A comparative study of Ar and H as carrier gases for the
2ž
/
growth of SiC films on Si 100 by electron cyclotron
resonance chemical vapor deposition at low temperature
Wen-Horng Lee
a, Jing-Cheng Lin
a, Chiapyng Lee
a,U, Huang-Chung Cheng
b,
Tri-Rung Yew
ca
Department of Chemical Engineering, National Taiwan Uni¨ersity of Science and Technology, Taipei 10672, Taiwan, ROC
b
Department of Electronics Engineering, National Chiao-Tung Uni¨ersity, Hsinchu 30050, Taiwan, ROC
c
United Microelectronics Corporation, Science-Based Industrial Park, Hsinchu, Taiwan, ROC
Received 11 November 2000; accepted 6 April 2001
Abstract
Ž .
Electron cyclotron resonance chemical vapor deposition ECR-CVD of SiC films from silane and methane gas mixtures at low temperature has been investigated using two different carrier gases, namely, argon and hydrogen. The results obtained are compared. The chemical composition and crystalline microstructure were investigated by Fourier transform infrared spectroscopy ŽFTIR and cross-sectional transmission electron microscopy XTEM , respectively. The results indicate that the carrier gases. Ž . have a greater influence on the film composition and microstructure as compared to the growth parameters like pressure, power and flow ratio. The deposition mechanism which controls the film characteristics is also presented.䊚 2001 Elsevier Science B.V. All rights reserved.
Keywords: Chemical vapor deposition; Electron cyclotron resonance; Silicon carbide; Transmission electron microscopy
1. Introduction
Ž .
Silicon carbide SiC is an attractive semiconductor material for temperature, power, and high-frequency device applications because of its superior properties such as high thermal conductivity, high-melt-ing point, high breakdown field, high saturated drift velocity, small dielectric constant, and large band gap,
w x
etc. 1᎐3 .
On the other hand, its chemical inertness, trans-parency over a broad range of wavelength, and
hard-UCorresponding author. Tel.: 27376623; fax:
q886-2-27376644.
Ž .
E-mail address: cl@ch.ntust.edu.tw C. Lee .
ness can be used as heat-sinking, optical filter anti-reflection hard coatings, X-ray mask, and corrosion-resistant materials. Furthermore, SiC can also be used as a thin buffer layer for the growth of diamond films
w x w x
on silicon 4 and growth of GaN films on ␣-Al O 5 .2 3
Conventional ways of depositing SiC films by CVD methods were carried out in a high-temperature envi-ronment. A high-temperature CVD process is not suit-able for growing SiC films, because it may cause auto-doping, redistribution of dopants in the Si substrate and non-abrupt heterojuctions between SiC and silicon. From the viewpoint of device fabrications, low temper-ature growth is desired. A relatively new technique, known as electron cyclotron resonance chemical vapor
Ž .
deposition ECR-CVD has the ability to deposit-SiC Ž3C-SiC at low temperatures 6,7 .. w x
0925-9635r01r$ - see front matter 䊚 2001 Elsevier Science B.V. All rights reserved. Ž .
Although there have been previously reported
inves-w x
tigations on the effects of growth parameters 7᎐9 , the effect of different carrier gases has not been discussed yet. In this paper, the effects of different carrier gases were investigated by varying growth parameters such as total pressure, microwave power, and CH4rSiH flow4 ratio. The purpose of this work is to correlate the variation of film chemical composition and crystalline microstructure to the carrier gases. A mechanism which governs the correlation is proposed.
2. Experimental
Ž .
Silicon carbide SiC films were deposited in a commercial Plasma-Quest Model-357 ECR-CVD reac-tor using CH4rSiH rH or CH rSiH rAr gas mix-4 2 4 4 tures. The ECR-CVD system configuration has been
w x described elsewhere 8 .
Ž .
The substrates used were 100 oriented, p-type sili-con wafers with a resistivity of 5᎐15 ⍀-cm, and were cut into 15=30-mm pieces. The substrates were ex situ
w x cleaned by a modified spin-etching method 10 to provide a hydrogen-terminated silicon surface and
pre-w x vent surface oxidation during air exposure 11 . The substrate was then loaded into the reactor within a few minutes after cleaning.
