J. CHEM. SOC., CHEM. COMMUN., 1995 2537
Synthesis of Furan-, Thiophene- and Pyrrole-fused Sultines and their Application in
Diels-Alder Reactionst
Wen-Sheng Chung," Wen-Ju Lin, Wen-Dar Liu and Liang-Gyi Chen
Department
o f
Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan 30050, R. 0. C.The synthesis of 1,4-dihydrofurano[3,4-d]-3,2-oxathiine 2-oxide 8, 5,7-dimethyl-I ,4-dihydrothieno[3,4-d]-3,2-oxathiine 2-oxide 9 and 1,4-dihydro-6-tosylpyrrolo[3,4-d]-3,2-oxathiine 2-oxide 10, precursors for nonclassical heteroaromatic o-quinodimethanes, and their application in the Diels-Alder reactions are reported.
The chemistry of heterocyclic analogues of o-quinodimethane (o-QDM, 1) has attracted a great deal of attention recently, and
several reports of the generation of the furan and pyrrole derivatives have appeared. The compounds 3,4-dimethyl- idenefuran 2, 3,4-dimethylidenethiophene 3 and 3,4-dimethyl-
idenepyrrole 4 are n-conjugated non-KekulC molecules that
have aroused theoretical and synthetic interest. 1,2 These 3,4-di-
methylidene-heteroaromatics 2-4 were generated from the
corresponding diazenes2 and have been detected by EPR, UV and NMR spectroscopy. They have also been shown by Berson and coworkers2 to react with a series of alkenes to form two types of cycloadducts: fused and bridged. The results are synthetically useful and have been elaborated upon by Takayama and coworkers in syntheses of multicyclic com- pounds. 1 b ~ c
Diazene precursors are usually unstable at room temperature, therefore the search for possible substitutes becomes important. Various methods for generation of these highly reactive dienes have been developed.3.4 Among the many known methods for their preparation, that involving cheletropic elimination of SO2 from heteroaromatic-fused 3-sulfolenes 5-7 has drawn the most
Durst e t a l . were the first to generate o-QDM by
thermal elimination of SO2 from a sultine, 1,4-dihydro- 2,3-benzoxathiine 3-oxide.5 A significant advantage of using sultines is that their thermolysis occurs at a much lower temperature than that of corresponding sulfolenes (80 vs.
170-220 "C). Recently other papers on using sultines as o-QDM precursors have appeared,6,7 however, the use of sultines in heteroaromatic analogues is still rare. We report here our work on the synthesis of furanosultine 8, thienosultine 9 and pyrrolosultine 10 and their applications in Diels-Alder reac-
tions with alkenes and alkynes.
Previously unknown furanosultine 8 and thienosultine 9 are synthesized in two steps with good yield, as shown in Scheme 1. The 3,4-bis(chloromethyl)furan 11 was prepared by the known
method.2b.8 The bis(chloromethy1)thiophene 12 was obtained
by chloromethylation of 2,5-dimethylthiophene adapted ac- cording to the procedures developed by Wynberg et al. in the
synthesis of corresponding sulfones 6.9 The 3,4-bis(chlor- omethyl)-N-tosylpyrrole 13 was synthesized in four steps (41 %
overall yield) as described by Berson and co-workers in the syntheses of corresponding diazenes.2 The key step in the syntheses of sultines 8-10 is the use of Rongalite6-7~ (sodium formaldehyde sulfoxylate) with the corresponding dichlorides The Diels-Alder reactions of furan-fused sultine 8 with several dienophiles are presented in Table 1 (entries 1-5) and Scheme 2. Heating 8 with 3 equivalents of dimethyl acetyl-
enedicarboxylate (DMAD) in benzene at 120-123 "C in a sealed tube for 1 h produced 5,6-dimethylidene-7-oxanorborn- ene 14a in 38% yield plus some polymer by-products (Table 1,
entry 1). Essentially the same types of reactions were observed with diethyl fumarate (DEF), dimethyl maleate (DMM) and fumaronitrile (FN) (Table 1, entries 2-4). With N-phenyl- maleimide (NPM), thermolysis at 134-140 "C gave a new type of product 15e in 24% yield. The low to medium yields of these reactions of furanosultine 8 with various dienophiles are disappointing, however, as their reaction products are different from those reported by Takayama and co-workers for the reactions of furan-fused sulfolene 5,1hpc where two types of products 14 and 15 were formed. Furthermore, the high yield of
intractable polymers in all cases also implies that radical or biradical-initiated polymerization is involved.
