Glutathione S-transferase M1*null genotype but
not myeloperoxidase promoter G – 463A polymorphism
is associated with higher susceptibility to endometriosis
Yao-Yuan Hsieh
1,4, Chi-Chen Chang
1, Fuu-Jen Tsai
2, Cheng-Chieh Lin
3, Jiun-Ming Chen
2and Chang-Hai Tsai
2,51
Department of Obstetrics and Gynecology,
2Department of Pediatrics and Medical Genetics,
3Department of Family Medicine,
China Medical University Hospital, Taichung, and
4Department of Biological Science and Technology, National Chiao Tung
University, Hsinchu, Taiwan
5
To whom correspondence should be addressed at: Department of Pediatrics and Medical Genetics, China Medical University
Hospital, No.2 Yuh-Der Road, Taichung, Taiwan. E-mail: d0704@www.cmuh.org.tw
Glutathione S-transferase M1 (GSTM1), one member of the GST family, is responsible for metabolism of xenobiotics and
carcinogens. Myeloperoxidase (MPO) plays an important role in the oxidation and activation of carcinogens and nitric oxide.
Allelic variants of GSTM1 and MPO gene polymorphisms might impair detoxification function and increase the susceptibility
to endometriosis. We aimed to investigate if these polymorphisms are useful markers for predicting endometriosis
suscepti-bility. Women were divided into two groups: (i) endometriosis (n 5 150); (ii) non-endometriosis (n 5 159). Polymorphisms for
GSTM1 and MPO were amplified by polymerase chain reaction and detected by electrophoresis after restriction digestion.
The relative frequencies of the GSTM1*wild (1 /1 , 1 /0)/null (0/0) genotypes and MPO – 463*G/A gene polymorphisms
between both groups were compared. The distribution of GSTM1 polymorphisms was significantly different between the two
groups. Proportions of GSTM1*wild/null alleles in both groups were: (i) 36.7/63.3%; (ii) 95/5% (P 5 0.001). In contrast,
MPO – 463 genotypes were not significantly different between the two groups. Proportions of MPO*A
homozygote/heterozy-gote/G homozygote in both groups were: (i) 2.7/17.4/79.9% and (ii) 1.9/17/81.1% (P > 0.05). We conclude that the GSTM1*null
genotype is associated with a higher risk of endometriosis development. MPO – 463*G/A gene polymorphism is not related to
the susceptibility of endometriosis.
Key words: endometriosis/gene polymorphism/glutathione S-transferase (GSTM1)/myeloperoxidase (MPO)
Introduction
Endometriosis, a common polygenic/multifactorial disease, might be
caused by an interaction between multiple genes as well as the
environment (Bischoff and Simpson, 2000). Endometriosis displays
features similar to malignancy, including local invasion and
aggres-sive spread to distant organs. Tumor suppressor genes play a role in
the regulation of cell growth and prevention of carcinogenesis. The
altered tumor suppressor genes might be related to the development
of endometriosis. Genetic alterations have been identified in
endo-metriotic lesions, which might contribute to their initiation and
pro-gression (Jiang et al., 1998). It is logical to suspect that somatic
genetic factors might contribute to the development of
endometrio-sis (Treloar et al., 1999).
The glutathione S-transferases (GSTs) are a family of enzymes
responsible for the metabolism of xenobiotics and carcinogens.
GSTM1, one member of the GST family, was formerly termed
GST1 or GST class ‘mu’ (Mannervik et al., 1992). GSTM1 is
criti-cal in the detoxification of the oxidative stress product during
ovu-lation (Baxter et al., 2001). Failure to detoxify these products may
result in rapid accumulation of genetic damage and increase
suscep-tibility to epithelial ovarian cancer (Baxter et al., 2001).
Endo-metriosis is characterized by cyclical degeneration and chronic
inflammation, which will result in the production of reactive oxygen
species and other toxins. Because of the detoxification properties of
the GST enzymes, it is logical to suspect the role of GSTM1-related
gene in endometriosis patients.
Many gene polymorphisms have been reported to be associated
with
endometriosis,
including
GSTM1
gene
polymorphism
(Baranova et al., 1997, 1999; Arvanitis et al., 2003). The GSTM1
gene is located on chromosome 1p13 (Zhong et al., 1992). GSTM1
gene deletions might influence an individual’s enzymatic function,
impair their detoxification system and further increase the risk when
exposed to carcinogens and toxic chemicals (Seidegard et al., 1990).