The effect of total pressure was investigated by keep-ing the temperature at 200⬚C, microwave power at 1200 W and CH , SiH , and H4 4 2rAr flow rates at 5, 2.5 and
100 sccm, respectively. Then, the effect of the mi-crowave power was investigated by keeping the temper-ature at 200⬚C, total pressure at 20 mtorr, and CH ,4 SiH , and H4 2rAr flow rates at 5, 2.5 and 100 sccm, respectively. The effect of CH4rSiH flow ratio was4 investigated by keeping the temperature at 200⬚C, mi-crowave at 1200 W, and H2rAr flow rates at 100 sccm. The CH4rSiH flow ratio was varied by changing CH4 4 flow rate while keeping SiH flow rate at 5 sccm. The4 deposition time was 30 min in all cases.
The gas phase species in the plasma were examined by optical emission spectroscopy during the SiC film
Ž
growth. An optical emission spectrometer OES, OEA-.
6850, Rees Instruments Ltd. was attached to the chamber through one of the ports. Optical emission from the plasma was measured through the side view-ing port with the quartz window by multi-channel pho-todetector system. This system can detect lights with wavelength between 200 and 900 nm. Fourier
trans-Ž .
form infrared spectroscopy FTIR spectra were ob-tained with a BIO-RAD FTS-40 spectrometer from 400 to 4000 cmy1, with a resolution of 4 cmy1 and 16 scan times.
The crystalline structure of the deposited film was examined in a JEOL 2000FX STEM. The samples used for cross-sectional transmission electron microscopy
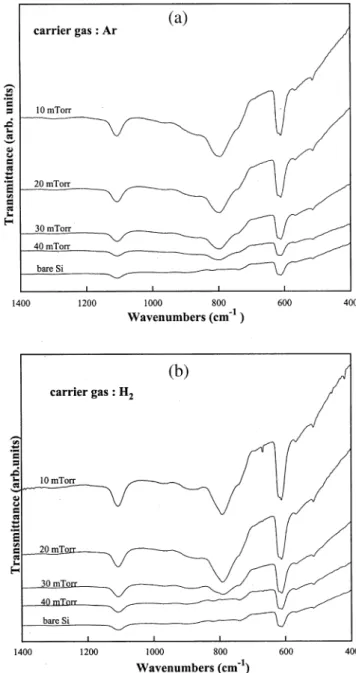
Fig. 1. FTIR transmission spectra of the Si substrate and the films deposited at 200⬚C, 1200 W, CH rSiH s2, various pressures, with4 4 Ž .a Ar as the carrier gas, and b H as the carrier gas.Ž . 2
ŽXTEM inspection were cut into 2. =5-mm size. The XTEM is a destructive analysis technique to observe the deposited film with electron beams perpendicular to the wafer surface normal. As XTEM can be used to observe the deposited film and the filmrsubstrate in-terface simultaneously, it becomes the most direct and precise way to determine the crystalline phase and lattice constant of the deposited films.
3. Results
Fig. 1a,b shows the FTIR spectra of the bare silicon wafer and films grown at 200⬚C, 1200 W, CH rSiH s4 4
2, and various total pressures for Ar and H used as2 the carrier gas, respectively. From the previous works w12᎐14 , it is known that the Si᎐C stretching vibrationx mode of -SiC occurs at approximately 800 cmy1. The weak peaks at approximately 600 and 1100 cmy1 are
Ž
due to Si and SiO , respectively. In Fig. 1a Ar is the2 .
carrier gas , all the spectra except bare Si substrate, exhibit the main peak at 800 cmy1 of the Si᎐C bond.