Thermal Diels-Alder reaction of 2,5-dimethylthiopheno- sultine 9 with several dienophiles was studied next (Table 1,
entries 6-9). In the absence of a dienophile, sultine 9 underwent thermolysis to give the sulfolene 6 in excellent yield (90%,
entry 6). Heating 9 with DMAD, DMF or NPM at 180 "C gave both the isomerized sulfolene 6 and fused adducts 16g and 16h
in 89-97% yields. Unexpectedly, no adducts were formed when the thiophene-fused sulfolene 6 was heated in the presence of
DMAD or DMF at the same reaction temperature (Table 1, entries 10-11). About 10% of the fused adduct 16h was
obtained only when a strong dienophile such as NPM was used (entries 9, 12). Wynberg et al.9a were the first to synthesize
thiophene-fused sulfolene 6b (R = COZMe), however, they did not detail its chemical reactivities other than pyrolysis. Our results show that thiophene-fused sulfolene 6 has very low
reactivity compared to the corresponding sultine 9 in the Diels-
11-13. $ 1 2 x = o 3 x = s 4 X = N R R R R R 5 X = O 8 R = H , X = O 11 R = H , X = O 7 X = NR 6 X = S 9 R = M e , X = S 1 2 R = M e , X = S 10 R = H, X = N - S O Z C ~ H ~ 13 R = H, X = N-SO2C7H7 11 8 13 10
Scheme 1 Reagents and conditions: i, A1Cl3, diethyl ether, room temp.; ii, Rongalite, TBAB, DMF, 25 "C; iii, C1CH20Me, SnC14-CS2
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:07:16.
2538
Alder reaction. Thus, in the reaction conditions studied here, the fused Diels-Alder adducts 16g-h can be obtained from the reaction of sultine 9 with dienophiles, but not from sulfolene 6.
It is interesting to observe the great similarity between our results with sultines with those with diazenes reported by Berson et aZ.2 For example, in the thiophene-biradical 3 trapping experiments, the sole adducts found (85-100% yields) had the fused structure 16 rather than the bridged structure
14.
Reactions of N-tosylpyrrolosultine 10 with a series of dienophiles (3 equiv.) at 150-170 "C run smoothly to give three types of products: sulfolene 7 (20-40%), 1 : 2 Diels-Alder adducts 15 ( 0 4 2 % ) and fused adducts 16 (5-73%) (Table 1, entries 13-18). Basically the same reaction products are obtained even at 110 "C (Table 1, entry 15), however to our surprise, only 7 and the fused adduct 16i were obtained when
E
+
111
EJ. CHEM. SOC., CHEM. COMMUN., 1995
DMAD was reduced to 1 equiv. (entry 14). The N-tosylpyrrolo- sulfolene 7 had been shown to be unreactivelo with dimethyl fumarate (DMF) even at 240 "C, however the N-tosylpyrrolo- sultine 10 reacted with DMF at 170 "C to give a fused adduct
16j (63%) and the isomerized 7 (32%) (Table 1 , entry 16 vs. 20).
It is also of interest to isolate the fused adduct 16i (5%) in the reaction of sultine 10 with DMAD compared with that of sulfolene 7 , l h ~ ' where 15i was the only product formed (entry 13
vs. 19).
These results can be explained by two mechanisms. The most obvious possibility is the formation of non-Kekulk biradicals
(2-4), followed by Diels-Alder reaction with a dienophile to
form either bridged (14) or fused adducts (16).2 Both adducts can add another dienophile to form the 1 : 2 adducts (15).
Alternatively, a Diels-Alder reaction may first occur on the aromatic moieties of sultines 8-10 to give 17, from which SO2
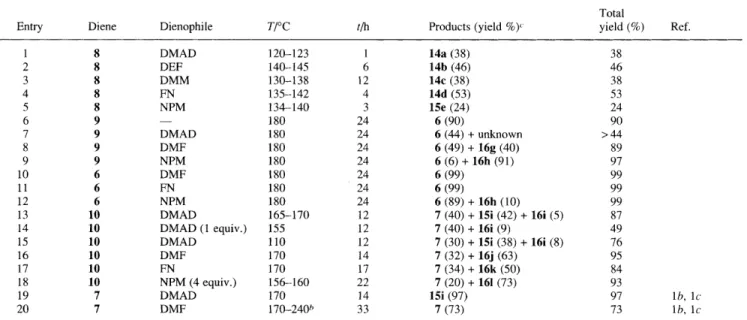
Table 1 The Diels-Alder reactions of sultines 8-10 and sulfolenes 6-7 with dienophiles"
Total
Products (yield %)c yield (%) Ref.
