Manuscript Number:
Title: Intrathecal propranolol displays long-acting spinal anesthesia with a more sensory-selective action over motor blockade in rats
Article Type: Research Paper
Section/Category: Neuropharmacology and analgesia Keywords: propranolol; spinal anesthesia
Corresponding Author: Mrs. Ching-Hsia Hung, Ph.D.
Corresponding Author's Institution: National Cheng Kung University First Author: Yu-Wen Chen, Ph.D.
Order of Authors: Yu-Wen Chen, Ph.D.; Chin-Chen Chu, M.D., Ph.D.; Yu-Chung Chen, M.S.; Ching-Hsia Hung, Ph.D.; Jhi-Joung Wang, M.D., Ph.D.
Abstract: To prevent cardiovascular effects of peripherally administered propranolol, the aim of this study was to evaluate the spinal anesthetic effect of intrathecal propranolol, a sodium channel blocker. After rats were injected 5 doses of propranolol intrathecally, the dose—response curve of spinal anesthesia was obtained. Then the spinal block potency and duration of propranolol was compared to lidocaine, which is known to produce local anesthesia. We found that propranolol produced a dose-dependent spinal blockade in motor, proprioception, and nociception. On a 50% effective dose (ED50) basis, the spinal anesthetic effect of propranolol was equal to lidocaine. On an equipotent basis (0.5, 1.0, and 2.5 μmol), the block duration on spinal anesthesia caused by propranolol was longer than that caused by lidocaine (p < 0.01 for each comparison). These preclinical findings reported that
propranolol produced similar spinal anesthesia to lidocaine. Propranolol with a more sensory-selective action over motor blockade produced longer spinal blockade than did lidocaine.
1
Intrathecal propranolol displays long-acting spinal anesthesia
with a more sensory-selective action over motor blockade in rats
Yu-Wen Chen, Ph.D.a,b, Chin-Chen Chu, M.D., Ph.D.c, Yu-Chung Chen, M.S.d,
Ching-Hsia Hung, Ph.D.e,*, Jhi-Joung Wang, M.D., Ph.D.c
a
Department of Physical Therapy, China Medical University, Taichung, Taiwan b
Graduate Institute of Neural and Cognitive Sciences, China Medical University, Taichung, Taiwan
c
Department of Medical Research, Chi-Mei Medical Center, Tainan, Taiwan d
Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan
e
Institute & Department of Physical Therapy, National Cheng Kung University, Tainan, Taiwan
Conflicts of interest: There is no conflict of interests for all authors.
*Address correspondence and reprint requests to: Ching-Hsia Hung, PhD, Department of Physical Therapy, National Cheng Kung University, No.1 Ta-Hsueh Road, Tainan, Taiwan
Tel: 886-6-2353535 ext 5939 Fax: 886-6-2370411
ABSTRACT
To prevent cardiovascular effects of peripherally administered propranolol, the aim of
this study was to evaluate the spinal anesthetic effect of intrathecal propranolol, a
sodium channel blocker. After rats were injected 5 doses of propranolol intrathecally,
the dose—response curve of spinal anesthesia was obtained. Then the spinal block
potency and duration of propranolol was compared to lidocaine, which is known to
produce local anesthesia. We found that propranolol produced a dose-dependent
spinal blockade in motor, proprioception, and nociception. On a 50% effective dose
(ED50) basis, the spinal anesthetic effect of propranolol was equal to lidocaine. On an
equipotent basis (0.5, 1.0, and 2.5 μmol), the block duration on spinal anesthesia
caused by propranolol was longer than that caused by lidocaine (p < 0.01 for each
comparison). These preclinical findings reported that propranolol produced similar
spinal anesthesia to lidocaine. Propranolol with a more sensory-selective action over
motor blockade produced longer spinal blockade than did lidocaine.
1. Introduction
Propranolol is discovered in 1964 and its introducing to the clinical practice has
been essential for the clinical usefulness in the therapy of cardiovascular diseases
(Frullani et al., 1970; Matthews and Baker, 1982). Propranolol was the first clinically
useful β-adrenergic receptor antagonist which was introduced by Sir James W. Black.