Ž
However, we can clearly observe in Fig. 1b H is the2
.
carrier gas that when the total pressure is 40 mtorr, the spectrum only exhibits peaks of Si and SiO , which2
are the same as those of the bare Si substrate. When the total pressure is lower than 40 mtorr, the peak appears at 800 cmy1 indicates the formation of SiC. The above results indicate that with Ar as a carrier gas,
Ž SiC can be grown in a larger pressure range 10᎐40
. Ž .
mtorr than with H as a carrier gas 102 ᎐30 mtorr . In order to investigate the difference between Ar and H2 in gas phase decomposition efficiency, optical emission spectra were recorded. Typical optical emis-sion spectra for CH4rSiH rAr and CH rSiH rH4 4 4 2 plasmas at 200⬚C, 1200 W, and CH rSiH s2 are4 4 shown in Fig. 2a. The major species observed are HU2 Ž220 nm , Si 244, 252, 288 and 390.6 nm , SiH 414. Ž . Ž
. Ž . Ž . Ž .
nm , CH 431 nm , H␣ 656 nm , H 486 nm and Ar Ž696.5, 750.4 and 811.5 nm 15 . The CH 431 nm.w x Ž .rH␣ Ž656 nm emission intensity ratio as a function of total.
Ž .
pressure is shown in Fig. 2b. The CH 431 nmrH␣ Ž656 nm emission intensity ratio changes slightly vs.. total pressure for Ar discharge, whereas for the H2
plasma the ratio changes significantly.
Figs. 3 and 4 show the dark-field and bright-field
Ž .
cross-sectional TEM XTEM micrographs with elec-tron diffraction patterns of the films grown under the same conditions as those of Fig. 1 when Ar and H2 were used as the carrier gas, respectively. The XTEM micrograph of polycrystalline silicon grains embedded
Ž
in amorphous SiC was shown in Fig. 3a Ar is the .
carrier gas , which was deposited at a total pressure of 40 mtorr. Fig. 3b indicates that at a total pressure of 30 mtorr, the deposited films on Si are of microcrystalline
Ž .
SiC c-SiC determined by the ring spacing of the electron diffraction pattern. The grains shown in the
Ž .
dark-field image Fig. 3b were of c-SiC, since they
² :
were taken from the SiC 111 ring of the diffraction pattern. When the total pressure was decreased to 20 mtorr and lower, the polycrystalline -SiC could be deposited as shown in Fig. 3c. Using the spot
diffrac-² :
tion pattern of 110 Si zone in Fig. 3c as a reference, the film is identified to be zinc-blend structure with a lattice constant of 0.436"0.005 nm, which is identical
w x
to that of bulk -SiC 16 . The grains shown in the dark-field image are of SiC since they were taken from
² :
the SiC 111 ring of the diffraction pattern.
Microstructures of the films grown at different total pressures using H as the carrier gas were also investi-2
gated and the results are shown in Fig. 4. When the total pressure is 40 mtorr, Fig. 4a indicates that only polycrystalline Si is deposited. At a total pressure of 30 mtorr, the film which is composed of amorphous SiC
Ž .
and embedded polycrystalline Si grains a-SiCqpoly-Si is deposited as shown in Fig. 4b. However, when the total pressure is decreased to 20 mtorr and lower, polycrystalline -SiC films are deposited as shown in
Ž .
Fig. 4c. Therefore, compared with Ar Fig. 3 a lower
Ž .
total pressure 20 mtorr is required to deposit poly-crystalline SiC when H is used as the carrier gas. The2 XTEM results are consistent with those obtained by
Ž .
FTIR Fig. 1 .
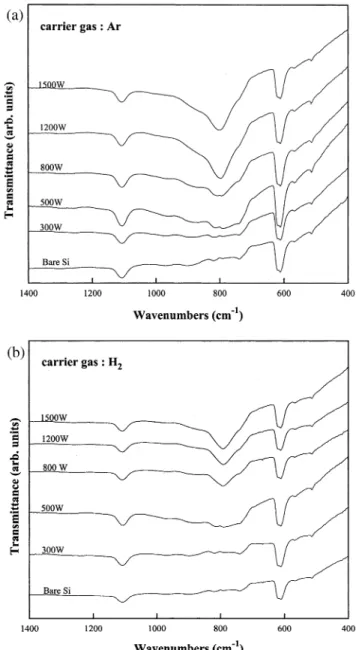
Experiments with various microwave powers were conducted at a fixed SiH4 flow rate of 2.5 sccm, 20 mtorr total pressure, and 200⬚C. The CH rSiH flow4 4
ratio was kept constant at 2. Compared with the FTIR
Ž .
spectrum obtained from the substrate bare Si , other spectra in Fig. 5a show that no Si᎐C bond is formed until microwave power is increased to 500 W. The full width at half-maximum of the peak at 800 cmy1 be-comes narrower when the microwave power is in-creased further above 500 W, which indicates that film crystallinity is improved with increasing microwave power. The results shown in Fig. 5b are similar to the results shown in Fig. 5a, when H is used as the carrier2 gas.
