3 to 0.4 mg/L in case nitrite accumulate in the reactor.
Overall, the SNAD process will offer a great future potential for removing nitrogen and organic compounds, it can save energy consumption and cost of adding extra chemical, from wastewater in the industrial application, especially from optoelectronics industrial wastewater in Taiwan.
![Table 1. Major anthropogenic sources of nitrogenous pollutants [10, 17-20]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8638204.192996/14.892.131.760.353.910/table-major-anthropogenic-sources-nitrogenous-pollutants.webp)


![Fig 3. Implementation of the SHARON-Anammox process at the WWTP, Rotterdam-Dokhaven [3]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8638204.192996/22.892.143.762.368.1014/fig-implementation-sharon-anammox-process-wwtp-rotterdam-dokhaven.webp)