An elevated frequency of the inactive GSTM1 gene has been
reported in endometriosis patients (Baranova et al., 1997, 1999).
Baranova et al. (1999) observed a significant excess of the GSTM
null genotype among women with endometriosis.
Myeloperoxidase (MPO), a 150 kDa hemoprotein secreted by
acti-vated macrophages, is involved in many pathological processes
(Rutgers et al., 2003). MPO plays an important role in the oxidative
pathway of neutrophils and monocytes by producing hypochlorous
acid (HOCl). MPO functions not only antimicrobiotically, but also
acts as a metabolic enzyme with many other substrates, which
pro-duces some reactive intermediates and consumes hydrogen peroxide
(Schabath et al., 2000). Moreover, the MPO – HOCl system has been
shown to oxidize low-density lipoprotein (LDL) (Winterbourn et al.,
Advance Access publication August 6, 2004 doi:10.1093/molehr/gah095
at National Chiao Tung University Library on April 27, 2014
http://molehr.oxfordjournals.org/
2000), activate carcinogens (Schabath et al., 2000) and reduce nitric
oxide (NO) bioavailability (Eiserich et al., 1998). A functional
MPO promoter polymorphism, 2 463G/A, has been associated with
incidence or severity of inflammatory diseases, including
athero-sclerosis, Alzheimer’s disease, and some cancers (Kumar et al.,
2004). MPO – 463G/A polymorphism could modify the binding site
for the SP1 transcription factor and significantly decrease the
expression of MPO as well as the severity of leukemia (Piedrafita
et al., 1996).
It is generally accepted that heritable genetic factors might
con-tribute to the development of endometriosis. Unlike mutations,
poly-morphisms are not directly linked to a certain disease. However,
they are useful tools in the study of multifactorial disorders
(Anderson et al., 1994). In our previous surveys, we observed the
correlation of endometriosis and some gene polymorphisms,
includ-ing p53 (Chang et al., 2002) and androgen receptor (Hsieh et al.,
2001). Based on these surveys, we tried to assess the risk of
endo-metriosis associated with GSTM1 and MPO gene polymorphism.
We aimed to evaluate whether these polymorphisms are attractive
markers for endometriosis susceptibility. To our knowledge, this
report is the largest survey for GSTM1 polymorphisms in
endo-metriosis. Furthermore, it is the first report about the MPO
polymor-phism in endometriosis.
Materials and methods
Pre-menopausal Taiwanese women with surgically diagnosed endometriosis and non-endometriosis were included. All patients were divided into two groups: (i) endometriosis stage III/IV (n ¼ 150); (ii) non-endometriosis (n ¼ 159). All individuals with endometriosis accepted laparoscopy or lapar-otomy management and were confirmed pathologically. All patients had normal blood pressure without obvious cardiovascular diseases. There were non-significant differences between both groups in age, weight and height. All women had consented to peripheral blood sampling for genotype ana-lyses. The studies were approved by the ethical committee and institutional review board of the China Medical University Hospital. Informed consents were signed by all women who donated their blood.
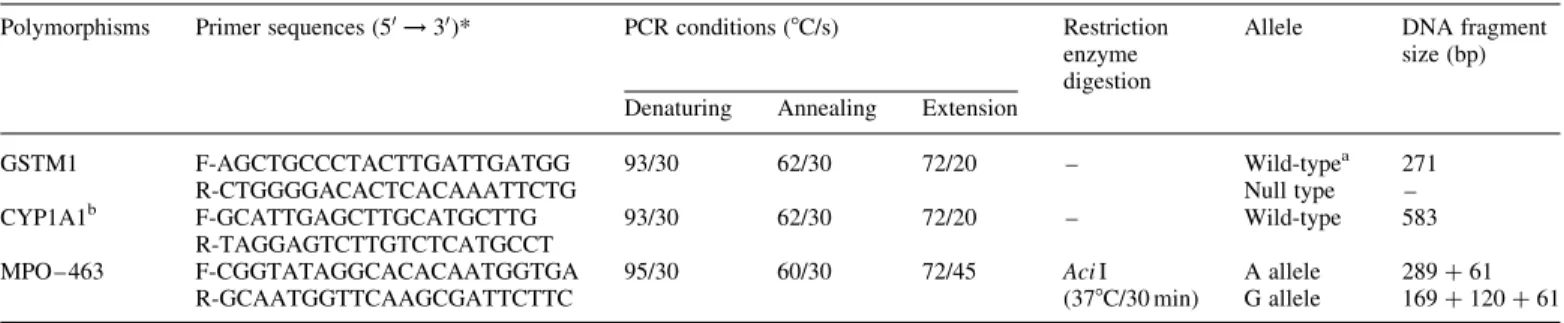
The GSTM1*wild/null and MPO – 463*G/A gene polymorphisms were determined according to previously described methods (Baxter et al., 2001; Table I. The primer sequences and PCR conditions for GSTM1 and MPO – 463 G/A gene polymorphisms
Polymorphisms Primer sequences (50! 30)* PCR conditions (8C/s) Restriction
enzyme digestion
Allele DNA fragment size (bp) Denaturing Annealing Extension
GSTM1 F-AGCTGCCCTACTTGATTGATGG R-CTGGGGACACTCACAAATTCTG 93/30 62/30 72/20 – Wild-typea Null type 271 – CYP1A1b F-GCATTGAGCTTGCATGCTTG R-TAGGAGTCTTGTCTCATGCCT 93/30 62/30 72/20 – Wild-type 583 MPO – 463 F-CGGTATAGGCACACAATGGTGA R-GCAATGGTTCAAGCGATTCTTC 95/30 60/30 72/45 Aci I (378C/30 min) A allele G allele 289 þ 61 169 þ 120 þ 61 * F and R indicate forward and reverse primers.