It revolutionized the medical management of angina pectoris and is considered to be
one of the most important contributions to clinical medicine and pharmacology in the
20th century (Zimmermann et al., 2010). Indications for the use of propranolol are
numerous, including the treatment of angina pectoris (Frishman et al., 1989;
Zimmermann et al., 2010), hypertension (Frishman et al., 1989), cardiac arrhythmias
(Matthews and Baker, 1982), hyperthrophic obstructive cardiomyopathy (Hess et al.,
1983), migraine (Linde and Rossnagel, 2004), and in the therapy of many
neuropsychiatric disorders (Tchivileva et al., 2010).
Recently, propranolol has been introduced as a novel modality for the treatment of
proliferating haemangiomas (Buckmiller, 2009; Maturo and Hartnick, 2010;
Zimmermann et al., 2010) and dental anxiety (Heaton et al., 2010). The response of
infantile haemangiomas to propranolol reported in the New England Journal of
Medicine by Léauté-Labréze et al. (Leaute-Labreze et al., 2008) catapulted the use of
al., 2008). In in vitro study, propranolol (100 µM) performed 78% inhibition of
veratridine-stimulated Na+ influx in a concentration-dependent manner in rat
cerebrocortical synaptosomes.(Chidlow et al., 2000) It is accepted that local
anesthetics reversibly block the conduction of electrical impulses in nerves by
blocking voltage-gated Na+ channels (Scholz, 2002; Sheets and Hanck, 2003).
Because propranolol can inhibit Na+ currents (Chidlow et al., 2000), it therefore has
been known to have a local anesthetic (termed topical) effect (Frullani et al., 1970;
Leszczynska and Kau, 1992; Zimmermann et al., 2010).
Spinal anesthesia is a relatively simple technique, which produces adequate
surgical conditions by injecting a small amount of local anesthetic with easy
landmarks, giving a wide popularity to this practice (Hung et al., 2009). However, to
the best of our knowledge, no study of spinal anesthesia of propranolol following
intrathecal puncture has been reported. The aim of this study was to evaluate the
spinal anesthetic effect of propranolol but also duration of action of drugs. Lidocaine,
a common local anesthetic, was used as control. Our results demonstrate that
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats weighting 300-350 g were obtained from the National
Laboratory Animal Centre in Taiwan, and then housed in a climate controlled room
maintained at 22℃ with approximately 50% relative humidity. Lighting was on a
12-h light/dark cycle (light on at 6:00 AM), with food and water available ad libitum
up to time of testing. The experimental protocols were approved by the animal
investigation committee of China Medical University, Taiwan, and conformed to the
recommendations and policies of the International Association for the Study of Pain
(IASP).
2.2. Drugs
(±)-Propranolol HCl and lidocaine HCl were purchased from Sigma-Aldrich
Chemical Co. (St. Louis, MO, USA). All drugs were freshly prepared in 5% dextrose
as solution before intrathecal injections.
2.3. Experimental protocol
Three experiments were carried out. In experiment 1, the potencies of propranolol
(0.27, 0.50, 1.00, 1.75, 2.50 µmol) and lidocaine (0.25, 0.50, 0.75, 1.00, 2.50 µmol)
on spinal anesthesia were evaluated (n=8 rats for each dose of each drug). In
spinal blockade of propranolol at the dose of 2.5 μmol was compared to lidocaine
(n=8 rats for each dose of each drug). In experiment 3, on an equipotent basis (0.5, 1.0,
and 2.5 μmol), the spinal block duration of propranolol was compared with that of
lidocaine. (n=8 rats for each dose of each drug).
2.4. Spinal anesthesia
2.4.1. Intrathecal drug injection
Intrathecal injections of drugs were performed in conscious rats. Following an
optimal flexion of the rat lumbar spine under prone position, each 50-µL of 1%
lidocaine was injected into the right and left side of paraspinal space (0.5 cm in depth)
which was 0.5 cm away from the mid-point of the longitudinal line of L4–5
intervertebral space. Two minutes later, a 27-gauge needle attached to a 50-µL
syringe (Hamilton, Reno, Nevada) was inserted into the mid-line of the L4–5
intervertebral space and advanced at a slightly caudal angle until a tail-flick indicated
entrance into the intrathecal space. Twenty-five microliters of drug were injected and
the rat was observed for the development of spinal blockade, indicated by paralysis of
both hind limbs (Chen et al., 2007; Leung et al., 2010). Rats, which showed unilateral
blockade, were excluded from the study and sacrificed by using an over dose of
isoflurane. All rats were injected intrathecally one time in this study.