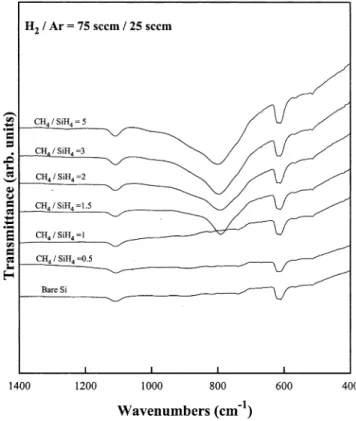
When the total pressure and microwave power were fixed at 20 mtorr and 1200 W, respectively, variation in the CH4rSiH flow ratio changed the composition of4
the film significantly as determined by the FTIR spec-tra and shown in Fig. 6. As the CH4rSiH flow ratio4
was varied from 0.5 to 10, the carrier gas flow rate Ž
remained constant at 100 sccm. In Fig. 6a Ar is the .
carrier gas , we can clearly observe that when the CH4rSiH flow ratio is 0.5, the spectrum only exhibits4
the peaks of Si and SiO . When the CH2 4rSiH flow4 ratio is 1 or higher, the peak, which appears at 800 cmy1 indicate the formation of SiC. However, it was observed that SiC films could be deposited at a CH4rSiH flow ratio of 2 or higher when H was the4 2
Ž .
carrier gas Fig. 6b .
The deposition at various CH4rSiH flow ratios was4 also studied by mixing 25 sccm Ar and 75 sccm H as2
the carrier gas. The result shown in Fig. 7 is very interesting because the formation of SiC can be observed at a CH4rSiH flow ratio of 1.5, which is4
between 1 and 2 when Ar and H2 are used as the carrier gas, respectively.
4. Discussion
In plasma-enhanced depositions of thin films, many reactions are involved both in plasma phase and on the
Ž . Ž . Ž . Fig. 2. a Typical in situ optical emission spectra of ECR plasmas for CH4rSiH rAr and CH rSiH rH gas systems, and b the CH 431 nm4 4 4 2
to H␣emission intensity ratio as a function of total pressure.
substrate surface. Therefore, in order to obtain a desir-able film composition and microstructure, it is impor-tant to understand the mechanism that governs the film
formation. It is known that the dissociation of SiH in4 a discharge essentially generates SiH and SiH radi-2 3
w x cals 17 .
Fig. 3. XTEM dark-field and bright-field micrographs with diffrac-tion patterns of the films deposited at 200⬚C, 1200 W, CH rSiH rAr4 4
Ž . Ž . Ž .
s5r2.5r100, and a total pressure of a 40, b 30, and c 20 mtorr. 4yx
Ž . SiH4Ž g .ªSiHxŽ g .q 2 H2Ž g . xs2,3 1 SiH and SiH radicals can decompose into Si by the2 3
w x
following reaction 18,19 .
Fig. 4. XTEM dark-field and bright-field micrographs with diffrac-tion patterns of the films deposited at 200⬚C, 1200 W,
Ž . Ž . CH4rSiH rH s5r2.5r100, and a total pressure of a 40, b 30,4 2
Ž .
and c 20 mtorr.
Fig. 5. FTIR transmission spectra of the Si substrate and the films deposited at 200⬚C, 20 mtorr, CH rSiH s2, various microwave4 4
Ž . Ž .
powers, with a Ar as the carrier gas, and b H as the carrier gas.2 x
Ž . SiHxŽ g .ªSi q HŽs . 2Ž g . xs2,3 2
2
In plasma, methyl can be formed by the direct disso-ciation of methane. Also, methane can react with the
w x hydrogen atom to form methyl 20 .
