THE EFFECT OF POTENTIAL GRADIENT ON ELECTRICAL ENHANCED
SLUDGE DEWATERING PROCESS: A SMALL PILOT SCALE STUDY
Ching Yuan
Chair and Associate Professor, Department of Civil and Environmental Engineering National University of Kaohsiung
Kaohsiung City, Taiwan
Chih-Huang Weng
Associate Professor, Department of Civil Engineering, I-Shou University Kaohsiung County, Taiwan
Key Words
: Electrical Kinetic Process, dewatering, potential gradient, sludgeABSTRACT
A sludge cake (73% of moisture content) after mechanically dewatering from a wastewater treatment plant was used to investigate the effects of potential gradient on the water removal by electrokinetic (EK) process. The potential gradient ranging from 2 to 5 V/cm were applied to induce the movement of bound water within the sludge specimen elapsed for 2 hr. Results showed that the direction of electroosmosis (EO) flow was from the anode to cathode. Due to the release of H+ and OH- through electrolysis of water, the sludge pH was maintained at 5.9 ~ 6.8 near the anode side and 9.8 ~ 10.8 near the cathode after EK treatment. As applied potential gradient of 2~5 V/cm to EK system for 2 hr, the moisture content of sludge decreased further to 47.5 %. The EO permeability and the power consumption throughout the test period are around 3.04 ~ 4.80 10-5 cm2/V-s and 14.4 ~ 66.8 kWh/m3, respectively. Up to 20.8 ~ 27.9% of total disposal cost saving was found with aid of EK technique. It is concluded that the water can be effectively and economically removed from sludge by EK process.
KEYWORDS: dewatering, electrokinetic process, potential gradient, sludge, sludge dewatering
INTRODUCTION
Sludge dewatering can be accomplished by freeze-thaw, centrifuge force, mechanical press and sun-drying. The freeze thaw method is capable of decreasing the water content of sludge to 50~60% (Knocke and Trahern, 1987; Lee and Lee, 1995) and even lower to 43% once added with polymer electrolytes (Huang et al., 1999) under low temperature condition. However, the consumption of energy and polymer electrolytes may become limiting factors under economical consideration. The technology by centrifuge force and mechanical press can’t remove the bound water efficiently. Use
of sunshine to remove water from sludge is an economical way but it needs a large space and sufficient time ( 7 days). In Taiwan, mechanical press and sunshine are the two common sludge dewatering methods being used in wastewater treatment plant. The water content of sludge can be decreased to 65 ~ 85% and 60 ~70% for the above-mentioned methods, respectively.
Electrokinetic (EK) process has been
demonstrated to be a cost-effective remediation technology to separate and extract heavy metals and organic contaminants from soils and sludges (Acar et
Table 1. The Physicochemical Properties of Tested Sludge
Characteristics Values Metals Values
Moisture content (%) 73.0 Cd (mg/kg) ND Organics (%) 50.1 Cr (mg/kg) ND Sludge pH 8.7 Cu (mg/kg) 68.4 pHzpc 4.6 Fe (mg/kg) 2227.6 Average particle Ni (mg/kg) 187.2 size(μm) 9.3 Pb (mg/kg) 6.2 Zn (mg/kg) 15.3
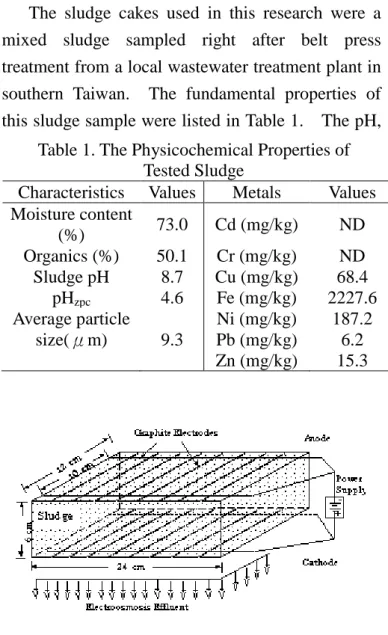
Figure 1. Schematic of Electrokinetic Apparatus It applies low voltage DC to the porous medium and the pollutants or water will be removed through the electroosmosis (EO) flow which is driven by an electrical field (Weng et al., 2000).
Hence, in this research, the EK process is used to
further improve the mechanical dewatering
efficiency. The present of this research was to investigate the effect of potential gradient on the sludge dewatering by EK process. Furthermore, the EK phenomenon during treatment was demonstrated.