aHomozygotes or heterozygotes could not be specified. b
Concomitant PCR with GSTM1 gene for internal controls.
Figure 1. Genotyping of (A) GSTM1*wild/null and (B) MPO– 463*G/A gene polymorphisms (M: Marker).
at National Chiao Tung University Library on April 27, 2014
http://molehr.oxfordjournals.org/
Reynolds et al., 2002). The genomic DNA was prepared from peripheral blood leukocytes by use of a genomic DNA isolation kit (Blossom, Taipei, Taiwan). A total of 50 ng genomic DNA was mixed with 20 pmol of each polymerase chain reaction (PCR) primer in a total volume of 25 ml con-taining 10 mM Tris – HCL pH 8.3, 50 mM potassium chloride, 2.0 mM magnesium chloride, 0.2 mM each deoxyribonucleotide triphosphate and 1 U DNA polymerase (Amplitag; Perkin-Elmer, Foster City, CA). A total of two gene polymorphisms were surveyed, including GSTM1*wild/null and MPO – 463*G/A. The SNP information for the genes involved was obtained via the NCBI website (http://www.ncbi.nlm.nih.gov/LocusLink/).
The PCR primer sequences and condition of each primer are listed in Table I. To confirm the successful amplification, an internal control was included in the PCR reaction of GSTM1. It consisted of a 583 bp amplicon from the CYP1A1 gene (Nicholl et al., 1999). The PCR amplification was performed in a programmable thermal cycler GenAmp PCR system 2400 (Perkin Elmer Applied Biosystems, Foster City, CA). After PCR amplifica-tion, the individual gene polymorphisms were analyzed after restriction digestion (New England Biolabs Inc., Beverly, MA). The base pairs for their wild-type, null type and SNP type are listed in Table I.
The PCR products were mixed together and 10 ml of this solution was loaded into 3% agarose gel containing ethidium bromide for electrophoresis. Each allele was recognized according to its size (Figure 1). Genotypes and allelic frequencies for GSTM1 and MPO gene polymorphisms in both groups were compared. Correlation of the GSTM1 and MPO genotype and endo-metriosis was evaluated. Allelic frequencies are expressed as a percentage of the total number of alleles. The SAS system with x2and Fisher’s exact tests were utilized for statistical analyses. A P-value of , 0.05 was considered statistically significant.
Results
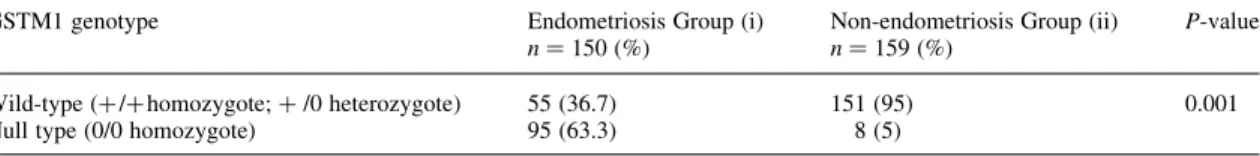
Proportions of the GSTM1 genotype in both groups were
signifi-cantly different. The GSTM1 null genotype frequency was strikingly
high among the individuals with endometriosis. Most normal
indi-viduals appear to have the wild genotype of GSTM1 (Table II).