Ž . CH4Ž g .qeªCH3Ž g .qH qeŽ g . 3
Ž . HŽ g .qCH4Ž g .lH2Ž g .qCH3Ž g . 4
Under the condition of excess hydrogen as in this study, the acetylene can be formed by the neutral
᎐neu-Fig. 6. FTIR transmission spectra of the Si substrate and the films deposited at 200⬚C, 1200 W, 20 mtorr, various CH rSiH flow ratios,4 4
Ž . Ž .
with a Ar as the carrier gas, and b H as the carrier gas.2
tral reaction of two methyl groups. This reaction can form C H then, result in C H2 6 2 2 following a series of
w x H abstraction reactions 20 .
Ž . CH3Ž g .qCH3Ž g .ªC H2 6Ž g . 5
Fig. 7. FTIR transmission spectra of the Si substrate and the films deposited at 200⬚C, 20 mtorr, 1200 W, H rArs75r25, and various2
CH4rSiH flow ratios.4
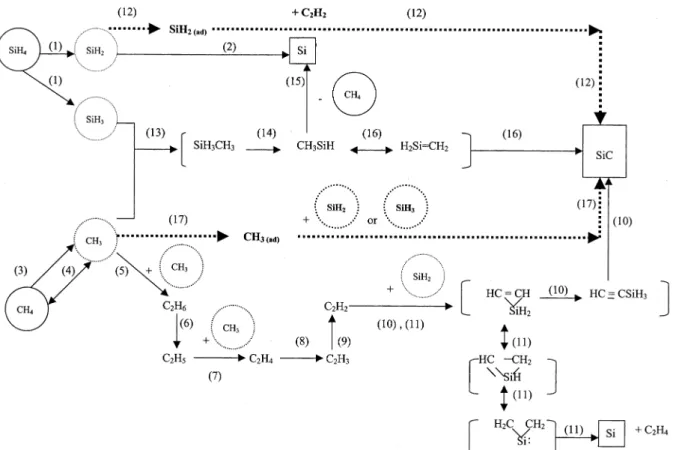
Ž . C H2 6Ž g .qH ªC HŽ g . 2 5Ž g .qH2Ž g . 6 Ž . C H2 5Ž g .qCH3Ž g .ªC H2 4Ž g .qCH4Ž g . 7 Ž . C H2 4Ž g .qH ªC HŽ g . 2 3Ž g .qH2Ž g . 8 Ž . C H2 3Ž g .qH ªC HŽ g . 2 2Ž g .qH2Ž g . 9 Ž . SiH2 may react with C H2 2 in two paths: i a gas-phase reaction between gaseous SiH2 and gaseous C H2 2 to form an intermediate product, probably
H C᎐CH2 2
SiH C3 ⬅CH or E then, adsorbing and decom-Si:
posing into SiC or Si, respectively, on the surface as
w x Ž .
Fig. 8. Proposed growth mechanism for SiC thin films with SiH and CH as the source gases.4 4
gaseous C H2 2 with SiH2 adsorbed on the surface to form SiC.
Ž . 2SiH2Ž ad .qC H2 2Ž g .ª2SiC q3HŽ s . 2Ž g . 12
On the other hand, SiH may react with CH in twox y Ž .
paths: i a gas-phase reaction between gaseous SiH3 w x and gaseous CH to form an intermediate product 22 ,3 most probably CH SiH or H Si3 2 ⫽CH resulting in Si2 or SiC, respectively, as proposed by Johnson et al. w23,24 ,x Ž . SiH3Ž g .qCH3Ž g .ªSiH CH3 3Ž g . 13 Ž . SiH CH3 3Ž g .ªCH SiH qH3 Ž g . 2Ž g . 14 Ž . CH SiH3 Ž g .ªSi qCHŽ s . 4Ž g . 15 Ž . CH SiH3 Ž g .lH Si⫽CH2 2Ž g .ªSiC q2HŽs . 2Ž g . 16
Ž .
and ii a reaction of the gaseous SiHx with CH3
adsorbed on the surface to give SiC 3qx
Ž . SiHxŽ g .qCH3Žad .ªSiC qŽ s . H2Ž g . 17
2
The dotted arrows indicate the reaction between the adsorbed and gaseous species. Based on the above discussion, the most probable film formation mecha-nism for the present work is summarized in Fig. 8.