MATERIALS AND METHODS
I. Sludge
The sludge cakes used in this research were a mixed sludge sampled right after belt press treatment from a local wastewater treatment plant in southern Taiwan. The fundamental properties of this sludge sample were listed in Table 1. The pH,
water content, organic content, and the metal content of the sludge were analyzed according to Taiwan EPA methods. The zeta potential of sludge particulates was determined using a zeta meter (Laser Zee 3.0, Pen Kem Inc., USA).
II. Electrokinetic Experiments
The EK experiments were performed with a small pilot scale apparatus (Figure 1) by 24 cm (L) 12 cm (W) 6cm (H) under electrical gradients of 2~5 V/cm for 2 hours. A filter cloth was inserted in between the cell wall and sludge specimen. A net of graphite rod electrodes (0.64 cm in diameter 10, Union Carbon Co., USA) was placed on the top and bottom layers of the EK cell, respectively. Then the anode and cathode of the DC power supply are connected to the top and bottom graphite layer, respectively. The detailed EK experimental conditions are listed in Table 2. The electric current, reservoir pH, and the amounts of effluent water were monitored during the tests. The sludge pH, residual water content profiles along the EK cell and the concentration of metals in the effluent were determined at the end of each test.
RESULTS AND DISCUSSIONS
I. Electroosmotic Permeability
The direction of EO flow was found as expected from the anode toward the cathode for all tests, i.e. the removed water was collected from the bottom layer of the EK apparatus. It is resulted from the surfaces of sludge particles are negatively charged during the test periods because the sludge pH profiles along the cell are all greater than pHzpc (4.6). The EO permeability, Ke (cm2/V-s), for a cylindrical sludge core of cross-section area of A (cm2) is calculated by Equation (1),
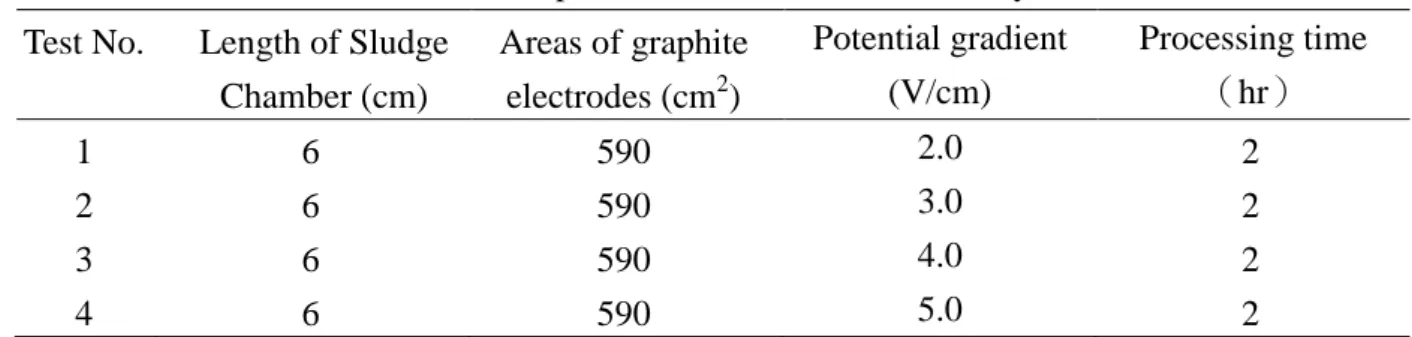
Table 2. The Experimental Conditions for EK Systems
Test No. Length of Sludge
Chamber (cm) Areas of graphite electrodes (cm2) Potential gradient (V/cm) Processing time (hr) 1 6 590 2.0 2 2 6 590 3.0 2 3 6 590 4.0 2 4 6 590 5.0 2 pH 2 4 6 8 10 12 14 N or malized D istance fr om Cathod e to A nod e 0.0 0.2 0.4 0.6 0.8 1.0 2 V/cm 3 V/cm 4 V/cm 5 V/cm Original pH 8.7 Anode Cathode Potential gradient where Qe (cm3/sec) is EO flow, ie (V/cm) is the
applied electric gradient. The results of EO flow collected efficiency in the cathode reservoir and Ke values for all tests were presented in Table 4. The obtained values of Ke (3.04 ~ 4.80 10
-5
cm2/V-s) are similar as those (5.2~ 8.2 10-5
cm2/V-s) in the researches of metal removal from sludge by EK process (Weng et al., 1999; Yuan and Weng, 2001a and 2001b).