Pro-portions of the wild/null alleles of the GSTM1 gene in the two
groups were: group (i) 36.7/63.3% and group (ii) 95/5%,
respect-ively (Table II, P ¼ 0.001). Null type (0/0 homozygote) of GSTM1
gene is associated with higher susceptibility of endometriosis.
Wild-type (þ / þ homozygote or þ /2 heterozygote) of GSTM1 gene is
associated with lower risk of endometriosis development.
In contrast, genotype proportions of different MPO polymorphisms
in both groups were not significantly different (Table III). Most
individuals in both groups appear to have the G-related genotype and
G allele. Proportions of MPO*A homozygote/heterozygote/G
homo-zygote in the two groups were: group (i) 2.7/17.4/79.9% and group
(ii) 1.9/17/81.1%, respectively (Table III). Furthermore, A and G
allele frequencies in the two groups were: (i) 11.4/88.6% and
(ii) 10.4/89.6%, respectively (Table III).
Discussion
Numerous chronic disorders, such as endometriosis, osteoporosis,
hypertension, diabetes and asthma, have been attributed to genetic
susceptibility. Endometriosis, a multifactorial disease, involves
complex interactions between hormones and cytokines activation,
immunoinflammatory processes and genetic factors (Vigano et al.,
1998). Recent experimental studies indicated that dioxin may be
involved in the pathogenesis of endometriosis (Gibbons, 1993).
Dioxin is widely present in the environment; most people absorb
traces of dioxin by exposure to pesticides in their diet. These toxins
might also contribute to an imbalance of sex hormones or alter
growth factors and the immune response (Mayani et al., 1997).
GSTM1 functions both as a detoxification enzyme and an
intra-cellular drug- and hormone-binding protein (Chasseaud, 1979).
GSTM1 catalyzes the detoxification of genotoxic chemicals,
includ-ing the products of chronic oxidative stress such as cytotoxic lipid
and DNA species (Hayes et al., 1995). GSTM1 enhances the
conju-gation of glutathione with several alkylating agents (Dulik et al.,
1990). The GSTM1 gene is specific for certain carcinogens,
includ-ing trans-stilbene oxide and a metabolite of benzopyrene contained
in smoke fumes (Seidegard et al., 1990). The GSTM1 gene might
influence the related isoenzyme expression as well as the host
sus-ceptibility to lung cancer among smokers (Seidegard et al., 1990).
Impaired GSTM1 function might result in increased risk to DNA
damage and malignant transformation. The null condition of
GSTM1 gene (0/0 genotype) represented an expanded deletion
(, 10 kb) of the gene, which might impair the further production of
mRNA and protein (Seidegard et al., 1988).
GSTM1 gene deletion (0/0 genotype) is a useful marker for the
early detection of many diseases, including endometriosis, ovarian
cancer (Baxter et al., 2001), cystic fibrosis (Baranov et al., 1996),
bladder (Brockmoller et al., 1984), lung (Nakachi et al., 1993)
and stomach cancers (Harada et al., 1992). Baranova et al. (1999)
reported a highly significant excess of the GSTM1 null genotype in
women with endometriosis versus controls (76.9 versus 45.8%).
Recently, Arvanitis et al. (2003) also demonstrated that the GSTM1
null deletion adds to this risk of endometriosis. In contrast, Baxter
et al. (2001) demonstrated that the GSTM1 null allele is not an
endometriosis susceptibility allele; however, it may predispose
endometriotic lesions to malignant transformation to endometrioid
and clear cell ovarian cancer. Some investigators demonstrated the
non-association between the individual diseases with GSTM1,
including cancers of the ovary (Cehisselbauer et al., 1992), bladder
(Brockmoller et al., 1994), lung (Brockmoller et al., 1993) and
stomach (Harada et al., 1992). These discrepancies might be due to
different illness classification, racial and disease variation.
Table II. Distributions of GSTM1 genotypes in women with and without endometriosis GSTM1 genotype Endometriosis Group (i)
n ¼ 150 (%)
Non-endometriosis Group (ii) n ¼ 159 (%)
P-value
Wild-type (þ /þ homozygote; þ /0 heterozygote) 55 (36.7) 151 (95) 0.001 Null type (0/0 homozygote) 95 (63.3) 8 (5)
Table III. Genotypes and allelic frequencies for MPO*–463 gene polymorphism in women with and without endometriosis Genotype Endometriosis Group (i) n ¼ 150 (%) Non-endometriosis Group (ii) n ¼ 159 (%) P-value* A/A 4 (2.7) 3 (1.9) NS A/G 26 (17.4) 27 (17) G/G 119 (79.9) 129 (81.1) Allele frequencies A 34 (11.4) 33 (10.4) NS G 264 (88.6) 285 (89.6) NS, not significant.