The bond energies of Si᎐H and C᎐H are 70.4 and w x
98.8 kcalrmol 25 , respectively. If the effective dis-charge powers for dissociation are equal, the dissocia-tion of CH is more difficult than that of SiH . From4 4
Ž .
the results of OES Fig. 2 , it is clear that the CHrH␣ emission intensity ratio decreases with the increasing gas pressure. This indicates that at higher total pres-sure the generation of atomic hydrogen is promoted and the formation of CH radicals is inhibited. How-3 ever, total pressure has a less effect on the decrease of CHrH emission intensity ratio, which represents the␣ concentration of CH when Ar is the carrier gas.3
As shown in Fig. 1b and Fig. 4, when H2 is the carrier gas, the deposited films become poly-Si as the total pressure is increased to 40 mtorr. This is because the generation of CH3 radicals is inhibited at higher pressure as mentioned earlier, as a result less amount of CH radicals impinging onto the substrate surface.3
Ž . Ž .
Reactions 1 and then 2 become the dominant route for the deposition of poly-Si. However, when Ar is the
Ž .
carrier gas, we can clearly observe Fig. 1a that all spectra except bare Si substrate exhibit the main peak at 800 cmy1 of the Si᎐C bond at all pressures. This
also indicates that total pressure has a less effect on the concentration of CH when Ar is the carrier gas.3
Ž .
The results of TEM Fig. 3 show that a film of poly-Si grains embedded in a-SiC was deposited at a higher
Ž .
pressure 40 mtorr , whereas ᎐SiC was formed at a
Ž .
lower pressure 20 mtorr and below . This also indi-cates that the number of CH3 radicals reaching the growing surface is smaller at high pressure. Therefore, FTIR and TEM results are consistent with those of OES due to the same reasons.
Based on the above discussion, the formation of various type films at different CH4rSiH flow ratios4
and microwave powers can also be explained as follows. From the results shown in Fig. 6b, in the case of CH4rSiH flow ratios below 2 when H is the carrier4 2
gas, most of the SiH decomposes into SiH , SiH and4 3 2
silicon. However, only a small quantity of CH decom-4 poses into CH . The decomposition reaction of SiH3 4 wEq. 1 should dominate and result in large amount ofŽ .x SiHx radicals. The SiHx radicals adsorb onto the Si surface where they decompose and polycrystalline
sili-w Ž .x
con is deposited Eq. 2 . At a CH4rSiH flow ratio of4 2, relatively larger amounts of the CH4 is added into the system. The amounts of CH3 radicals and C H2 2
Ž . are comparable with that of SiH , so that Eqs. 10 ,x Ž12 , 16 and 17 of the mechanism could occur to. Ž . Ž . form SiC. However, the SiC films could be deposited at 1 and higher CH4rSiH flow ratios when Ar is the4
Ž .
carrier gas Fig. 6a . This is because CH4 is more effectively decomposed in ECR plasma when Ar is the carrier gas and the heavy mass of Ar produces signifi-cant increase in the momentum of radicals and cause
Ž . Ž . Ž . Ž .
Eqs. 10 , 12 , 16 and 17 to occur at CH4rSiH sl.4 For the same reason, the formation of SiC using a mixture of 25% Ar in H2 as the carrier gas at a
Ž .
CH4rSiH flow ratio of 1.5 Fig. 7 is obvious.4
When the microwave power is as low as 300 W, the
Ž .
deposited film is still poly-Si Fig. 5 even at a CH4rSiH flow ratio of 2. That is because the energy4
needed for SiH formation is lower than that of CHx 3
and the energy is enough for the subsequent decompo-sition of SiH to occur. However, at 500 W and abovex the energy supplied by plasma may be enough for the dissociation of CH4 so that most SiHx radicals can react with CH radicals and C H to form SiC. This is3 2 2
because CH4 has a lower dissociation efficiency than SiH . The results obtained at different flow ratios and4 microwave powers indicate that the dissociation of CH4 should be the rate-limiting step.