After intrathecal drug injection, three neurobehavioral examinations, which
consisted of evaluations of motor, proprioception, and nociception, were conducted
(Chen et al., 2010a; b; Hung et al., 2009). For consistency, one trained examiner was
responsible for handling of all rats and behavioral evaluations. The magnitude of
spinal blockade (motor, proprioception, and nociception) was described as the
percentage of possible effect (% PE). The maximum blockade in a time course of
spinal anesthesia of drugs was described as the percent of maximum possible effect
(% MPE). In brief, the motor function was evaluated by measuring 'the extensor
postural thrust' of the right hind limb of each rat on a digital scale. The evaluation of
proprioception was based on the resting posture and postural reactions (‘tactile placing’ and ‘hopping’). The functional deficit was graded as 3 (normal or 0% MPE), 2 (slightly impaired), 1 (severely impaired), and 0 (completely impaired or 100%
MPE). The nociception was evaluated using the withdrawal reflex or vocalization
elicited via pinching a skin fold on each rat's back at 1 cm from the proximal part of
the tail, the lateral metatarsus of the right hind limb, and the dorsal part of the
mid-tail.
2.5. The 50% effective dose (ED50)
After intrathecally injecting the rats with different doses of each drug (n = 8 for
each dose of each drug, The curve was then fitted using a computer-derived SAS
NLIN analysis (SAS Institute Inc., Carey, NC), and the values of 50% effective doses,
defined as the doses that caused 50% spinal anesthesia, were obtained (Hung et al.,
2010; Chen et al., 2010a; Chen et al., 2010b).
2.6. The spinal block duration
The blockade duration (n = 8 rats for each dose of each drug) caused by each drug
was also evaluated on an equipotent basis (0.5, 1.0, and 2.5 μmol). The duration of
each blockade, defined as the interval from drug injection to full recovery, was
measured and compared. In this study, the area under curve (AUC) of motor,
proprioception, and nociception of propranolol and lidocaine at the same dose of 2.5 μmol was estimated using Kinetica version 2.0.1 (InnaPhase Corporation, Philadelphia, PA).
2.7. Statistical Analysis
Values are presented as means ± SE or ED50 values with 95% confidence interval
(95% CI). Data were evaluated by either student-t test (experiment 1 and 2) or 2-way
(experiment 3) analysis of variance (ANOVA) followed by pairwise Tukey's honest
significance difference (HSD) test. The full recovery time and AUCs of motor,
proprioception, and nociception was evaluated by 1-way (experiment 2) ANOVA
(version 17.0, SPSS, Inc, Chicago, IL, USA), was used, and a P value less than 0.05
3. Results
3.1. The spinal blockade of propranolol
Intrathecal propranolol, as well as lidocaine, produced dose-dependent effects on
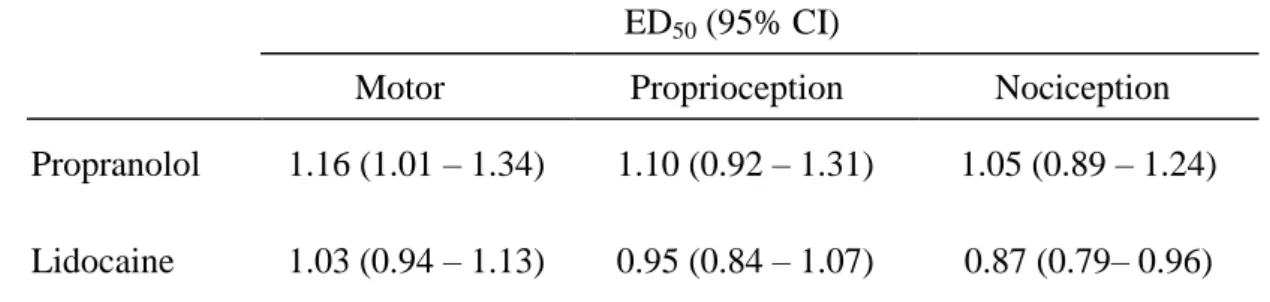
spinal anesthesia in rats (Fig. 1). The ED50s of propranolol and lidocaine in motor,
proprioception, and nociception are shown in Table 1. On the ED50 basis, the spinal
blockade of propranolol in motor, proprioception, and nociception is similar to
lidocaine (Table 1).