*P-value was calculated by x2tests.
at National Chiao Tung University Library on April 27, 2014
http://molehr.oxfordjournals.org/
In this study, we observed that the genotype distribution for
GSTM1 gene polymorphism was significantly different between the
individuals with and without endometriosis. The null genotype is
related with higher susceptibility of endometriosis; whereas the
wild-type is related with lower risk of endometriosis development.
Our data strongly suggest that the lack of GSTM1 gene products
might substantially contribute to the pathogenesis of endometriosis.
However, our finding only suggested their connection as well as
possibility. The related scientific proof for the underlying
mecha-nisms is still warranted.
In this survey, the GSTM1 null genotype frequency (63.3%) in
individuals with endometriosis was significantly lower than that
observed by Baranova et al. (1997, 1999; 75 – 86%) and higher than
that observed by Arvanitis et al. (2003; 58.5%). The discrepancies
between Baranova et al. (1997, 1999) and Arvanitis et al. (2003)
suggested racial differences within the French and Greek
popu-lations, even though they are both Caucasian populations. However,
the conclusions of Baranova et al. (1997, 1999) were based on
fewer case numbers (, 100). Our study of 150 endometriosis cases
and 159 controls provides the stronger support for the claim that the
GSTM1 null allele is a predisposing factor for endometriosis.
Fur-thermore, our finding is the first indication that GSTM1 gene
deficiency predisposes to endometriosis in an Asian population.
However, we also observed a different distribution of null
GSTM1 genotype between normal Asians and Caucasians (Board,
1990; Groppi et al., 1991; Harada et al., 1992). The fluctuation of
GSTM1 deficiency found in most Caucasians is in the range of
40 – 52% (Baranov et al., 1996; Arvanitis et al., 2003). In contrast,
in our study we found the null genotype in 5% of the normal
con-trols. The discrepancy might be mainly due to the ethnic differences
between Asians and Caucasians. Furthermore, whether the GSTM1
0/0 genotype in different populations results from identical deletion
or from other alterations of the GSTM1 gene remains unknown.
MPO is a 150 kDa hemoprotein stored exclusively in the
azurophi-lic granules of monocytes and neutrophils (PMNs). MPO produces
not only the strong oxidant bleach (hypochlorous acid) from
hydro-gen peroxide and chloride ions but also oxidizes LDL into a
macrophage high-uptake form, inactivates protease inhibitors, and
consumes nitric oxide (Rutgers et al., 2003). These may contribute to
endothelial dysfunction and damage (Rutgers et al., 2003).
Addition-ally, MPO produces a strong oxidant and procarcinogens such as
ben-zo(a)pyrene and aromatic amine intermediates (Hazen et al., 1996).
MPO may act as a cocarcinogen by generating free radicals and
acti-vating aromatic amines and carcinogens (Josephy, 1996).
The combination of genetic factors involved in oxidative stress
response with environmental carcinogens may play an important
role in carcinogenesis (Hung et al., 2004). MPO* – 463G/A gene
polymorphism may be related to numerous diseases, including lung
cancer (Chevrier et al., 2003), atherosclerosis (Makela et al., 2003),
coronary artery disease (Makela et al., 2004), bladder carcinogenesis
(Hung et al., 2004), sarcoidosis (Rothkrantz-Kos et al., 2003),
Alzheimer’s disease (Leininger-Muller et al., 2003), hepatoblastoma
(Pakakasama et al., 2003), among others. MPO – 463**A
geno-type/allele are related to lower MPO expression (Chevrier et al.,
2003) as well as reduced risk of individual cancers (London et al.,
1993). The ‘A’ allele is a protective factor with regard to the risk of
hepatoblastoma (Pakakasama et al., 2003). Furthermore, the MPO
expression might be regulated by an estrogen-dependent mechanism
involving the 2 463G/A promoter polymorphism (Kumar et al.,
2004). The effects of hormone replacement therapy (HRT) on
ather-osclerosis progression in subjects with the GG genotype seem to be
especially beneficial compared with controls with the same genotype
but without HRT (Makela et al., 2003).