II. Sludge pH Profiles
Water electrolysis reaction which H+ and OH- are continuously released at the anode and cathode, respectively (Equations 2 and 3) is considered as the predominant reaction under an electric field.
Anode: 2H2O → O2(g) + 4H+ + 4e- (2) Cathode: 2H2O + 2e- → H2(g) + 2 OH- (3)
During the EK process, the movement of H+ and OH- would change the sludge pH drastically. The sludge pH profile along the EK apparatus affected by the potential gradient is shown in Figure 2. A general trend of low pH at the anode and high pH at the cathode was found. The occurrence of these phenomena were attributed to the acid front generated at the anode reservoir flushed across the
sludge specimen and a great amount of OH
-produced at the cathode. The results show that higher potential gradient in the EK process would lower the sludge pH further near the anode side and increase the sludge pH further near the cathode side.
III. Metal Concentration in the Effluent
The results of metals collected in the cathode reservoir are listed in Table 3. Up to 0.084% of Cu, 0.050% of Fe and 0.438% of Zn were collected from the cathode effluent. It shows the amount of metals obtained in the effluent is of insignificant concern. However, the EK process might result in an accumulation of soluble organic matter (in terms of
Figure 2. Effect of Potential Gradient on Sludge pH Profile.
Table 3. The Amount and Percentage of Metals in the Effluent Cu (mg) Fe (mg) Zn (mg) 1 0.0321 1.1054 0.067 (0.047%) (0.050%) (0.438%) 2 0.0572 0.1827 0.0157 (0.084%) (0.008%) (0.103%) 3 0.0550 0.1519 0.0122 (0.080%) (0.007%) (0.080%) 4 0.0516 0.1664 0.0113 (0.075%) (0.007%) (0.074%)
Ps: The value in the parenthesis = (Metals in the effluent Total amount of metal)100.
TOC or COD) and even odor in the effluent. It needs to be further investigated and considered for effluent treatment if a rather high organic matter content of sludge, i.e. biosolid, is founded in the effluent.
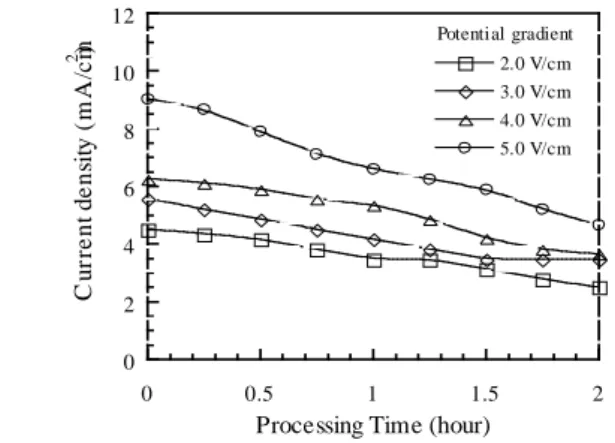
VI. Current Density
The effect of potential; gradient on current density is shown in Figure 3. The current density is decreased as processing time increased under potential gradient of 2~5 V/cm. It may resulted from the sludge pore clogging by metal hydroxide precipitates formed in the base environment near the cathode. The higher potential gradient would conquer electrical resistance so it resulted in higher current density.