Entry Diene Dienophile T I T tlh
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 8 8 8 8 8 9 9 9 9 6 6 6 10 10 10 10 10 10 7 7 DMAD DEF DMM FN NPM DMAD DMF NPM DMF FN NPM DMAD DMAD (1 equiv.) DMAD DMF FN NPM (4 equiv.) DMAD DMF - 120-1 23 140-145 130-138 135-142 134-140 180 180 180 180 180 180 180 165-170 155 110 170 170 156-160 170 170-240h 1 6 12 4 3 24 24 24 24 24 24 24 12 12 12 14 17 22 14 33 14a (38) 14b (46) 14c (38) 14d (53) 15e (24) 6 (90) 6 (44) + unknown 6 (49) + 16g (40) 6 (6) + 16h (91) 6 (99) 6 (99) 6 (89) + 16h (10) 7 (40)
+
1 5 (42) + 16i ( 5 ) 7 (40)+
16i (9) 7 (30)+
15i (38)+
16i (8) 7 (32)+
16j (63) 7 (34)+
16k (50) 7 (20)+
161 (73) 15i (97) 7 (73) 38 46 38 53 24 90 > 44 89 97 99 99 99 87 49 76 95 84 93 97 lb, l c 73 lb, l ca Reactions were run with 3 equiv. of dienophiles in benzene (sealed tube) unless otherwise specified. b Reaction was run in xylene. Isolated yield. For structures of products 14a-161 see Scheme 2.
R \ n-
* b f "
-SO* "+o I Ad
8-1 0 7 - 2-4 R + dienophile * + dienophile * R x y J E E R 16a-I R E R 14a-d R 5-7 R R 15a-I RScheme 2 Possible reaction pathways
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:07:16.
J. CHEM. SOC., CHEM. COMMUN., 1995 2539
is eliminated instantaneously to give bridged adducts 14.
Compound 14 further reacts with another dienophile to give the
1 : 2 adducts 15, and finally a retro-Diels-Alder reaction of 15
would occur to form 16 (Scheme 2). The latter mechanism was
proposed by Takayama to explain results with corresponding sulfolenes 5 and 7. We conclude that the furano-, thieno- and N -
tosylpyrrolo-fused sultines 8-10 reacted under milder condition
than the corresponding sulfones 5-7 and their reaction products were different in many cases. When generated in the presence of a dienophile, they can provide elegant synthons for the formation of [4
+
21 cycloadducts. If less reactive trapping agents were used, the diene was recaptured by SO2 to afford the sulfones 5-7 (path 3 in Scheme 2).”We thank the National Science Council of the Republic of China for financial support (Grant No. NSC 84-2113-M- 009-002). W. S. C. would like to thank Professor J. A. Berson for his encouragement and valuable suggestions.
Received, 11 th September 1995; Com. 5105968B
Footnotes
?- Presented in the 15th International Congress of Heterocyclic Chemistry, Taipei, R. 0. C., August, 1995, Book of Abstracts, PO3-257.
$ Dichlorides 11,2 Wh and 132 have been reported in the literature and our samples correspond in all respects with the reported properties. For all products satisfactory spectral data were obtained. Selected data for 8: light yellow oil; lH NMR (300 MHz, CDC13) 6 7.40 (1 H, s), 7.36 (1 H, s), 5.28