However,
some
investigators
indicated
the
non-association
between MPO – 463G/A polymorphism with individual diseases,
including lung cancer (Dally et al., 2003), vasculitis (Fiebeler et al.,
2004), sarcoidosis (Rothkrantz-Kos et al., 2003), and others. The
functional MPO promoter polymorphism is not related to the disease
severity in the sarcoidosis population (Rothkrantz-Kos et al., 2003).
In this study, we observed the non-association between the MPO
promoter 2 463 gene polymorphisms and the susceptibility to
endo-metriosis. We also observed the frequency of the A allele (10 – 11%)
in our population was significantly lower than the reported
frequen-cies in Caucasian populations [23.4% (London et al., 1997), 25.7%
(Le Marchand et al., 2000), 21.2% (Cascorbi et al., 2000), 29.8%
(Schabath et al., 2000) and 30.6% (Misra et al., 2001)]. These
dis-crepancies might be due to racial variation.
In conclusion, an association between endometriosis and GSTM1
gene polymorphism exists. The GSTM1 null genotype is related to
an increased susceptibility to endometriosis. The GSTM1 gene
poly-morphism likely contributes to the pathogenesis of endometriosis. It
also suggests the defects in carcinogen detoxification may be
involved in the pathogenesis of endometriosis. In contrast, the
MPO* – 463G/A gene polymorphisms are not related to the
suscepti-bility of endometriosis. Although the real roles of GSTM1 and
MPO gene polymorphism have not yet been clarified, these
poly-morphisms deserve more attention to realize their roles upon
endo-metriosis development. This study could be extended to investigate
whether and how the detoxification affects the endometriosis
for-mation. Furthermore, after the clarification of these issues, GSTM1
gene polymorphism may become a useful marker to predict the
future development of endometriosis and to permit early therapeutic
intervention in women at high risk for endometriosis.
References
Anderson TI, Heimdal KR, Skrede M, Tveit K, Berg K and Borresen AL (1994) Oestrogen receptore (ESR) polymorphisms and breast cancer susceptibility. Hum Genet 94,665– 670.
Arvanitis DA, Koumantakis GE, Goumenou AG, Matalliotakis IM, Koumantakis EE and Spandidos DA (2003) CYP1A1, CYP19, and GSTM1 polymorphisms increase the risk of endometriosis. Fertil Steril 79,702– 709.
Baranov VS, Ivaschenko T, Bakay B et al. (1996) Proportion of the GSTM1 0/0 genotype in some Slavic populations and its correlation with cystic fibrosis and some multifactorial diseases. Hum Genet 97,516 – 520. Baranova H, Bothorishvilli R, Canis M, Albuisson E, Perriot S,
Glowaczower E, Bruhat MA, Baranov V and Malet P (1997) Glutathione S-transferase M1 gene polymorphism and susceptibility to endometriosis in a French population. Mol Hum Reprod 3,775 – 780.
Baranova H, Canis M, Ivaschenko T, Albuisson E, Bothorishvilli R, Baranov V, Malet P and Bruhat MA (1999) Possible involvement of arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1 genes in the development of endometriosis. Mol Hum Reprod 5,636 – 641.
Baxter SW, Thomas EJ and Campbell IG (2001) GSTM1 null polymorphism and susceptibility to endometriosis and ovarian cancer. Carcinogenesis 22, 63 – 65.
Bischoff FZ and Simpson JL (2000) Heritability and molecular genetic studies of endometriosis. Hum Reprod Update 6,37 – 44.
Board PG (1990) Biochemical genetics of glutathione S-transferase in man. Am J Hum genet 33,36 – 43.
Brockmoller J, Kerb R, Drakoulis N, Niz M and Webb G (1993) genotype and phenotype of glutathione S-transferase class mu and kappa in lung cancer patients and controls. Cancer Res 53,1004 – 1009.
Brockmoller J, Kerb R, Drakoulis N, Staffeldt B and Roots I (1994) Glutathione S-transferase M1 and its variants A and B as host factors of bladder cancer susceptibility, a case-control study. Cancer Res 54, 4103 – 4111.
Cascorbi I, Henning S, Brockmoller J, Gephart J, Meisel C, Muller JM, Loddenkemper R and Roots I (2000) Substantially reduced risk of cancer
at National Chiao Tung University Library on April 27, 2014
http://molehr.oxfordjournals.org/
of the aerodigestive tract in subjects with variant 2 463A of the myeloper-oxidase gene. Cancer Res 60,644 – 649.