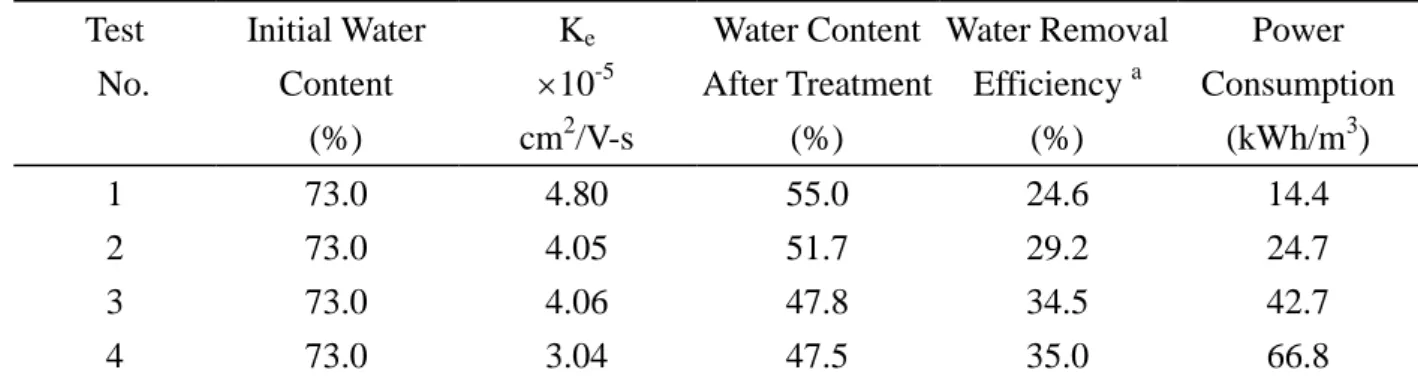
V. Water Removal Efficiency
The efficiency of water removal from sludge by EK process is summarized in Table 4 . As for the test of applied processing time of 2 hours, the water removal efficiency was 24.6%, 29.2%, 34.5% and 35.0% for the potential gradient of 2, 3, 4 and 5 V/cm, respectively. The results obviously indicated that EK process condition operated at higher potential gradient would enhance water removal from sludge as potential gradient less than 4 V/cm. However, the water removal efficiency increased insignificantly as potential gradient greater than 4 V/cm.
The residual water content profile along the sludge specimen was shown in Figures 4. In general, higher bound water removal efficiency was found at the anode side rather than that at the cathode side. The water contents at the anode were 48.9%, 45.8%, 42.4% and 38.6% as applied potential gradient of 2, 3, 4 and 5 V/cm, respectively, under an elapsed time of 2 hours. The residual water content of soil specimen gradually increased to the initial value of 73% as further near the cathode. It shows the water front was moved from anode to cathode
under electrical driving force and the water removal efficiency indeed increased accordingly under higher potential gradients. Although the highest total water removal efficiency for this research is only
0 2 4 6 8 10 12 0 0.5 1 1.5 2 2.0 V/cm 3.0 V/cm 4.0 V/cm 5.0 V/cm C u rr e n t d e n si ty ( m A /c m 2 )
Processing Time (hour)
Potential gradient
Figure 3. Effect of Potentia Gradient on Current Density
Residual Water Content (%)
30 40 50 60 70 80 N or mal ized D istan ce fr om Cath od e to A no de 0.0 0.2 0.4 0.6 0.8 1.0 2 V/cm 3 V/cm 4 V/cm 5 V/cm Initial Water Content 73% Anode Cathode Potential gradient
Figure 4. The Residual Water Content Profile Along the EK Cell
0 5 10 15 20 25 30 35 40 2 10-5 2.5 10-5 3 10-5 3.5 10-5 4 10-5 4.5 10-5 5 10-5 0 10 20 30 40 50 60 70 80 P o te n ti al g ra d ie nt ( V /c m ) E O p er m e a bi lity (c m 2 /V -s ) P ower consumption (kW h/m3)
Figure 5. Relationship of Power Consumption with Potential Gradient and EO Permeability,
35.0%, it reaches as low as water content of 38.5% for the sludge near the anode.
VI. Power Consumption
The energy consumption can be calculated based on the following equation:
Eu = P/W = (∫VI dt) / Vs (4)
where Eu = energy consumption per unit weight of
sludge (watt-hr/ton) ; P = energy expenditure (watt-hr);W = the weight of sludge (ton); V = the voltage (V); I = the current (A); t = prcessing time (hr). The energy consumption upon the termination of the experiments is in the range of 14.4 ~ 66.8 kWh/m3. A phenomenon was found for the case of constant processing time: the higher the potential gradient, the more the power consumption (Figure 5).
However, as applied higher potential gradient, it resulted in lower EO permeability (Figure 5) due to the clogging of metal oxides formed under the basic environment in the cathode.
The economic analysis for EK process is listed in Table 5. Approximate 21.1 ~ 27.9% of total cost saving for sludge dewatering by EK process is estimated. As potential gradient increased to 5.0 V/cm, it resulted in a lower cost saving due to higher power consumption.