3.2. The spinal blockade of propranolol and lidocaine at the dose of 2.5 μmol
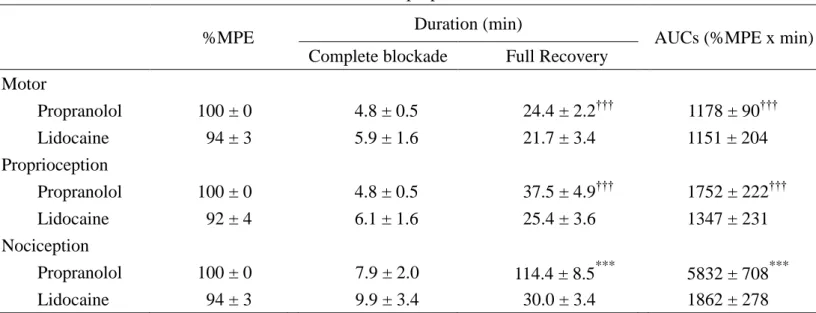
The time course of spinal blockade of propranolol and lidocaine in motor function,
proprioception, and nociception has been demonstrated in Figure 2. At this given dose
of 2.5 μmol, propranolol showed 100, 100, and 100% of blockades (% MPE) in motor
function, proprioception, and nociception with duration of action of about 24, 38, and
114 min, respectively. At the same given dose, lidocaine performed 94, 92, and 94%
of blockades in motor function, proprioception, and nociception with duration of
action of about 22, 25, and 30 min, respectively (Fig. 2 and Table 2). Of note,
propranolol at the dose of 2.5 μmol performed complete blockade (100% MPE) of
motor function, proprioception, and nociception, but not lidocaine (Fig. 2 and Table
2).
anesthesia
The full recovery time and AUCs of nociceptive blockade are longer than the
motor or proprioceptive blockade for propranolol, but not lidocaine (P < 0.001 for the
differences; Fig. 2 and Fig. 3). The full recovery time and AUCs of spinal blockade of
propranolol significantly are greater than those of lidocaine in nociception (Fig. 2 and
Table 2). However, the duration of complete spinal blockade of propranolol is similar
to that of lidocaine in motor function, proprioception, and nociception (Fig. 2 and
Table 2).
3.4. On an equipotent basis, the spinal block duration in nociception caused by
propranolol was longer than that caused by lidocaine
Durations were measured as an interval from the time zero at the time of injection
to the time of complete functional recovery. On an equipotent basis (0.5, 1.0, 2.5
µmol), the spinal block duration in nociception caused by propranolol was longer than
that caused by lidocaine (Fig. 3). All rats recovered completely after intrathecal drug
4. Discussion
This study indicated for the first time that intrathecal propranolol produced a
dose-dependent spinal anesthesia in rats. Propranolol was similar to lidocaine at
producing spinal anesthesia. On an equipotent basis, propranolol showed the longer
action of spinal blockade than lidocaine.
The adrenergic system is a prime controller of blood pressure. Though we have
made no study about the known cardiovascular effects, the previous study showed that centrally active propranolol (β1+2+[3], 44 μmol/kg) had little effect on blood pressure, cardiac output, heart rate, and total peripheral vascular resistance in
normotensive rats (Berg et al., 2010). Furthermore, our study performed the lower dose of 7.7μmol/kg of centrally administered propranolol to produce spinal anesthesia. We suggest that the lower dose of intrathecal propranolol may have no effect on
cardiovascular baselines (e.g. blood pressure), and it is worth studied in the future.
Adrenergic antagonists have long been known to affect nerve function.