(lH,AB,J14.4Hz),5.02(1H,AB,J14.4Hz),3.87(1 H,A’B’,J15.6Hz) and 3.65 (1 H, A‘B’, J 15.3 Hz); I3C NMR (75.4 MHz, CDC13) 6 141.01 (CH), 136.38 (CH), 113.92 (C,), 108.00 (C,), 55.03 (CH2) and 46.64 (CH2). For 9: white solid, mp 70-71 “C; ‘H NMR, 6 5.16 (1 H, A B,J 14.1 Hz), 4.92 (1 H, AB, J 13.5Hz), 3.88 ( I H, A’B’, J 15.6Hz), 3.58 ( 1 H, A‘B’, J 15.0 Hz) and 2.27 (6 H, s); 13C NMR, 133.15 (C,), 128.72 (C,), 125.40 (C,), 119.13 (C,), 58.73 (CH,), 50.92 (CH2) and 12.1 1 (CH3); mlz 202 (M+), 138 (loo%), 123 and 91; (Found: M+ 202.01 16 C8HI0O2S2 requires 202.0122). For 10, white solid, mp 152-153 OC; ‘H NMR, 6 7.76 (2 H, d, J 8.8 Hz), 7.31 (2 H, d , J 8.1 Hz), 7.07 ( I H, s), 7.01 ( l H , S), 5.20 (1 H, AB, J 14.2 Hz), 4.91 (1 H, AB, J 14.2 Hz), 3.88 (1 H, A’B’, J 15.6 Hz), 3.56 (lH, A’B’, J 15.6 Hz) and 2.42 (3 H, s); 13C NMR, 145.50 (C,), 135.54 (C,), 130.15 (CH), 127.03 (CH), 119.19 (CH), 117.12 (C,), 114.44 (CH), 110.97 (C,), 55.99 (CH2), 48.21 (CH2) and 21.64 (CH3); mlz 31 1 (M+), 247 (loo%), 155 and 92. References 1 2 3 4 5 6 7 8 9 10 11
For recent review see; ( a ) R. A. Aitken, I. Gosney and J. I. G. Cadogan, Prog. Heterocycl. Chem., 1992, 4, I ; 1993, 5 , 1; ( b ) T.-S. Chou, Rev. Heteroatom Chem., 1993, 8, 65; ( c ) K. Ando and H. Takayama, Heterocycles, 1994,37, 1417; (4 K. Ando, M. Kankake, T. Suzuki and H. Takayama, Tetrahedron, 1995, 51, 129.
( a ) L. C. Bush, R. B. Heath and J. A. Berson, J . Am. Chem. Soc., 1993, 115,9830; (b) M. M. Greenberg, S. C. Blackstock, K. J. Stone and J. A. Berson, J . A m . Chem. SOC., 1989, 111, 3659; (c) K. J. Stone, M. M. Greenberg, S. C. Blackstock and J. A. Berson, J . Am. Chem. Soc., 1989, 111, 3671 and references cited therein.
S. Braverman, Y. Duar and D. Segev, Tetrahedron Lett., 1976, 3181;
P. J. Garratt and S. B. Neoh, J. Org. Chem., 1979, 44, 2667. J. L. Charlton and M. M. Alauddin, Tetrahedron, 1987, 43, 2873; R. L. Funk and K. P. C. Vollhart, Chem. SOC. Rev., 1980,9,41; W. Oppolzer, Synthesis, 1978,793; K. C. Nicolaou, W. E. Barnette and P. Ma, J . Org. Chem., 1980,45, 1463.
T. Durst, J. L. Charlton and D. B. Mount, Can. J . Chem., 1986,64,246; J. L. Charlton and T. Durst, Tetrahedron Lett., 1984,25, 5287; F. Jung, M. Molin, R. Van Den Elzen and T. Durst, J . Am. Chem. Soc., 1974,96, 935.
For a review of sultines see: D. C. Dittmer and M. D. Hoey, The Chemistry of Sulphinic Acids, Esters and Their Derivatives, Wiley, Chichester, 1990, pp. 239-273.
( a ) M. D. Hoey, D. C. Dittmer, J . Org. Chem., 1991,56,1947; ( b ) W. F. Jarvis, M. D. Hoey, A. L. Finocchio and D. C. Dittmer, J . Org. Chem., 1988, 53, 5750; ( c ) G. Attardo, W. Wang, J.-L. Kraus and B. Belleau, Tetrahedron lett., 1994, 35, 4743.
M. P. Cava and A. A. Deana, J . Am. Chem. SOC., 1959,81,4266; C. E. Doecke, P. J. Garratt, H. Shahriari-Zavareh and R. Zahler, J . Org. Chem., 1984, 49, 1412.
( a ) H. Wynberg and D. J. Zwanenburg, J . Org. Chem., 1964,29, 1919;
( b ) D. J. Zwanenburg and H. Wynberg, J . Org. Chem., 1969, 34, 333. K. Ando, M. Kankake, T. Suzuki and H. Takayama, Synlett., 1994,741; K. Ando, M. Kankake, T. Suzuki and H. Takayama, J . Chem. Soc., Chem. Commun., 1992, 1100.
Sulfur dioxide appears to be an especially reactive trapping agent which can intercept the furan, thiophene and pyrrole xylenes. See for example: P. M. S. Chauhan, A. P. A. Crew, G. Jenkins, R. C. Storr, S. M. Walker and M. Yelland, Tetrahedron Lett., 1990, 31, 1487; 1491.
Published on 01 January 1995. Downloaded by National Chiao Tung University on 28/04/2014 16:07:16.