CONCLUSIONS
The results of water removal from sludge by a small pilot scale EK process were concluded as follows:
1.The obtained values of Ke in this research (3.04 ~ 4.80 10-5 cm2/V-s) are similar as those in Table 4. The Experimental Results of Water Removal from Sludge by Electrokinetic Process
Test No. Initial Water Content (%) Ke 10-5 cm2/V-s Water Content After Treatment (%) Water Removal Efficiency a (%) Power Consumption (kWh/m3) 1 73.0 4.80 55.0 24.6 14.4 2 73.0 4.05 51.7 29.2 24.7 3 73.0 4.06 47.8 34.5 42.7 4 73.0 3.04 47.5 35.0 66.8
a: Water Removal Efficiency = [(Initial Water Content in the sludge Residual Water Content After Treatment) Initial Water Content in the Sludge] 100%
Table 5. The Economical Analysis of EK Process for Sludge Dewatering
Test No. Sludge Water Content (%) Disposal Costa (USD/ton) Energy Costb (USD/ton) Total Costc (USD/ton) Cost Saving With EKd (%) No EK With EK No EK With EK No EK With EK No EK With EK
1 73.0 55.5 84 66 0 0.5 84 66.5 20.8
2 73.0 51.7 84 62 0 1.0 84 63.0 25.0
3 73.0 47.8 84 59 0 1.6 84 60.6 27.9
4 73.0 47.4 84 59 0 2.5 84 61.5 26.8
Ps: a. The disposal fee includes the shipping fee.
b: The energy fee in Taiwan is estimated as 0.05USD/kWh.
c:Total Cost = (Disposal Cost + Energy Cost); The total cost excludes the capital cost of EK apparatus. d. Cost Saving With EK= (Total Cost without EK – Total Cost with EK) Total Cost without EK
previous researches of metal removal from sludge by EK process.
2.In this research, the sludge in the vicinity of anode was acidified, while the sludge near the cathode becomes more basic after EK treatment.
3.The results indicate that higher potential gradient in the EK systems would enhance the water removal from sludge. Under potential gradient of 2~5 V/cm, the moisture content of sludge can be lowered from 73.0% to 47.5~55.0% for 2 hr of processing time.
4.Approximate 20.8 ~ 27.9% of total cost saving for sludge dewatering by EK process in this research is estimated. As potential gradient increased to 5.0 V/cm, it resulted in a lower cost saving due to higher power consumption.
ACKNOWLEDGEMENTS
This research was supported in part by the National Science Council of Republic of China under Grant No. NSC 89-2211-E-214-001. The authors would like to thank Miss. Tzu-Shin Chiang and Miss Mei-Lan Wu foranalysis assistance.
REFERENCES
Acar, Y. B., and A. N. Alshawabkeh, 1996. Electrokinetic remediation. I: pilot-scale tests with lead-spiked kaolinite. J. of Geotec. and Geoenviron. Engrg., 122(3), 173 ~ 185.
Huang, C. P., R. S.Yuan, C. K. Peng, and M. Y. Chen, 1999. The effect of low temperature on the dewatering characteristics of sludge,” Proc. of The 24th Conf. of Wastewater Treatment Technol., 771 ~ 775 (in Chinese).
Knocke, W. R., and P. Trahern, 1987. Freeze-thaw conditioning of chemical and biological sludge. Wat. Res., 23, 35 ~ 42.
Lee, D. J. and S. F. Lee, 1995. Measurement of bound water in sludge : the use of differential scanning calorimetric (DSC). J. Chem. Tech. Biotechnol. 62, 359 ~ 364.
Weng, C. H., Y. H. Lin, and Y. H. Hsieh, 2000. Electrokinetic remediation of trichloroethylene contaminated kaolinite. J. of Chinese Institute of Environ. Engrg., 10(4), 279 ~ 289.
Weng, C. H., C. Yuan, W. C. Chen, H. P. Chuang, and R. C. Chen, 1999. The feasibility study of
metal removal from sludge by
surfactant-electrokinetic process. Proc. of The
14th Conf. of Waste Treatment Technol.,
5-9~5-15.
Yuan C., C. H. Weng, H. P. Chuang, and W. C. Chen, 2001. J. of Chinese Institute of Environ. Engrg., in press.
Yuan C. and C. H. Weng, 2001a “Electrokinetic removal of heavy metals from industrial sludge with various processing fluids,” Water Science
and Technology, in press.
Yuan, C and C. H. Weng, 2001b. “Sludge dewatering by electrokinetic technique: effect of processing time and potential gradient “, Advances in