Beta-blockers inhibit nerve conduction at millimolar-range concentrations (Sada and
Ban, 1981). The high concentrations of adrenergic antagonists markedly potentiate the
duration of block of tetrodotoxin, by an effect that does not appear to be adrenergic
receptor-specific (Kohane et al., 2001). Co-injection with 20 mM propranolol
al., 2001). Local anesthetics produce neural blockade via blocking the Na+ currents in
the nervous tissues through the voltage-gated Na+ channels (McLure and Rubin,
2005). The beta-blockers propranolol blocks, moreover, Na+ current in a manner
similar to the blocking of local anesthetic drugs (Bankston and Kass, 2010), and
propranolol efficacy is dependent on the inactivated state of the channel and blocks
late non-inactivating current more effectively than peak Na+ current (Bankston and
Kass, 2010). These data can support that propranolol produced spinal anesthesia in
rats and propranolol administration into the sciatic nerve area produced
neuromuscular blocking activity and local anesthesia (Leszczynska and Kau, 1992) in
mice. In this report, we also demonstrated that propranolol produced the similar
potency of spinal blockades to lidocaine.
Propranolol contains both hydrophobic and hydrophilic groups. In addition, it
contains a secondary amine, and the same chemical characteristic exists in some local
anesthetics. We demonstrated that intrathecal propranolol showed motor, sensory and
proprioceptive blocking effects, suggesting the local anesthetic characteristics of
propranolol. Intrathecal propranolol (2.5 μmol) produced complete spinal anesthesia
with drug action of about 5-8 min and duration of spinal blockades with drug action
of about 24-114 min.
control is frequently practiced (Gurlit et al., 2004). The duration of spinal blockade is
defined as the interval from drug injection to full recovery of blockades. Intrathecal
propranolol and lidocaine at equipotent doses (0.5, 1.0, and 2.5 μmol) were studied.
Our results showed that the duration of spinal blockade in nociception caused by
propranolol was longer than that caused by lidocaine on an equipotent basis (Fig. 3).
Besides, sensory block duration of propranolol was longer than that of lidocaine on an
equivalent dose (2.5 μmol) (Fig. 2 and Table 2). Treatment with long-acting local
anesthetic propranolol for surgery and postoperative pain control is worth studied in
the future.
Intrathecal propranolol produced a longer duration of sensory blockade than the
motor blockade, but not lidocaine (Fig. 2 and Fig. 3). This is in resemblance to the
clinical impression that lidocaine is not the drug of choice when a more
sensory-selective action over motor blockade. Besides, the AUC of propranolol in
nociceptive blockade was almost 5.0-folds larger than that in motor function. We
suggested that the pharmacokinetic differences, such as absorption, distribution and
metabolism of propranolol and lidocaine, may account for the differences in the
duration of action of the two drugs.
We did not evaluate whether propranolol had spinal nerve toxicity, however, it is
intrathecal drug injection. Furthermore, there is no an emerging sedative effects of
centrally administered propranolol. All rats recovered completely. Histologic studies
must be performed in the future before the possible use of propranolol as spinal
analgesic in humans.
In conclusion, intrathecal propranolol produced similar potency to lidocaine on
spinal anesthesia, and propranolol showed longer spinal anesthetic action with a
Acknowledgements
The authors gratefully acknowledge the financial support provided for this study
by the National Science Council of Taiwan (NSC 98-2314-B-006-017-MY3; NSC
References
Bankston, J.R., Kass, R.S., 2010. Molecular determinants of local anesthetic action of
beta-blocking drugs: Implications for therapeutic management of long QT
syndrome variant 3. J Mol Cell Cardiol 48, 246-253.
Berg, T., Piercey, B.W., Jensen, J., 2010. Role of beta1-3-adrenoceptors in blood
pressure control at rest and during tyramine-induced norepinephrine release in
spontaneously hypertensive rats. Hypertension 55, 1224-1230.
Buckmiller, L.M., 2009. Propranolol treatment for infantile hemangiomas. Curr Opin
Otolaryngol Head Neck Surg 17, 458-459.
Chen, Y.W., Chen, Y.C., Lin, C.N., Chu, C.C., Lin, M.T., Wang, J.J., Kao, C.H., 2007.
The spinal anaesthetic effect of dextromethorphan, dextrorphan, and
3-methoxymorphinan. Eur J Pharmacol 569, 188-193.
Chen, Y.W., Chu, C.C., Chen, Y.C., Wang, J.J., Hung, C.H., 2010a. The
dose-dependent study of verapamil and diltiazem on spinal anesthesia in the rat.