5. Conclusion
ECR-CVD has been employed to prepare ᎐SiC films using CH4rSiH rH or CH rSiH rAr mixtures.4 2 4 4
The deposited films were characterized with XTEM, OES, and FTIR. The results indicate that the carrier gases have a greater influence on the film composition and microstructure as compared to the growth parame-ters like pressure, power and flow ratio. Based on the proposed film formation mechanism in combination with experimental results, we found that the dissocia-tion of CH is the rate-limiting step for the growth of4 SiC irrespective of whether Ar or H as the carrier gas.2 At higher total pressure, the generation of atomic hydrogen is promoted and the formation of CH radi-3
cals is inhibited. In contrast to H , total pressure has2
less effect on the concentration of CH when Ar is the3
carrier gas. Ar is more effective in the dissociation of reactant gases than H .2
Acknowledgements
This work was supported by Republic of China Na-tional Science Council under Contract No. ‘NSC87-2214-011-011’.
References
w x1 M. Bhatnagar, B.J. Baliga, IEEE Trans. Electron Dev. 40 Ž1993 645..
w x2 J.D. Hwang, Y.K. Fang, Y.J. Song, D.N. Yaung, Jpn. J. Appl. Ž .
Phys. 34 1995 1447.
w x3 H.J. Kim, R.F. Davis, J. Appl. Phys. 60 1986 2897.Ž . w x4 E.G. Wang, Physica B 185 1993 85.Ž .
w x5 T. Takeuchi, H. Amano, K. Hiramatsu, N. Sawaki, I. Akasaki, J. Ž .
Cryst. Growth 115 1991 634.
w x6 M. Diani, J.L. Bischoff, L. Kubler, D. Bolmont, Appl. Surf. Sci. Ž .
68 1993 575.
w x7 M. Katsuno, T. Futagi, Y. Ohta, H. Mimura, K. Kitamura, Ž .
Appl. Surf. Sci. 70r71 1993 675.
w x8 K.L. Cheng, H.C. Cheng, C.C. Liu, C. Lee, T.R. Yew, Jpn. J. Ž .
Appl. Phys. 34 1995 5527.
w x9 T. Futagi, M. Katsuno, N. Ohtani, H. Mimura, K. Kawamura, Ž .
Appl. Phys. Lett. 58 1991 2948.
w x10 C.H. Chen, C.M. Wan, T.R. Yew, Appl. Phys. Lett. 62 1993Ž . 3126.
w x11 P.J. Grunthaner, F.J. Grunthaner, R.W. Fathauer et al., Thin Ž .
Solid Films 183 1989 197.
w x12 Y. Sun, T. Miyasato, J.K. Wigmore, J. Appl. Phys. 85 1999Ž . 3377.
w x13 A. Chayahara, A. Masuda, T. Imura, Y. Osaka, Jpn. J. Appl. Ž .
Phys. 25 1986 L564.
w x14 P. Rai-Choudhury, N.P. Formigoni, J. Electrochem. Soc. 116 Ž1969 1440..
w x15 D.S. Kim, Y.H. Lee, J. Electrochem. Soc. 142 1995 3493.Ž . w x16 C.C. Liu, C. Lee, K.L. Cheng, H.C. Cheng, T.R. Yew, Appl.
Ž . Phys. Lett. 66 1995 168.
w x17 M.D. Shieh, C. Lee, C.H. Cheng, T.R. Yew, C.Y. Kung, Appl. Ž .
Phys. Lett. 63 1993 1252.
w x18 J.H. Lee, S.K. Park, C.S. Kim, Jpn. J. Appl. Phys. 34 1995Ž . L1191.
w x19 M. Zhang, Y. Nakayama, S. Nonoyama, K. Wakita, J. Non-Cryst. Ž .
w x20 W.L. Hsu, J. Appl. Phys. 72 1992 3102.Ž .
w x21 J.W. Erwin, M.A. Ring, H.E. O’Neal, Int. J. Chem. Kinet. 17 Ž1985 1067..
w x22 Y. Catherine, G. Turban, B. Grolleau, Thin Solid Films 76 Ž1981 23..
w x23 A.D. Johnson, J. Perrin, J.A. Mucha, D.E. Ibbotson, Mater. Ž .
Res. Soc. Symp. Proc. 282 1993 451.
w x24 A.D. Johnson, J. Perrin, J.A. Mucha, D.E. Ibbotson, J. Phys. Ž .
Chem. 97 1993 12937.