Cehisselbauer JC, Hogan WM, Buctow KH and Twe KD (1992) Hetero-geneity of glutathione S-transferase enzyme and gene expression in ovar-ian carcinoma. Pharmacogenetics 2,63 – 72.
Chang CC, Hsieh YY, Tsai FJ, Tsai CH, Tsai HD and Lin CC (2002) The proline form of p53 codon 72 polymorphism is associated with endo-metriosis. Fertil Steril 77,43 – 45.
Chasseaud LF (1979) The role of glutathione S-transferase in the metabolism of chemical carcinogenes and other electrophilic agents. Adv Cancer Res 29,175 – 274.
Chevrier I, Stucker I, Houllier AM, Cenee S, Beaune P, Laurent-Puig P and Loriot MA (2003) Myeloperoxidase, new polymorphisms and relation with lung cancer risk. Pharmacogenetics 13,729– 739.
Dally H, Bartsch H and Risch A (2003) The myeloperoxidase ( – 463)G ! A polymorphism does not decrease lung cancer susceptibility in Caucasians. Cancer Epidemiol Biomarkers Prev 12,683.
Dulik DM, Colvin OM and Fenselau C (1990) Characterization of gluta-thione conjugates of chlorambucil by fast atom bombardment and thermo-spray liquid chromatography/mass spectrometry. Biomed Environ Mass Spectrom 19,248 – 252.
Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B and van der Vliet A (1998) Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391, 393 – 397.
Fiebeler A, Borgmann S, Woywodt A, Haller H and Haubitz M (2004) No association of G-463A myeloperoxidase gene polymorphism with MPO-ANCA-associated vasculitis. Nephrol Dial Transplant 19,969– 971. Gibbons A (1993) Dioxin tied to endometriosis. Science 362,1373.
Groppi A, Coutelle CH, Fleury B, Iron A, Begueret J and Couzigou P (1991) Glutathione S-transferase class mu in French alcoholic cirrhotic patients. Hum Genet 87,628 – 630.
Harada S, Misawa S, Nakamura T, Tanaka N, Ueno E and Nozoe M (1992) Detection of GST1 gene deletion by the polymerase chain reaction and its possible correlation with stomach cancer in Japanese. Hum Genet 90, 62 – 64.
Hayes JD and Strange RC (1995) Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res 22,193– 207.
Hazen SL, Hsu FF, Duffin K and Heinecke JW (1996) Molecular chlorine generated by the myeloperoxidase hydrogen peroxide chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem 271,23080– 23088.
Hsieh YY, Chang CC, Tsai FJ, Wu JY, Tsai CC and Tsai HD (2001) Andro-gen receptor trinucleotide polymorphism in endometriosis. Fertil Steril 76,412 – 413.
Hung RJ, Boffetta P, Brennan P, Malaveille C, Gelatti U, Placidi D, Carta A, Hautefeuille A and Porru S (2004) Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis.
Jiang X, Morland SJ, Hitchocock A, Thomas EJ and Campbell IG (1998) Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res 58,1707– 1712.
Josephy PD (1996) The role of peroxidase-catalyzed activation of aromatic amines in breast cancer. Mutagenesis 11,3 – 7.
Kumar AP, Piedrafita FJ and Reynolds WF (2004) Peroxisome proliferator-activated receptor gamma ligands regulate myeloperoxidase expression in macrophages by an estrogen-dependent mechanism involving the 2 463GA promoter polymorphism. J Biol Chem 279,8300– 8315. Le Marchand L, Seifried A, Lum A and Wilkens LR (2000) Association of
the myeloperoxidase – 463G ! A polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev 9,181 – 184.
Leininger-Muller B, Hoy A, Herbeth B, Pfister M, Serot JM, Stavljenic-Rukavina M, Massana L, Passmore P, Siest G and Visvikis S (2003) Myeloperoxidase G – 463A polymorphism and Alzheimer’s disease in the ApoEurope study. Neurosci Lett 349,95 – 98.
London SJ, Lehman TA and Taylor JA (1997) Myeloperoxidase genetic polymorphism and lung cancer risk. Cancer Res 57,5001– 5003.
Makela R, Dastidar P, Jokela H, Saarela M, Punnonen R and Lehtimaki T (2003) Effect of long-term hormone replacement therapy on
atherosclero-sis progression in postmenopausal women relates to myeloperoxidase pro-moter polymorphism. J Clin Endocrinol Metab 88,3823– 3828.