Neurosci Lett 482, 76-80.
Chen, Y.W., Chu, C.C., Chen, Y.C., Wang, J.J., Hung, C.H., 2010b. Isobolographic
analysis of caramiphen and lidocaine on spinal anesthesia in rats. Neurosci Lett
469, 174-178.
antagonist, reduces Na(+) influx into cortical synaptosomes by direct interaction
with Na(+) channels: comparison with other beta-adrenoceptor antagonists. Br J
Pharmacol 130, 759-766.
Frishman, W.H., Shapiro, W., Charlap, S., 1989. Labetalol compared with propranolol
in patients with both angina pectoris and systemic hypertension: a double-blind
study. J Clin Pharmacol 29, 504-511.
Frullani, F., Merigo, A., Novelli, G.P., 1970. [Anesthetic local effect of propranolol
and practololol (ICI 50. 172) and related cardiac implications]. Minerva
Anestesiol 36, 41-44.
Gurlit, S., Reinhardt, S., Mollmann, M., 2004. Continuous spinal analgesia or
opioid-added continuous epidural analgesia for postoperative pain control after
hip replacement. Eur J Anaesthesiol 21, 708-714.
Heaton, L.J., McNeil, D.W., Milgrom, P., 2010. Propranolol and D-cycloserine as
adjunctive medications in reducing dental fear in sedation practice. SAAD Dig
26, 27-35.
Hess, O.M., Grimm, J., Krayenbuehl, H.P., 1983. Diastolic function in hypertrophic
cardiomyopathy: effects of propranolol and verapamil on diastolic stiffness. Eur
Heart J 4 Suppl F, 47-56.
oxybuprocaine and proxymetacaine produced potent and long-lasting spinal
anesthesia in rats. Neurosci Lett 454, 249-253.
Kohane, D.S., Lu, N.T., Crosa, G.A., Kuang, Y., Berde, C.B., 2001. High
concentrations of adrenergic antagonists prolong sciatic nerve blockade by
tetrodotoxin. Acta Anaesthesiol Scand 45, 899-905.
Leaute-Labreze, C., Dumas de la Roque, E., Hubiche, T., Boralevi, F., Thambo, J.B.,
Taieb, A., 2008. Propranolol for severe hemangiomas of infancy. N Engl J Med
358, 2649-2651.
Leszczynska, K., Kau, S.T., 1992. A sciatic nerve blockade method to differentiate
drug-induced local anesthesia from neuromuscular blockade in mice. J
Pharmacol Toxicol Methods 27, 85-93.
Leung, Y.M., Wu, B.T., Chen, Y.C., Hung, C.H., Chen, Y.W., 2010. Diphenidol
inhibited sodium currents and produced spinal anesthesia. Neuropharmacology
58, 1147-1152.
Linde, K., Rossnagel, K., 2004. Propranolol for migraine prophylaxis. Cochrane
Database Syst Rev, CD003225.
Matthews, J.C., Baker, J.K., 1982. Effects of propranolol and a number of its
analogues on sodium channels. Biochem Pharmacol 31, 1681-1685.
treatment for infantile airway hemangiomas. Int J Pediatr Otorhinolaryngol 74,
323-325.
McLure, H.A., Rubin, A.P., 2005. Review of local anaesthetic agents. Minerva
Anestesiol 71, 59-74.
Sada, H., Ban, T., 1981. Frequency-dependent block of nerve conduction by
beta-adrenergic blocking agents. Arch Int Pharmacodyn Ther 254, 134-144.
Scholz, A., 2002. Mechanisms of (local) anaesthetics on voltage-gated sodium and
other ion channels. Br J Anaesth 89, 52-61.
Sheets, M.F., Hanck, D.A., 2003. Molecular action of lidocaine on the voltage sensors
of sodium channels. J Gen Physiol 121, 163-175.
Siegfried, E.C., Keenan, W.J., Al-Jureidini, S., 2008. More on propranolol for
hemangiomas of infancy. N Engl J Med 359, 2846; author reply 2846-2847.
Tchivileva, I.E., Lim, P.F., Smith, S.B., Slade, G.D., Diatchenko, L., McLean, S.A.,
Maixner, W., 2010. Effect of catechol-O-methyltransferase polymorphism on
response to propranolol therapy in chronic musculoskeletal pain: a randomized,
double-blind, placebo-controlled, crossover pilot study. Pharmacogenet
Genomics 20, 239-248.
Zimmermann, A.P., Wiegand, S., Werner, J.A., Eivazi, B., 2010. Propranolol therapy
Table 1. The 50% effective doses (ED50s) of propranolol and lidocaine on spinal blockades of motor, proprioception, and nociception
ED50 (95% CI)
Motor Proprioception Nociception
Propranolol 1.16 (1.01 – 1.34) 1.10 (0.92 – 1.31) 1.05 (0.89 – 1.24) Lidocaine 1.03 (0.94 – 1.13) 0.95 (0.84 – 1.07) 0.87 (0.79– 0.96) ED50s of drugs (μmol) were obtained from Figure 1. CI = confidence interval. The differences between propranolol and lidocaine on ED50s of motor, proprioception, and nociception are not significant.
Table 2. The %MPE, duration, and AUCs of intrathecal propranolol and lidocaine
%MPE Duration (min) AUCs (%MPE x min)
Complete blockade Full Recovery Motor Propranolol 100 ± 0 4.8 ± 0.5 24.4 ± 2.2††† 1178 ± 90††† Lidocaine 94 ± 3 5.9 ± 1.6 21.7 ± 3.4 1151 ± 204 Proprioception Propranolol 100 ± 0 4.8 ± 0.5 37.5 ± 4.9††† 1752 ± 222††† Lidocaine 92 ± 4 6.1 ± 1.6 25.4 ± 3.6 1347 ± 231 Nociception Propranolol 100 ± 0 7.9 ± 2.0 114.4 ± 8.5*** 5832 ± 708*** Lidocaine 94 ± 3 9.9 ± 3.4 30.0 ± 3.4 1862 ± 278
Percent of maximal possible effect (%MPE), duration of drug action, and area under curves (AUCs) of motor, proprioception, and nociception (means SE) for propranolol and lidocaine at the same dose of 2.5 μmol (n = 8). Of note, all of the rats in the propranolol group showes complete blockade (100% MPE) of any function tested. Symbols (***) indicate P < 0.001 when propranolol compared to lidocaine; Symbols (†††) indicate P < 0.001 when nociception compared to motor or proprioception.
0.1 1 10 % MPE (ma xi ma l po ssib le e ffe ct) 0 20 40 60 80 100 Lidocaine Propranolol 0.1 1 10 % MPE (ma xi ma l po ssib le e ffe ct) 0 20 40 60 80 100 Dose (mol) 0.1 1 10 % MPE (ma xi ma l po ssib le e ffe ct) 0 20 40 60 80 100
Motor
Proprioception
Nociception
Fig. 1.
0 15 30 45 60 75 90 105 120 135 150
%PE
(
po
ssible
e
ffe
ct
)
0 20 40 60 80 100 Motor Proprioception NociceptionTime (min)
0 15 30 45 60 75%PE
(
po
ssible
e
ffe
ct
)
0 20 40 60 80 100Propranolol
Lidocaine
Fig. 2.
0.5 1.0 1.5 2.0 2.5 Full Recover y Tim e (m in) 0 40 80 120 Propranolol (P) Lidocaine (L) Dose (mol) 0.5 1.0 1.5 2.0 2.5 Full Recover y Tim e (m in) 0 40 80 120 0.5 1.0 1.5 2.0 2.5 Full Recover y Tim e (m in) 0 40 80 120
Motor
Proprioception
Nociception
P > L P = L P = LFig. 3.
Figure Legends
Fig. 1. The dose—response curves of propranolol and lidocaine on spinal blockades
of motor, proprioception, and nociception (% MPE) in rats (n = 8 at each testing
point). Data are means ± SE.
Fig. 2. Time courses of spinal anesthesia of propranolol and lidocaine at the same
dose of 2.5 μmol in rats. Values are expressed as means SE. Each testing point of
the time course study contained eight rats.
Fig. 3. Full recovery time of drug action on spinal blockades (% MPE) of motor,
proprioception, and nociception at three doses of 0.5, 1.0, and 2.5 μmol (n = 8 at each