Makela R, Laaksonen R, Janatuinen T, Vesalainen R, Nuutila P, Jaakkola O, Knuuti J and Lehtimaki T (2004) Myeloperoxidase gene variation and coronary flow reserve in young healthy men. J Biomed Sci 11,59 – 64. Mannervik B, Awasthi YC, Board PG, Hayes JD, Di Ilio C, Ketterer B,
Listowsky I, Morgenstern R, Muramatsu M, Pearson WR et al. (1992) Nomenclature for human glutathione transferases. Biochem J 282,305 – 306. Mayani A, Barel S, Soback S and Almagor M (1997) Dioxin concentrations
in women with endometriosis. Hum Reprod 12,373– 375.
Misra RR, Tangrea JA, Virtamo J, Ratnasinghe D, Andersen MR, Barrett M, Taylor PR and Albanes D (2001) Variation in the promoter region of the myeloperoxidase gene is not directly related to lung cancer risk among male smokers in Finland. Cancer Lett 164,161 – 167.
Nakachi K, Imai K, Hayashi S and Kwasjiri K (1993) Polymorphism of the CYP1A1 and glutathione S-transferase genes associated with susceptibility to lung cancer in relation to cigarette dose in a Japanese population. Cancer Res 53,2994 – 2999.
Nicholl DJ, Bennett P, Hiller L, Bonifati V, Vanacore N, Fabbrini G, Marconi R, Colosimo C, Lamberti P, Stocchi F et al. (1999) A study of five candidate genes in Parkinson’s disease and related neurodegenerative disorders. European Study Group on Atypical Parkinsonism. Neurology 53,1415 – 1421.
Pakakasama S, Chen TT, Frawley W, Muller C, Douglass EC and Tomlinson GE (2003) Myeloperoxidase promotor polymorphism and risk of hepatoblastoma. Int J Cancer 106,205 – 207.
Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M and Reynolds WF (1996) An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem 271,14412– 14420.
Reynolds WF, Stegeman CA and Tervaert JW (2002) – 463 G/A myeloper-oxidase promoter polymorphism is associated with clinical manifestations and the course of disease in MPO-ANCA-associated vasculitis. Clin Immunol 103,154– 160.
Rothkrantz-Kos S, Drent M, Rutgers A, Heeringa P, De Vries J, van Dieijen-Visser MP and Cohen Tervaert JW (2003) Relationship between myelo-peroxidase promotor polymorphism and disease severity in sarcoidosis. Eur J Intern Med 14,296– 301.
Rutgers A, Heeringa P, Giesen JE, Theunissen RT, Jacobs H and Tervaert JW (2003) Neutrophil myeloperoxidase activity and the influence of two single-nucleotide promoter polymorphisms. Br J Haematol 123,536 – 538. Rutgers A, Heeringa P and Tervaert JW (2003) The role of myeloperoxidase
in the pathogenesis of systemic vasculitis. Clin Exp Rheumatol 21, S55 – S63.
Schabath MB, Spitz MR, Zhang X, Delclos GL and Wu X (2000) Genetic variants of myeloperoxidase and lung cancer risk. Carcinogenesis 21, 1163 – 1166.
Seidegard J, Pero RW, Markowits MM, Roush G, Miller DG and Beatti EJ (1990) Isozyme of glutathione transferase (class Mu) as a marker for the susceptibility to lung cancer, a follow up study. Carcinogenesis 11,33 – 36. Seidegard J, Vorachek WR, Pero RW and Pearson WR (1988) Hereditary
differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci USA 85,7293 – 7297.
Treloar SA, O’Connor DT, O’Connor VM and Martin NG (1999) Genetic influences on endometriosis in an Australian twin sample. Fertil Steril 71, 701 – 710.
Vigano P, Gaffuri B, Somigliana E, Busacca M, Di Blasio AM and Vignali M (1998) Expression of intercellular adhesion molecule (ICAM)-1 mRNA and protein is enhanced in endometriosis versus endometrial stromal cells in culture. Mol Hum Reprod 4,1150 – 1156.
Winterbourn CC, Vissers MC and Kettle AJ (2000) Myeloperoxidase. Current Opinion in Hematology 7,53 – 58.
Zhong S, Wolf CR and Spurr NK (1992) Chromosomal assignment and link-age analysis of the human glutathione S-transferase mu gene (GSTM1) using intron specific polymerase chain reaciton. Hum Genet 90,435 – 439.
Submitted on March 27, 2004; revised on June 2, 2004; accepted on July 11, 2004
at National Chiao Tung University Library on April 27, 2014
http://molehr.oxfordjournals.org/